35 Care of the cardiac surgical patient
Allograft (Homograft): Tissue from another human, commonly procured from cadaver aortic valves with a portion of the attached aorta. The aortic allograft is obtained in a sterile manner; tested to rule out communicable disease, malignant disease, diabetes, and other pathologic conditions; treated with antibiotics; measured; placed in a sterile bag; and cryopreserved in a special freezer. When needed for surgery to replace the aortic valve or a portion of the ascending aorta and valve, the correct size of allograft is thawed and implanted.
Annuloplasty: The surgical repair of a dilated valve annulus, which causes valvular regurgitation. A cloth-covered prosthetic annuloplasty ring is sewn to the valve annulus (usually mitral or tricuspid) to reduce the overall circumference of the annular tissue, thereby reducing the amount of regurgitant blood flow.
Aortic Aneurysm: A localized or diffuse dilation of the arterial wall. Weakening and degeneration of the medial layer of the aortic wall leads to progressive enlargement of all layers of the aorta. Thoracic aortic aneurysms can occur in the ascending aorta, the aortic arch, or the descending aorta and extend into the abdominal aorta and branch vessels.
Aortic Dissection: A unique condition that affects the aortic media in which repeated stress (often from hypertension) and a congenital predisposition produce a tear in the intimal layer. Blood enters the tear, creating a dissecting hematoma within the tunica media and forming a false lumen. As the false lumen enlarges, with more blood entering the tear, the true lumen compresses and eventually compresses branches of the aorta. The intimal tear usually forms in the ascending aorta, although it can originate in any portion of the thoracic aorta. Surgery (excision of the aorta that contains the tear and replacement with a graft) is indicated for ascending aortic and transverse aortic arch dissections.
Aortic Insufficiency (AI): Also called aortic regurgitation; a condition that occurs when the aortic valve leaflets do not close properly (or have tears) and the valve becomes incompetent, thereby allowing regurgitation of blood from the aorta back into the left ventricle during diastole. AI can be the result of a primary valve disorder or aortic root disease. Valve disorders may be the result of congenital or rheumatic heart disease, infective endocarditis, torn leaflets, or trauma; aortic root disease may include aneurysmal dilatation, aortic dissection, Marfan syndrome, or some other condition that predisposes the root to dilatation (e.g., ectasia).
Aortic Stenosis (AS): The development of stiff and fibrotic valve leaflets or a narrowing of the orifice of the aortic valve itself. Valvular AS is often characterized by a fusion of the commissures of the valve leaflets that leaves only a small opening. A calculated aortic valve orifice of less than 1 cm2 is considered critical AS. Cardinal symptoms are syncope, angina pectoris, and dyspnea; these symptoms are related to insufficient cerebral blood flow, myocardial blood flow, and impaired left ventricle function, respectively. AS can occur as a congenital process, as in the bicuspid valve (a common congenital anomaly first diagnosed in adulthood), as an acquired disease process related to a history of rheumatic heart disease, or as calcific AS.
Aortocoronary Bypass Grafts: See Myocardial Revascularization.
Atrial Septal Defect (ASD): An opening in the atrial septum, ASDs are among the frequently seen congenital anomalies in adulthood. The most common form in the adult is the ostium secundum defect. Depending on the size of the defect (commonly the size of a quarter coin), there is a shunting of blood from the left atrium to the right atrium and subsequent increased pulmonary flow.
Balloon Valvotomy or Valvuloplasty: A catheter-based interventional (i.e., nonsurgical) procedure to enlarge a (stenotic) valve opening. For aortic valvuloplasty, the balloon tip is positioned across the aortic valve, and the balloon is inflated to dilate the aortic valve orifice. Balloon valvotomy for the mitral valve is performed with a catheter percutaneously inserted into a large vein and threaded to the right atrium, where a septal puncture is made to allow access to the mitral valve.
Bicuspid Aortic Valve: See Aortic Stenosis.
Cardioplegia: Literally, “paralysis of the heart”; pharmacologically induced cardiac arrest during surgery on the heart. Potassium is the most commonly used arresting agent, but the solutions may also contain electrolytes, buffers to maintain appropriate pH, glucose, metabolic substrates, calcium antagonists, tromethamine, heparin, and antiarrhythmic agents; the carrying solution may be crystalloid or blood (preferable for its oxygen carrying capacity). The purposes of cardioplegia are protection of the myocardium against irreversible ischemic injury during the aortic cross-clamp period when the heart is arrested and conservation of energy resources that can be used after removal of the cross clamp.
Cardiopulmonary Bypass (CPB): A temporary substitution for the heart and lungs with a mechanical device that drains systemic venous return (thereby decompressing the heart) into an oxygenator that removes excess carbon dioxide and adds oxygen (simulating the lungs) and pumps arterialized blood into the systemic circulation (simulating the heart). The development of CPB enabled surgeons to isolate the heart by cross clamping the aorta and to pharmacologically induce cardiac arrest to create a dry, quiet operative field in which to perform the surgical repairs. During the period of cardiac arrest, the rest of the body can be adequately perfused via CPB.
Coarctation of the Aorta: A congenital narrowing of the thoracic aorta, usually in the area of the ligamentum arteriosum (formerly the fetal patent ductus arteriosus, which shunts blood from the pulmonary artery to the aorta).
Commissurotomy: The opening or separation of fused valve leaflet commissures (in the adult, generally the mitral or pulmonary valve).
Congenital Heart Disease: Cardiac anomalies apparent at birth or later in life. In this section, some of the congenital anomalies that may be seen in adulthood are discussed.
Dysrhythmia Surgery for Atrial Fibrillation (AF): Surgery designed to block or redirect aberrant atrial fibrillatory impulses toward the atrioventricular node to achieve normal sinus rhythm.
Hybrid Suite: Procedure or operating room that integrates interventional and traditional surgical equipment and supplies, and imaging and monitoring capability.
Hypothermia: Cooling of the patient during cardiac surgery. Hypothermia can be induced (elective) or inadvertent. Induced systemic hypothermia is achieved with a heat exchanger incorporated into the cardiopulmonary bypass circuit; induced cardiac hypothermia is achieved with infusion of cooled cardioplegia solution into the coronary circulation. Topical cardiac cooling is achieved by pouring cold solutions onto the heart.
Implantable Cardioverter-Defibrillator (ICD): A device that provides a shock to the heart for tachycardia above a predetermined rate or ventricular fibrillation and also has the capability to provide bradycardia therapy. The device is usually inserted transvenously in the electrophysiology or cardiac catheterization laboratory. Patients with ICDs who undergo surgery have a magnet placed over the generator (usually in the right or left upper chest) to turn off the device and avoid frequent shocks during induced cardiac arrest. After surgery, the magnet is removed and the ICD is again activated.
Minimally Invasive Cardiac Surgery: Operations that use smaller incisions, percutaneous and or endoscopic access, endovascular techniques, or off-pump (i.e., no cardiopulmonary bypass) procedures. A common procedure in minimally invasive surgery is the endoscopic video-assisted excision of saphenous vein coronary bypass grafts. In other cases, smaller sternal or thoracic chest incisions or ports may be used for target areas of the heart that can be accessed through these minimal incisions (e.g., certain coronary bypass operations for single vessel disease and some valve procedures).
Mitral Regurgitation (MR): A condition that occurs when one or more components of the mitral valve apparatus prevents competent closure of the valve, with subsequent regurgitation of blood into the left atrium.
Mitral Stenosis (MS): A narrowing of the mitral valve orifice that creates an impedance to flow across the valve.
Myocardial Protection Techniques: Techniques or procedures designed to conserve myocardial energy resources and limit the amount of ischemic tissue injury that can occur. The use of cardioplegia and induced hypothermia (see previous definition) to arrest and preserve the heart form the foundation of myocardial protection. Additional myocardial protective measures can include minimization of the risk of an air embolus from ambient room air trapped inside the heart with insufflation of CO2 gas through a catheter into the pericardial well. The CO2 gas not only displaces ambient air but also dissolves approximately 25 times faster in the blood than room air and is less harmful to cardiac tissue.
Myocardial Revascularization: Also called coronary artery bypass grafting. Surgical procedure performed for coronary artery disease in which conduits of the patient’s own tissue (autograft), most commonly the internal mammary artery or other arteries (e.g., radial artery, gastroepiploic artery) and the greater saphenous vein, are attached directly to the affected coronary artery at a site distal to the narrowed atherosclerotic lesion. Generally, coronary narrowing of 70% or greater in arteries that are 1 mm or larger is an indication for bypass grafting; narrowing of less than 70% can create competitive flow between the conduit and the native artery and reduce the amount of blood that perfuses the bypass graft (and the anastomosed coronary artery).
Patent Ductus Arteriosus (PDA): A duct between the pulmonary artery and the aorta, present in fetal life. The duct usually closes within 1 or 2 weeks after birth and becomes the ligamentum arteriosum.
Percutaneous Transluminal Coronary Angioplasty (PTCA) with Stent Insertion: A catheter-based interventional technique performed with fluoroscopy in the cardiac catheterization (see previous definition) laboratory for acute or chronically obstructed coronary arteries. A percutaneously inserted balloon catheter is threaded to the right or left coronary os, where the catheter is positioned across the coronary artery at the site of the narrowing. The balloon is inflated to compress the atherosclerotic lesion and enlarge the coronary lumen. A stent (bare metal or coated) is commonly inserted in conjunction with the PTCA to maintain coronary artery patency.
Pericardiectomy, Pericardial Window: Pericardiectomy is the partial excision of an adhered, thickened fibrotic pericardium to relieve constriction of the heart and great blood vessels. In patients with chronic cardiac effusions, the creation of a pericardial window (excision of a portion of the pericardial or pleural wall) between the pericardial sac and the pleural space drains the fluid. In patients with chronic effusions, the window to the pleural space generally allows future fluid accumulation to be reabsorbed.
Pulmonary Stenosis: Fusion of the pulmonary valve cusps at the commissures, which creates an obstruction to the right-ventricular outflow tract. Another form of pulmonary stenosis occurs in the infundibular portion where fibromuscular obstruction occurs proximal to the valve, creating right ventricular outflow tract obstruction.
Tetralogy of Fallot (TOF): A congenital entity with four distinctive features: (1) a high ventricular septal defect (VSD); (2) pulmonary stenosis that affects the valve or the infundibular region; (3) overriding of the ventricular septal defect by the aorta; and (4) hypertrophy of the right ventricle. In the adult and commonly in the child, correction includes closure of the VSD with a patch and enlargement of the pulmonary valve (or stenosed infundibular region) with dilators, via commissurotomy, valve replacement, infundibular resection, or insertion of an allograft, depending on the underlying pathology.
Transplantation: Replacement of the patient’s heart with a human donor heart; replacement of one or both lungs with lungs from a donor, or one lung from a living donor). Heart donors are persons with irreversible catastrophic brain injury who do not have atherosclerotic heart disease; age limitations have become less restrictive, and donors and recipients may be 60 years or older in certain cases. Severe pulmonary disease is a contraindication for lung donation.
Tricuspid Regurgitation: A condition that occurs when the tricuspid valve does not totally close because the leaflets do not completely approximate in diastole.
Tricuspid Stenosis: A narrowing of the orifice of the tricuspid valve.
Valve Replacement: A surgical procedure in which the native valve is replaced with a mechanical or biologic prosthesis; allograft valve replacement also may be performed.
Valvuloplasty: The repair of one or more components of the right (tricuspid) or left (mitral) atrioventricular valve complex that consists of the valve annulus (see Annuloplasty), the valve leaflets, the chordae tendineae, the papillary muscles, and the endoventricular wall.
Ventricular Assist Device (VAD): Mechanical devices to support the left ventricle (LVAD), the right ventricle (RVAD), or both ventricles (BiVAD) can be used long term as a bridge to transplant or destination therapy, depending on the underlying pathology. VADs decrease the workload of the heart by diverting blood from the ventricle to an artificial pump that maintains systemic (LVAD) or pulmonary (RVAD) perfusion. Currently, patients can be ambulatory with implantable VADs powered by batteries, which has substantially improved the quality of life for many VAD patients.
Ventricular Septal Defect (VSD): Consists of a hole through the ventricular septum. VSDs may be congenital or acquired (i.e., postinfarction VSD).
The first suture repair of a right ventricular stab wound in 1896 demonstrated that the heart could be manipulated and sutured without causing ventricular fibrillation and without entering the pleural cavity, thereby creating a pneumothorax. The introduction of positive-pressure endotracheal anesthesia obviated the dangers of a pneumothorax because clinicians could access the heart and enter the pleural cavities without causing the lungs to collapse. Gibbon’s introduction in 1953 of extracorporeal circulation (i.e., cardiopulmonary bypass) enabled surgeons to isolate the heart and lungs from the circulation without producing irreversible tissue anoxia.1 The development of electric defibrillation and induced chemical cardiac arrest made stopping and restarting the heart possible with consistent predictability; myocardial protection techniques protected and conserved myocardial energy resources during the period of induced cardiac arrest. Newer diagnostic imaging and monitoring devices have allowed clinicians to visualize the heart, identify specific pathoanatomic changes, record the heart’s electric activity, measure blood pressure and flow, and assess ventricular function. These imaging and monitoring improvements, along with the refinement of mechanical and bioprosthetic materials, improved anesthetic agents, percutaneous and newer minimally invasive surgical techniques, and additional technologic advances have made development of more effective procedures possible to revascularize the myocardium, repair and replace valves, treat aneurysms, alter conduction disturbances, and assist and replace a failing heart.1 The reader will find related information in Chapters 11, 34, and 36.
Preoperative considerations
Before surgery, documentation of the history and physical examination, surgical consents, the results of diagnostic and laboratory tests, and additional pertinent information are reviewed by the nurse. Among the significant factors are estimates of left ventricle (LV) function (e.g., ejection fraction), a history of medications that may affect perioperative bleeding (e.g., aspirin, anticoagulants), oxygen-carrying capacity (e.g., hematocrit, hemoglobin), renal function (creatinine), pulmonary function (spirometry), cardiac anatomy (e.g., of the valves, coronary arteries), and the presence of concomitant carotid artery disease.2 Many patients are admitted the morning of surgery, which shortens the period of time for teaching. Often preoperative laboratory testing and requests for packed red blood cells are performed a few days before the scheduled surgery; patient teaching can be accomplished at this time (Table 35-1).
Table 35-1 Patient Teaching for Coronary Artery Bypass Graft Surgery and Valve Surgery
| TOPIC | CABG SURGERY | VALVE SURGERY |
|---|---|---|
| Perioperative Pointers | ||
| Medical diagnosis | Coronary artery occlusive disease | Valve regurgitation, stenosis, or mixed lesion |
| Diagnostic tests | ECG, chest radiograph, nuclear imaging, cardiac catheterization, carotid duplex studies | Same, plus echocardiogram |
| Routine preoperative tests | CMP, ECG, T&C, pulmonary function, PT, PTT, INR, urinanalysis | Same |
| Incision site | Midsternal or anterior thoracotomy; multiple leg incisions for vein harvest, arm incision for radial artery harvest | Mini-sternotomy, full sternotomy, or mini-thoracotomy |
| Resumption of eating | After removal of ET and NG tubes (postoperative day 1) clear liquids, then advance to high calorie/high protein diet | Same |
| Pain control | IM, PO, PCA | Same |
| Estimated length of procedure | 3-6 hours | Same |
| Estimated length of hospital stay | 4-6 days | 5-7 days |
| Long-term effects of surgery | Possible loss of saphenous vein or internal mammary or radial arteries; possible intermittent lower leg ischemia | Possible chronic anticoagulation therapy; differences between biologic and mechanical prostheses, valve repair |
| Drains or tubes | 2 days: mediastinal chest tube, pleural tube; 2-3 days: leg drains, urinary drainage catheter; remove tubes when drainage >100 mL for 8 hours | 2 days: mediastinal and pleural tubes, urinary catheter |
| Postoperative Pointers and Home Instructions | ||
| Food | Cardiac diet | Same |
| Wound care | Wounds covered if draining; redress after shower or bath; contact clinician if signs of infection | Same |
| Bathing | Daily | Same |
| Driving | 4-6 weeks (automatic shift only) | Same |
| Sex | Restricted by limits of ability to bear weight on upper arms and chest | Same |
| Return to work | 8-12 weeks | Same |
| Medications | Aspirin anticoagulant, cardiac drugs | Warfarin (Coumadin), cardiac drugs |
| Follow-up | 7-14 days | Same, plus laboratory tests for determination of bleeding times |
| Special restrictions | Upper body movement restricted for 6 weeks for sternal healing | Same |
| Lifestyle changes | Reduction of coronary risk factors; cardiac rehabilitation | Risk factor reduction; cardiac rehabilitation |
| Worrisome but normal | Fatigue, swelling in leg; leg discomfort 4-6 weeks; weakness, emotional let down | Fatigue; sound of mechanical valve; weakness; emotional let down |
ECG, Electrocardiogram; CMP, comprehensive metabolic panel (includes glucose, blood urea nitrogen, sodium, potassium, chloride, creatinine, albumin, bilirubin, calcium, alkaline phosphatase, total protein); T&C, type and cross match (blood); PT, prothrombin time; PTT, partial thromboplastin time; INR, International Normalized Ratio; ET, endotracheal; NG, nasogastric; IM, intramuscular; PO, by mouth; PCA, patient-controlled analgesia.
Adapted from Lemmer JH, Vlahakes GJ: Handbook of patient care in cardiac surgery, ed 7, Philadelphia, 2010, Lippincott Williams & Wilkins; Bojar RM: Manual of perioperative care in adult cardiac surgery, ed 5, Oxford, 2011, Wiley-Blackwell.
Anesthesia
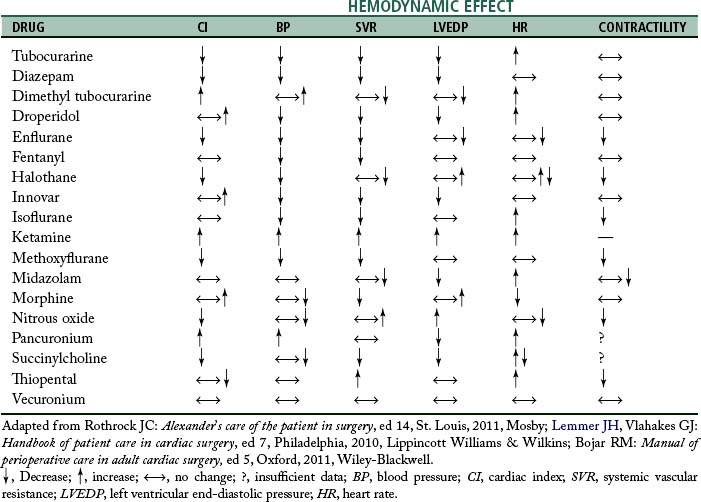
Anesthesia for cardiac surgery varies by hospital and can also vary by the type of cardiac repair. Ideally, anesthetic management includes drugs that have rapid onset and termination, minimize ischemia, and are nontoxic to myocardial and other tissue.3 For example, in patients with significant pulmonary involvement, agents that increase pulmonary vascular resistance should be avoided.2 Hemodynamic effects of some anesthetic drugs are shown in Table 35-2.
Procedures
Monitoring
Monitoring (Table 35-3) of the patient’s electrocardiogram (ECG) and direct blood pressure (commonly via the radial artery in the nondominant wrist) results is initiated when the patient arrives in the preoperative area. Central lines are usually inserted after transport into the operating room (OR), but occasionally are inserted in another designated location, such as the preoperative area. Whether the patient first undergoes intubation before insertion of central lines (i.e., central venous pressure, pulmonary artery catheter) or after line insertion is at the discretion of the anesthesia provider; the sequence varies among institutions. Increasingly, an anesthesia provider inserts a transesophageal echocardiography (TEE) probe to illustrate cardiac function. Use of TEE for valve surgery and for patients with compromised LV function is routine.2,3
Table 35-3 Physiologic Monitoring
| MONITORING DEVICE | LOCATION | ASSESSES/MEASURES |
|---|---|---|
| Cardiovascular System | ||
| Electrocardiogram (ECG) | Electrodes placed on shoulders, hips, and left axillary line | Electrical activity of heart: lead II useful to monitor cardiac rhythm (good visualization of P wave and QRS) and myocardial ischemia (inferior surface); lead V5 useful to detect myocardial ischemia (anterior surface) |
| Intraarterial catheter | Radial artery (also femoral artery) aorta, bypass circuit; in children, may use superficial temporal or dorsalis pedis arteries; in neonates, may use umbilical artery | Direct arterial BP; blood gases; blood chemistries |
| Blood pressure cuff | Right or left arm | Indirect BP |
| CVP line | RA | RA pressure (CVP); RV filling pressure; RV preload |
| PA catheter (addition of fiberoptics provides additional information about mixed venous oxygen saturation [SvO2]) | PA (proximal and distal) | PA pressures: systolic, diastolic, mean, wedge; pulmonary vascular resistance; LV filling pressure; LV preload; CO; assessment of stroke volume, stroke work, systemic vascular resistance; mixed venous saturation (continuous indirect assessment of CO and reflection of tissue oxygenation); RV function |
| LA catheter (when used) | LA | LA pressure (direct); LV filling pressure; LV preload |
| TEE | Esophagus | Valve function before and after repair; LV wall motion, failure; intracardiac air bubbles |
| Urinary drainage catheter | Urinary bladder | Urinary output, renal perfusion; indirect measure of CO |
| Respiratory System | ||
| Mass spectrometry | Anesthesia circuit | Inspired or expired O2, CO2, and anesthetic gases; used to avoid hypoxia, hypercarbia, anesthetic overdose |
| Pulse oximeter | Finger or toe cot; earlobe, nose | Oxygen saturation of arterial hemoglobin; tissue oxygenation |
| Capnography | Anesthesia circuit | End-tidal CO2; used to detect integrity of anesthesia circuit; avoid disconnections of monitor, endotracheal tube; detect spontaneous ventilation, rebreathing, obstructive pulmonary disease |
| Central Nervous System | ||
| Temperature | Esophagus, nasopharynx, urinary bladder, rectum, ventricular septum, bypass circuit, PA catheter | Core, and peripheral temperature of heart, brain, and other organs |
| Electroencephalogram | Scalp electrodes | Detect cerebral ischemia, embolus; indication of depth of anesthesia |
| Renal System | ||
| Urinary discharge catheter | Bladder | Urinary output; indirect measure of cardiac output |
BP, Blood pressure; CVP, central venous pressure; RA, right atrial; RV, right ventricular; PA, pulmonary artery; LA, left atrial; CO, cardiac output; TEE, transesophageal echocardiography.
Adapted from Lemmer JH, Vlahakes GJ: Handbook of patient care in cardiac surgery, ed 7, Philadelphia, 2010, Lippincott Williams & Wilkins; Bojar RM: Manual of perioperative care in adult cardiac surgery, ed 5, Oxford, 2011, Wiley-Blackwell.
Incisions
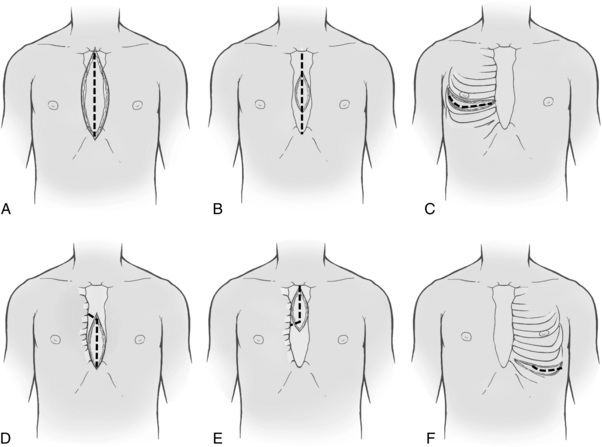
After the patient has been anesthetized, positioned, and washed (prepared), the chest is opened. The most commonly used chest incision in cardiac surgery is the median sternotomy; this is the preferred incision when a thorough assessment of, and complete access to, the heart (as with global coronary artery disease) is needed. However, ministernotomy, anterolateral or posterolateral thoracotomy incisions, or transverse sternotomy also may be used (Fig. 35-1). The surgeon splits the sternum with a saw from the sternal notch to the xiphoid process. After the sternum is opened, the exposed pericardial sac is incised anteriorly, and the pericardial edges are tacked up along the chest wall incision to allow complete access to the entire pericardium without the necessity of entering the pleural cavities. Minimally invasive surgery uses smaller incisions and can speed patient recovery, enable the patient to return faster to normal activities, and reduce costs.
Cardiopulmonary bypass
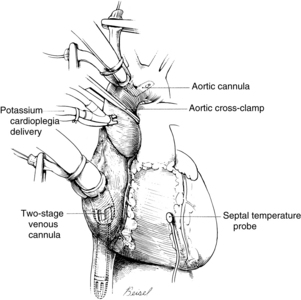
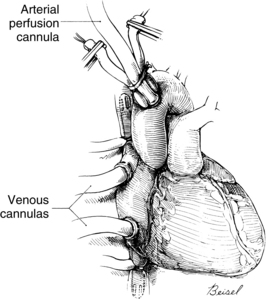
Systemic venous return to the heart flows by gravity drainage (the level of the patient needs to be above that of the bypass machine to facilitate drainage) into one or two large-bore (e.g., 32F to 36F) cannulas inserted into the right atrium. With the single two-stage cannula (Fig. 35-2), openings in the distal tip drain blood returning from the inferior vena cava (IVC) and openings in the middle portion of the cannula drain venous blood from the superior vena cava (SVC) and the coronary circulation exiting from the coronary sinus. Some blood enters the right atrium (RA) and the pulmonary circulation. With double cannulation (Fig. 35-3), individual cannulas are inserted into the IVC and the SVC, thereby forcing all venous return into the cannulas. Generally the two-stage cannula is used for coronary artery bypass grafting and aortic valve surgery when total right side decompression is not generally needed and blood entering the right side of the heart does not obscure the surgical field. Individual (double) venous cannulation can be used for procedures in the right side (e.g., tricuspid valve repair) to keep the right heart free of blood. Occasionally, two cannulas can be used when greater decompression of the RA is necessary (e.g., mitral valve surgery).
After the blood is oxygenated, it is pumped into the systemic circulation via an arterial cannula, commonly located in the aorta (see Fig. 35-3). When aortic pathology (e.g., aortic aneurysm) prevents cannulation, the femoral artery can be cannulated (retrograde flow) for arterial return. The axillary artery may be needed when the aorta or both of the femoral arteries are unavailable.
In addition to the standard cannulation techniques for cardiopulmonary bypass (CPB), minimally invasive systems use endovascular catheters (Fig. 35-4). A venous catheter is inserted into the femoral vein, and the arterial cannula is placed into the femoral artery. The ipsilateral femoral artery is also used for insertion of a multilumen catheter. One lumen serves as an endovascular cross clamp that occludes the aorta with inflation of an intraaortic balloon at the tip of the catheter, and the other lumen can be used for infusion of antegrade cardioplegia. Pressure lines, venting catheters, and a coronary sinus retrograde cardioplegia catheter can be inserted via the jugular vein. This system does not require median sternotomy and is an important adjunct for minimally invasive surgery.4
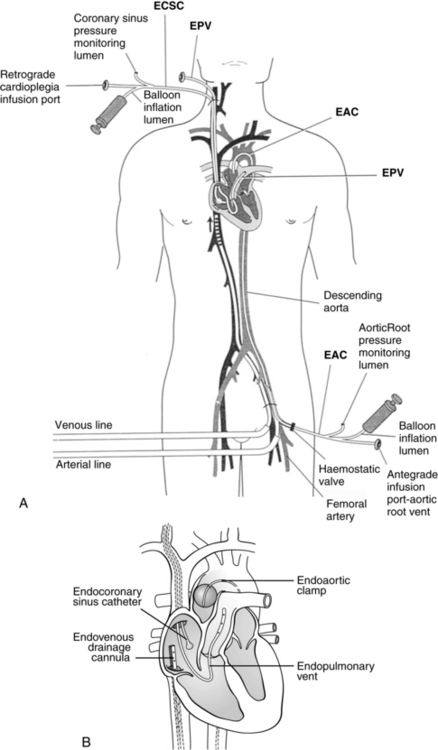
FIG. 35-4 Minimally invasive cardiopulmonary bypass technique. A, Positioning of endovascular catheters). B, Correct positions of endocoronary sinus catheter, endoaortic clamp, endovenous drainage cannula, and endopulmonary vent for port-access cardiopulmonary bypass. EAC, Endoaortic clamp; ECSC, endocoronary sinus catheter; EPV, cardiopulmonary vent.
(A, From Toomasian M, et al: Extracorporeal circulation for port-access cardiac surgery, Perfusion 12:83, 1997. B, From Kaplan J, editor: Kaplan’s cardiac anesthesia, ed 5, Philadelphia, 2006, Saunders.)
A number of risks are associated with the use of CPB (e.g., particulate or air embolus), and some form of checklist is often used before CPB is instituted (Box 35-1) and before the patient is weaned from CPB (Box 35-2). Such checklists help to enhance the safety of CPB. In addition, the clinical sequelae of CPB can affect all body systems. Caregivers in the postoperative period should be aware of the possible effects of CPB (Table 35-4).
BOX 35-1 Checklist for Initiation of Pump
• Activated coagulation time checked and at least 350 to 400 seconds
• Muscle relaxant adequate (or infusion turned on for maintenance)
• Inotropic infusions turned off
• Pupil symmetry assessed for later comparison (unilateral dilation may indicate unilateral carotid perfusion on cardiopulmonary bypass)
• Pulmonary artery catheter (if used) pulled back 5 cm
• Urinary output emptied before bypass (start anew with initiation of bypass)
From Lemmer JH, Vlahakes GJ: Handbook of patient care in cardiac surgery, ed 7, Philadelphia, 2010, Lippincott Williams & Wilkins; Bojar RM: Manual of perioperative care in adult cardiac surgery, ed 5, Oxford, 2011, Wiley-Blackwell.
BOX 35-2 Checklist for Weaning from the Pump
• Patient is warm (to at least 36° C)
• All monitors are functioning; electrocardiogram, pulmonary artery tracing, arterial line tracing, central venous pressure reflect heart filling
• Heart rate is 70 to 100 beats/min (<60 beats/min is reduced cardiac output; ≥120 beats/min is detrimental to left ventricular filling)
• Infusions are prepared as necessary
• Lungs are inflated, but kept out of the surgeon’s field
• Pressure becomes pulsatile as ventricles fill
• Blood is drawn for measurement of electrolytes, hematocrit, arterial blood gases, and activated clotting time
• The surgeon clamps venous drainage cannula and removes the cannula
From Lemmer JH, Vlahakes GJ: Handbook of patient care in cardiac surgery, ed 7, Philadelphia, 2010, Lippincott Williams & Wilkins; Bojar RM: Manual of perioperative care in adult cardiac surgery, ed 5, Oxford, 2011, Wiley-Blackwell.
Table 35-4 Effects of Cardiopulmonary Bypass
| EFFECTS | CONTRIBUTING FACTORS |
|---|---|
| Cardiovascular System | |
| Perioperative myocardial infarction | Inadequate myocardial protection and emboli |
| Low cardiac output syndrome after surgery | Preexisting heart disease, inadequate myocardial protection, alteration in colloidal osmotic pressure, left ventricular dysfunction, hypoperfusion injury, hypothermia, long pump run |
| Increased afterload | Catecholamine release |
| Hypertension | Elevated rennin, angiotensin, and aldosterone levels |
| Hypotension | Postoperative diuresis, sudden vasodilation (rewarming), third spacing |
| Pulmonary System | |
| Respiratory insufficiency | Alterations in colloidal osmotic pressure, interstitial pulmonary edema, decreased perfusion, alterations in ventilatory patterns, decreased surfactant production, pulmonary microemboli |
| Atelectasis | Complement activation and inflammatory response, emboli, alveolar-capillary membrane damage |
| Neurologic System | |
| Cerebrovascular accident | Cerebral emboli |
| Transient motor defects | Decreased cerebral blood flow |
| Cerebral hemorrhage | Systematic heparin administration |
| Neuropsychological deficits | Microemboli, ischemia, altered perfusion flow of bypass |
| Gastrointestinal System | |
| Gastrointestinal bleeding | Hormonal stress and coagulation diatheses |
| Intestinal ischemia or infarction | Emboli and decreased perfusion |
| Acute pancreatitis | Pancreatic vasculature emboli |
| Renal System | |
| Acute renal failure | Decreased renal blood flow, microemboli, and myohemoglobin release |
| Hemoglobinuria | Red blood cell hemolysis |
| Fluid and Electrolyte Balance | |
| Interstitial edema, weight gain | Increased extravascular fluid and organ dysfunction, fluid shifts, decreased plasma protein concentration, increased capillary permeability |
| Intravascular hypovolemia | Decreased intravascular volume, bleeding, and interstitial edema |
| Hypokalemia | Dilution, polyuria, intracellular shifts of potassium ions |
| Hyperkalemia | Potassium cardioplegia and increased intracellular exchange of glucose and potassium, cellular destruction |
| Hyponatremia, hypocalcemia, and hypomagnesemia | Dilution, fluid shifts, diureses |
| Endocrine System | |
| Water and sodium retention | Increase in antidiuretic hormone |
| Hypothyroidism | Increased levels of thyroxine (T4) and decreased levels of triiodothyronine (T3) and thyroid-stimulating hormone |
| Hyperglycemia | Depressed insulin response, stimulation of glycogenesis |
| Immune System | |
| Infection | Exposure to multiple pathogens, decreased immunoglobin levels, and hypothermia |
| Postperfusion syndrome | Release of anaphylactic toxins, complement activation |
| Hematologic Factors | |
| Bleeding | Blood cell hemolysis, heparin rebound, reduction in platelet count and coagulation factors, coagulopathy, systemic heparin administration, depressed liver function from hypothermia |
Adapted from Lemmer JH, Vlahakes GJ: Handbook of patient care in cardiac surgery, ed 7, Philadelphia, 2010, Lippincott Williams & Wilkins; Bojar RM: Manual of perioperative care in adult cardiac surgery, ed 5, Oxford, 2011, Wiley-Blackwell.
Myocardial protection
The goal of myocardial protection is to conserve cardiac energy resources; the goal is achieved with induced hypothermia and rapid diastolic arrest. Hypothermia reduces the metabolic rate (and therefore the energy demands) of the tissue being cooled; effective intraoperative myocardial protection positively affects the patient’s postoperative recovery. For most cardiac procedures of less than 1 hour of induced cardiac arrest (e.g., coronary artery bypass grafting), the perfusionist cools the systemic circulation from a normal temperature of 37° C (98.6° F) to approximately 32° C (89.6° F). The cardioplegia solution is cooled to a lower temperature (near freezing) for intracardiac cooling as a method of myocardial protection. For procedures with a longer cross-clamp time, the systemic temperature may be further reduced to protect the brain, kidneys, and other organs while the heart is arrested by reducing energy demands and limiting ischemic injury. Topical cooling of the heart with cold lavage or frozen “slush” placed on the heart and in the pericardial well helps to achieve transmural cooling. Although induced hypothermia plays an important role in minimization of tissue ischemia during selected portions of cardiac procedures, inadvertant hypothermia has become an important consideration for cardiac patients because of associated risks for perioperative complications. Before surgery, shivering increases myocardial oxygen demands and further taxes hearts that have diminished myocardial energy supplies. During surgery, before and after CPB, inadvertent cooling of the patient is associated with increased surgical bleeding and a diminished immune response. After surgery, hypothermia contributes to patient discomfort, impaired wound healing, prolonged bleeding, longer lengths of stay, and cardiac events such as ischemia and tachyarrhythmias.2,3 Active measures, such as the application of warm blankets before surgery and during the intraoperative period (before and after induced hypothermia), the postoperative use of forced warm air devices and intravenous fluid warmers, and an increased ambient room temperature, can help to maintain normothermia.
Rapid diastolic arrest conserves existing cellular energy resources (e.g., adenosine triphosphate) by avoiding the energy-expensive state of ventricular fibrillation before the heart achieves an arrested state. Quick arrest of a beating heart (e.g., 10 to 20 seconds) enhances myocardial energy conservation. Potassium is the most commonly used arresting agent, but the solutions may also contain electrolytes, buffers to maintain appropriate pH, glucose, metabolic substrates, calcium antagonists, tromethamine, heparin, and antiarrhythmic agents. The carrying solution can be crystalloid or blood (preferable for its oxygen carrying capacity).4
Methods of infusing cardioplegia include the antegrade and retrograde routes and the direct coronary ostial route of infusion. With the antegrade method, a needle catheter is inserted into the anterior aorta proximal to the aortic cross clamp (see Fig. 35-2). The cardioplegia solution is infused with sufficient pressure to close the aortic valve. With the cross clamp applied distally and a competent aortic valve proximally, the only paths for the solution to travel are the right and left coronary ostia lying between the cross-clamped aorta and the closed aortic valve. In patients with aortic valve insufficiency, cardioplegia flow into the coronary ostia is significantly reduced because the incompetent aortic valve provides a lower pressure pathway into the inner ventricular chamber, thereby distending the heart and increasing myocardial wall tension. Retrograde delivery is indicated in the presence of coronary artery lesions that impair the transmural distribution of the cardioplegia when delivered solely by the antegrade route. For the retrograde route, the surgeon inserts a catheter through a stab wound into the right atrial wall and threads the catheter into the coronary sinus. The cardioplegic solution infused into the coronary sinus flows retrograde through the cardiac veins and arteries. The solution exits via the coronary ostia, where it is removed by suction.
Surgery for coronary artery disease
Coronary artery disease remains the leading cause of death among men and women.5 Left heart cardiac catheterization is commonly performed for identification of areas affected by obstructive atherosclerotic coronary artery disease. Selective coronary angiography with an injection of contrast medium into the right and left coronary ostia illustrates coronary anatomy, distal coronary perfusion, the location of atherosclerotic lesions, the status of coronary collateral circulation, and the percentage of narrowing of coronary arteries affected by coronary artery disease (CAD). Dye is injected into the LV (ventriculography) to determine wall motion (e.g., hypokinesia, dyskinesia, paradoxic motion), and valvular function (e.g., mitral regurgitation, prosthetic valve function). Generally, a right-heart catheterization yields data concerning the inferior and superior vena cava, the right atrium and ventricle, tricuspid and pulmonary valves, and the pulmonary artery.
Percutaneous coronary interventions, specifically percutaneous coronary interventions angioplasty (PTCA) with stent insertion, can be performed for discrete lesions. Equalized pressure measurements above and below the lesion indicate a successful angioplasty. The restenosis rate for angioplasty at 6 months has been approximately 30%, necessitating additional angioplasties or surgery. Complications from the interventional procedure include prolonged chest pain, myocardial infarction, coronary spasm, or coronary artery dissection that can necessitate emergency coronary artery bypass grafting (CABG). Evidence supports surgical revascularization (i.e., CABG) over percutaneous coronary interventions in patients with left main coronary artery disease.6,7
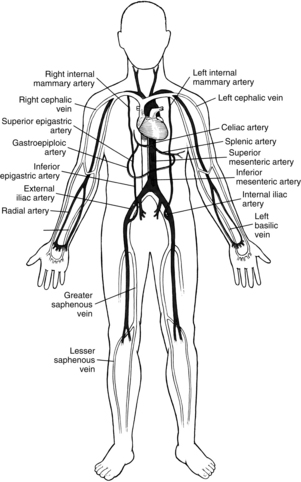
When CABG is recommended for patients, the number and type of bypass grafts (e.g., saphenous vein, internal mammary artery) are assessed. Fig. 35-5 illustrates possible arterial and venous autologous conduits. Grafts from cadaver human saphenous vein, human and bovine umbilical vein, and synthetic grafts have been used when other conduits were unavailable. CABG does not cure CAD; its purpose is to increase blood flow to the myocardium beyond the obstructive lesions. CAD is a progressive disease, and retardation of the atherosclerotic process requires life style changes and pharmacologic support (e.g., statins). Gender-based differences in CABG outcomes are under scrutiny, and guidelines have been published to provide evidence for the selection of techniques.5
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree