49 Care of the pediatric patient
Anesthetic: An agent used to produce anesthesia.
Anesthesia: Partial or complete loss of sensation, with or without loss of consciousness; referred to in this chapter as the administration of an anesthetic agent via injection or inhalation.
Antagonist: To counteract the action of something else, such as a drug that binds to a receptor site and prevents receptor stimulation.
Anticholinergic: A parasympatholytic; blocks the parasympathetic nerve fibers and parasympathetic nerve impulse conduction.
Anxiolytic: Medication used to reduce, relieve, or counteract anxiety.
Apnea/Apneic: Suspension of breathing.
Aspiration: The general use of this term is to draw in or out via suction; however, specifically referred to in this chapter are situations in which an individual is at risk for entry of gastric secretions, oropharyngeal secretions, or exogenous food or fluids into tracheobronchial passages, because of loss of the normal protective mechanisms as occurs with induction of general anesthesia.
Atelectasis: A collapse, lack of expansion, or airless condition of the lungs.
Barotrauma: An injury caused by a change in atmospheric pressure relative to a potentially closed space within a surrounding area.
Child: Younger than 13 years of age; before puberty.
Conception: Onset of pregnancy with implantation of a fertilized ovum in the uterine wall; fertilization.
Delirium: An acute and reversible condition characterized by agitation, confusion, disorientation, hallucinations or delusions, difficulty focusing attention, and inability to rest.
Desaturation: When oxygen is dissociated from hemoglobin.
Dissociative: A type of anesthesia with marked catalepsy, amnesia, and analgesia.
Dysphoria: A mood disorder of restlessness without apparent cause, anxiety, dissatisfaction, and discomfort.
Emergence: To evolve or rise out of anesthesia to a level of consciousness and status of protective reflexes, motor activity, and orientation.
Emergence Delirium: Occurs during initial cessation from general anesthesia to an awake state and initial transfer into the postanesthesia care unit.
Erythropoiesis: Forming red blood cells.
Gestation: Period of intrauterine fetal development from conception to birth.
Hemostasis: To stop bleeding; stasis refers to standing still.
Hypercarbia, Hypercapnia: Elevated above normal levels of carbon dioxide in the blood (>45 mm Hg).
Hyperflexion: Increased flexion of a joint; in this text, refers to the neck.
Hyperoxia: Increased levels of oxygen in the blood.
Hyperthermia, Hyperpyrexia: An elevated body temperature greater than the normal range.
Hypervolemia: An abnormal increase in circulating blood volume.
Hypothermia: A lower body temperature below normal range.
Hypovolemia: An abnormal decrease in circulating blood volume.
Hypoxemia: Decreased levels of oxygen in the blood.
Induction: Anesthetization, onset of general anesthesia.
Infant: Includes the neonatal period and extends through 12 months of age.
Inhalation: To draw a breath, vapor, or gas into the lungs.
Inspiratory Pressure: An active positive pressure ventilatory maneuver in which a delivered volume of gas is given to a set peak level of pressure before passive expiration.
Isotonic: In this chapter, pertains to an intravenous solution with the same osmotic pressure as normal body fluid.
Laryngospasm: A spasm of the laryngeal muscles.
Larynx: The musculocartilaginous organ at the upper end of the trachea, below the root of the tongue, and part of the airway and vocal apparatus.
Macroglossia: An abnormally small tongue.
Maintenance: Stage of anesthesia in which relaxation of muscles and loss of sensation and consciousness are adequate for the performance of surgery.
Micrognathia: Refers to the jaw; abnormal smallness, particularly of the lower jaw.
Neonatal Period: The first 28 days of life.
Newborn (“Newly Born”): Younger than 72 hours.
Occiput: The back part of the skull.
Parenteral: Any route of administration for a medication other than alimentary; such as intravenous, subcutaneous, intramuscular, or mucosal.
Pediatrics: The medical science specific to the care of children and treatment of diseases that occur in childhood.
Pharynx: Refers to the passageway from the nasal and oral cavity to the larynx and esophagus.
Postconceptual Age: Postgestational age (number of weeks since birth) plus conceptual age (number of weeks at delivery).
Premature Newborn: Birth before 37 weeks’ gestation.
Rebreathing: Inhalation of a gas or gases previously exhaled.
Retrognathia: When the mandible lies behind the frontal plane of the maxilla.
Thermogenesis: Heat production. Nonshivering thermogenesis is a physiologic response of the newborn infant during periods of hypothermia with stimulation of the sympathetic catabolism of brown fat with release of energy in the form of heat. Brown fat is primarily located in the neck and chest of the infant.
Anatomic and physiologic considerations
Respiratory system
Understanding the differences between the adult and pediatric respiratory systems is essential to properly manage the pediatric airway. There are several distinctions of the pediatric airway that make these patients more susceptible to airway obstruction and hypoxemia.1,2–5 Respiratory distress will occur quickly in the pediatric patient if respiratory complications are not managed quickly and properly.2
The newborn has small nares, a large tongue, a small mandible, a short neck, and a large amount of upper airway lymphoid tissue.2–6 Newborns are considered obligate or preferential nose breathers4–6; therefore anything that partially or fully blocks the nares can result in respiratory compromise. In the neonate, the epiglottis is at the level of the first cervical vertebra (C1); however, the epiglottis usually moves down to the level of C3 by 6 months of age (this makes oral breathing more feasible).3 The epiglottis of the newborn is U shaped versus being flatter in the adult. A straight laryngoscope blade may be more maneuverable in the pediatric airway and is most commonly used for intubation in pediatric patients. The tracheal length is relatively short in children, which makes proper placement and securing of the endotracheal tube critical to avoid bronchial intubation (or accidental extubation).7 When the endotracheal tube is secured (and anytime the patient is repositioned), the presence of bilateral breath sounds and end-tidal carbon dioxide (ETCO2) should be reconfirmed.
The vocal cords of the newborn are more anterior (C4) than in adults (C6)3–5; this makes it more difficult to properly align the airway for ventilation and intubation.5,6 Historically, the shape of the child’s larynx has been thought to resemble that of an inverted cone with the narrowest portion of the trachea residing at the cricoid cartilage.1,3,4,6 Recent research suggests that the narrowest portion of the pediatric trachea may actually be the glottis.5 No matter where the smallest diameter lies, the diameter of the endotracheal tube that can be used is limited. In addition, because the diameter of the pediatric airway is small, airway edema may lead to significant narrowing (and potential occlusion) of the airway.
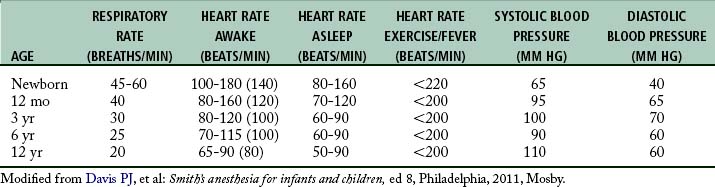
Newborns are diaphragmatic breathers.5,6 The ribs of newborns are situated horizontally in a cylindrical thorax, which limits thorax expansion. Consequently, ventilatory efforts are the result almost entirely of the movement of the diaphragm. Newborns are susceptible to ventilator problems when excursion of the diaphragm is impeded. As a result, gastric distention caused by faulty bag and mask ventilation, improper positioning, or bowel obstruction can produce inadequate ventilation.5 In the pediatric patient, the sternum and anterior rib cage are compliant, and the intercostal and accessory muscles of respiration are poorly developed. The respiratory rates of infants and young children (Table 49-1) are faster than those of adults.5 This faster rate is a result of (1) the lung volumes in infants being extremely small in relation to their body size and (2) the higher metabolic rates in infants (oxygen consumption per unit body weight is double that of adults). This is the main reason that pediatric patients rapidly desaturate during short periods of hypoventilation or apnea.
The control of breathing in infants during the first several weeks of life differs significantly from that of the adult patient. As in the adult, the newborn’s primary drive to ventilation is carbon dioxide; however, hypoxemia depresses rather than stimulates respiration in the newborn.5 This secondary response is potentiated further by hypothermia, a condition that can occur at any point in the perioperative period.
The respiratory control center in both full-term and premature infants can fatigue easily; therefore ventilatory reaction to high carbon dioxide tensions or to low percentage of oxygen is not as rapid in the newborn.5 As a result, the newborn might not be able to compensate for rapid changes in arterial blood gas levels. By 3 weeks of age, hypoxemia induces sustained hyperventilation, as in older children and adults.5 In addition, newborns and infants may breathe irregularly because of the lack of a mature respiratory center. Periodic breathing is often seen in this age group.5
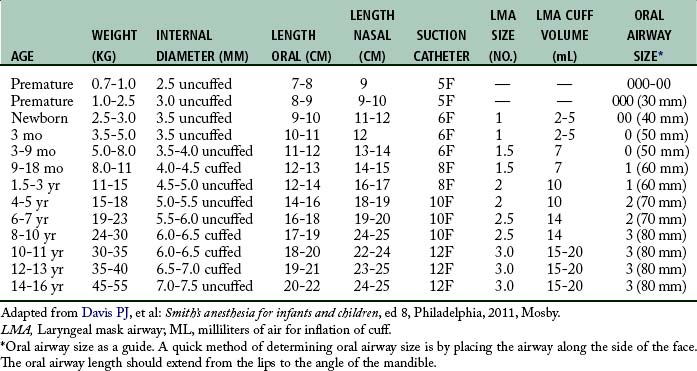
Endotracheal intubation is more widely used in pediatric anesthesia today and is considered the preferable airway management technique for general anesthesia in premature infants and most neonates. The reason for this change is that the premature infant and neonate can prove difficult to ventilate by mask, which increases the risk of filling the stomach with air during mask ventilation.1 Both endotracheal tube and laryngeal mask airways have been used safely in children of all ages (Table 49-2). The advantages of endotracheal intubation include decreased dead space, avoidance of laryngospasm and gastric distention, and prevention of aspiration; however, the incidence rate of post intubation edema from trauma and infection may be increased.
Cardiovascular system
As the pediatric patient matures, the cardiovascular system undergoes substantial changes. Normally the respiratory rate and heart rate decreases with increasing age.5 Advancing age and increasing body size result in increases in the systolic and diastolic blood pressure. The cardiovascular age-related changes for newborns, infants, and children are summarized in Table 49-1. The newborn heart functions near its peak ventricular function and therefore has little cardiac reserve. Thus, in the newborn, heart rate plays a major role in determination of cardiac function.5 The newborn is relatively unable to compensate for suboptimal conditions such as hypoxemia, acidosis, or myocardial depression.5 With the advent of more sophisticated blood pressure monitoring devices, measurements in infants can be taken with greater accuracy. The pediatric patient ordinarily has the usual signs of impending shock or airway obstruction, but physiologic status deteriorates rapidly if the problem is not rectified quickly.1–3,5,6 The perianesthesia nurse should closely observe children for subtle changes in cardiovascular status. If abnormalities arise, prompt intervention is essential.
At birth, fetal hemoglobin levels are high compared with those in the adult patient; however, the fetal hemoglobin does not readily release the oxygen it carries to tissues. Hemoglobin values decrease progressively and reach their lowest values by 2 to 3 months of age.5 By 4 to 6 months of age, the amount of oxygen available to tissues begins to increase and reaches the highest value usually by 10 months of age. This increase remains steady during the first decade of life. Research regarding the physiologic anemia of childhood suggests that, although children’s hemoglobin levels are lower than adults, oxygen unloading at the tissue level is increased in children.5 This allows a lower level of hemoglobin in infants and children to be as efficient in tissue oxygenation as a higher hemoglobin in adult patients (Table 49-3).
Composition and regulation of body fluids
Maturation of the kidneys in newborns occurs rapidly. In the neonate, renal function is characterized with obligate salt loss, slow clearance of fluid overload, and an inability to conserve fluid.8 Consequently, newborns are intolerant of both dehydration and fluid overload. The newborn can conserve sodium to some degree despite a low glomerular filtration rate and limited tubular function5; however, premature infants are prone to hyponatremia and water overloading. Dehydration in the neonate of any gestational age has harmful effects on renal function.5 Moreover, decreased renal function can delay the excretion of drugs primarily eliminated by renal clearance. At 20 weeks after birth, maturation of glomerular filtration and tubular function is nearly complete.4,5
The blood volume of the newborn younger than 1 month of age is approximately 80 to 90 mL/kg3; however, the blood volume of the premature newborn is as high as 100 mL/kg. The estimated blood volume of an infant from 3 months until 3 years of age is 75 to 80 mL/kg. In children older than 6 years, the estimated blood volume approximates that of an adult (65 mL/kg in the adult female; 70 mL/kg in the adult male).3
Water distribution in the various body compartments is markedly different among the premature newborn, the full-term newborn, the child, and the adult. Water distribution is significant because body water composition affects the volume of distribution of drugs. Premature infants have the highest percentage of fluid in the extracellular fluid compartment. A progressive decrease in total body water and distribution to the extracellular fluid compartment is seen during the first year of life. Complete maturation of renal function occurs when the child reaches 2 to 3 years of age. The fluid requirements for infants and children are reviewed in Table 49-4.
Table 49-4 Formula for Hourly Maintenance Fluid Requirements in Infants and Children
| BODY WEIGHT (KG) | HOURLY FLUID REQUIREMENT* |
|---|---|
| 0-10 | 4 mL/kg/h for each 1 kg body weight |
| 10-20 | 40 mL + 2 mL/kg/h for each 1 kg >10 kg |
| >20 | 60 mL + 1 mL/kg/h for each 1 kg >20 kg |
* Based on 1 mL of fluid per 1 kcal of caloric expenditure.
From Davis PJ, et al: Smith’s anesthesia for infants and children, ed 8, Philadelphia, 2011, Mosby.
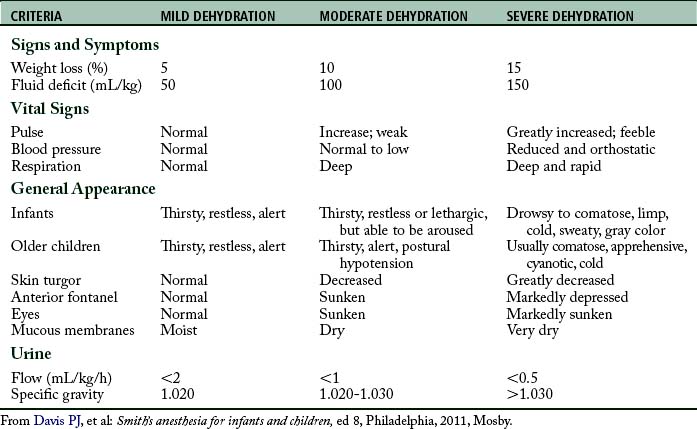
It is important to assess the hydration status of the pediatric patient to formulate an appropriate therapeutic strategy. Guidelines for assessing dehydration in children are provided in Table 49-5. Laboratory data, history and physical, and assessment of fluid input and output should be used to aid in the diagnosis of dehydration and guide therapy.
Thermal regulation
Newborns and infants are sensitive to heat loss because they have a relatively large body surface area, a relatively small amount of subcutaneous fat, poor vasomotor control, and a decreased ability to produce heat.9 The primary mechanism of heat production in a neonate is nonshivering thermogenesis mediated by brown fat.1,5,10 Shivering is of little significance to thermal regulation. When ambient temperature falls (<33° C), epinephrine is released by the sympathetic nervous system to activate thermogenesis. The preterm newborn needs a higher ambient temperature (at least 35° C) to minimize oxygen consumption.5 Ordinarily, to maintain a body temperature within normal limits, infants metabolize brown fat, cry, and move about vigorously. Newborns and infants respond to a cold environment by increasing their metabolism, which ultimately leads to an increase in oxygen consumption and the production of organic acids.
Prematurity
A premature newborn is defined as birth before 37 weeks’ gestation.3,5,6,8 The often labile condition of a premature neonate demands meticulous and vigilant perianesthesia care. Careful attention must be given to airway maintenance, medication dosage, fluid management, and temperature regulation. Premature infants and infants younger than 6 months are prone to airway obstruction and apneic episodes.3,5 Most infants in whom postanesthesia apnea develops are less than 46 weeks of postconceptual age; however, apnea has been reported in infants up to 60 weeks of postconceptual age.2 In addition to apneic spells, pulmonary complications include hyaline membrane disease and bronchopulmonary dysplasia. In addition, the premature neonate is immunocompromised and at greater risk for postoperative infection. In the sick premature neonate, the likelihood of blood transfusions, artificial ventilation, and the need for parenteral nutrition is greater.2 The risk of apnea in the postanesthesia care unit (PACU) may be decreased with intravenous (IV) administration of caffeine (10 mg/kg).5 In neonates, the half-life of caffeine is 37 to 231 hours.5 By 4 months of age, the half-life of caffeine decreases dramatically to approximately 6 hours and is similar to that in an adult. In addition, several authors cite the initial discovery of xanthine derivatives, such as theophylline or aminophylline, as a respiratory stimulant that can be used to decrease the frequency of apneic episodes in the newborn.2,5,8
Retinopathy of prematurity
Retrolental fibroplasia is a fibrovascularization and scarring of the retina. Although this disease is associated with hyperoxia (too much oxygen), a multitude of other risk factors may be involved, and the role of oxygen therapy is controversial.5,8 The risk of this retinal disorder is to newborns, especially premature infants who are born before 36 weeks’ gestation and weigh less than 1000 to 1500 g.5 Vascularization of the retina is complete at approximately 44 weeks’ gestation.8 The extreme prematurity may be the single most important factor in the development of retinopathy of prematurity. The normal PaO2 in neonates is between 60 and 80 mm Hg. Oxygenation is recommended to be continuously monitored with pulse oximetry, and hyperoxia should be avoided; therefore a saturation of 90% to 95% results in a PaO2 in the range of 60 to 80 mm Hg.5,6,8 In addition, the pulse oximeter probe must be placed on the right upper extremity or ear lobe, in case of a patent ductus arteriosus. Placement of two pulse oximeter probes on the premature infant may be helpful. Moreover, when an arterial catheter is indicated, it also should be placed in the right upper extremity.
In susceptible patients who are exposed to a hyperoxic environment, blood gas tension should be measured and an oxygen analyzer used to confirm the oxygen concentration. It is not known what level of oxygenation, or what exact length of exposure time, might lead to the development of retinopathy of prematurity.8 One must consider that attempts to prevent arterial hyperoxia and visual impairment must be tempered with the realization that unrecognized arterial hypoxemia can result in irreversible brain damage.
Infant respiratory distress syndrome
Infant respiratory distress syndrome (IRDS), once called hyaline membrane disease, is a severe disorder of the lungs of the newborn.11 The incidence rate of IRDS increases in premature infants. The basis of the pathogenesis of IRDS is insufficient surfactant levels.8 Surfactant is beneficial for the following two functions: (1) reduction of surface tension so that less pressure is required to hold the alveoli open and (2) maintenance of alveolar stability with adjustment of surface tension to changes in alveolar size. Insufficient surfactant levels increase surface tension at the alveolar air-liquid interface, resulting in alveolar collapse, an inordinate increase in the work of breathing, and impaired gas exchange. This impaired gas exchange results in hypoxemia and hypercarbia. In addition, the pulmonary vascular resistance is increased and leads to hypoperfusion of pulmonary and systemic circulation. This hypoperfusion, along with hypoxemia, causes tissue hypoxia and metabolic acidosis. An increase in survival with a decrease in serious complications is associated with administration of surfactant into the lungs at birth.5 As lung compliance improves, a progressive decrease in tidal volume and positive inspiratory pressure helps to prevent further lung injury.
Treatment for neonates with severe IRDS includes oxygen therapy, maintenance of intravenous fluids and nutritional support, temperature regulation, arterial blood gas monitoring, and laboratory sampling.8,11,12 Extremely preterm infants and those with severe disease often need intubation during delivery room resuscitation or shortly after birth.8 In addition, intermittent positive-pressure mechanical ventilation with positive end-expiratory pressure may be necessary to ventilate the exceptionally stiff lungs of these neonates. Chronic air trapping in preterm infants can occur, and excessive inflation pressures must be avoided.5
Pediatric perianesthesia considerations and techniques
Preoperative period
The preoperative meeting with the pediatric patient and his or her guardians begins the foundation of the perioperative care for this child. A thorough preoperative examination of the pediatric patient and the child’s medical record enable the nurse to assess the patient’s general state of health and identify chronic or acute disease processes. The preoperative evaluation includes (1) reviewing the patient’s chart; (2) reviewing current and past medical history of the patient with the patient and guardian(s); (3) determining medication, latex, and food allergies; (4) determining the fasting (nothing by mouth [NPO]) status of the patient; (5) formulating a list of the patient’s current medications (including herbal medications); and (6) reviewing any lab tests. Most importantly, the preoperative interview gives the nurse the opportunity to gain the confidence of the patient and caregiver which can help to alleviate anxiety. Depending on the age of the child, after the interview the nurse may be responsible for starting the intravenous catheter for the pediatric patient. If the child is healthy, this task may be postponed until the operating room, after mask induction with general anesthesia has been initiated.
Before initiating any preoperative interventions, the NPO status of the patient must be confirmed. NPO guidelines have been formulated to help prevent the aspiration of stomach contents into the lungs during surgery. The most current guidelines suggest the following: (1) solids are prohibited within 6 to 8 hours of surgery, (2) formula is prohibited within 6 hours of surgery, (3) breast milk is prohibited within 4 hours of surgery, and (4) clear liquids are prohibited within 2 hours of surgery.1
Even if the nurse gains the trust of the child and guardians, this may not be enough to alleviate all of the pediatric patient’s anxiety. Premedication with various anxiolytic medications might aid in these situations. It must be remembered, however, that the most common fear for children concerning hospitalization is the pain from needles or venopuncture; therefore the route of administration should be considered carefully. Children 1 year of age and older benefit from anxiolytic premedication to decrease preoperative anxiety and modify behavioral changes after discharge.13 Many anesthesia practitioners use oral midazolam premedication in pediatric patients 1 year of age and older, in whom a greater likelihood of uncontrollable separation anxiety exists. In addition, midazolam can be given intramuscularly, intravenously, rectally, or intranasally as an alternative route of administration for pediatric premedicant.13 However, the oral route is generally preferred unless preoperative intravenous access is available. The effect of premedication in children varies from sedation to extreme excitation. Flumazenil is a competitive antagonist at the benzodiazepine receptor and is used as a reversal agent for midazolam.5,8,14 An initial dose has been recommended of 0.2 mg of flumazenil IV (8 to 15 mcg/kg IV) given over 15 seconds14; this usually reverses the central nervous system effects of midazolam within 2 minutes. If need, additional doses of 0.1 mg IV to a total of 1 mg IV can be administered at 60-second intervals.14
An anticholinergic drug, such as glycopyrrolate or atropine, may be given preoperatively to protect against bradycardia, which can occur with the induction of general anesthesia.5,8,14 The most popular inhalation anesthetic agents used for pediatric anesthesia are halothane, sevoflurane, isoflurane, and desflurane. Halothane and sevoflurane are the most common induction inhalation agents, although halothane is no longer available for use in the United States. Sevoflurane may be superior to halothane for mask induction of general anesthesia because of (1) a quicker induction of anesthesia, (2) less incidence of cardiac arrhythmias, (3) more rapid psychomotor recovery postoperatively, and (4) less nausea and vomiting.15 After induction of general anesthesia with either sevoflurane or halothane, and when the child reaches the maintenance phase of general anesthesia, the anesthesia provider may switch to either isoflurane or desflurane. To help prevent emergence delirium, an analgesic should be administered before emergence from general anesthesia and transfer to the PACU.
Ketamine, a dissociative anesthetic, is sometimes used in pediatrics as an induction agent or for short procedures, such as painful dressing changes that do not require muscle relaxation. Emergence time depends on route of administration and whether the drug was repeated during the operation. The most serious disadvantage to ketamine is a high incidence rate of emergence delirium, hallucinations, and possible psychosis.14 Nystagmus can also occur. After the use of ketamine, the postanesthesia recovery area should be quiet and conducive to a slow peaceful emergence and recovery. A premedicant with a sedative, such as midazolam, can significantly reduce these side effects.8 It is suggested that, if possible, ketamine should always be administered with a drug such as midazolam1,8,14; this helps to reduce the emergence delirium. Ketamine has the advantage of providing analgesia and supporting spontaneous respirations.14 Caution should be used when additional opioids are given in the PACU. For uncooperative children or patients with Down syndrome or mental retardation, an intramuscular injection of midazolam combined with ketamine may be helpful.2