Chapter 59 Care of Patients with Noninflammatory Intestinal Disorders
Safe and Effective Care Environment
1. Prioritize nursing care for the patient with abdominal trauma.
2. Identify community-based resources for patients with colorectal cancer (CRC).
3. Describe the importance of collaborating with health care team members to provide care for patients with CRC.
Health Promotion and Maintenance
4. Teach patients health promotion practices to prevent CRC.
5. Plan health teaching for patients to promote self-management when caring for a colostomy.
7. Develop a teaching-learning plan for patients with irritable bowel syndrome (IBS).
8. Differentiate the most common types of hernias.
9. Develop a plan of care for a patient undergoing a minimally invasive inguinal hernia repair.
10. Identify risk factors for CRC.
11. Interpret assessment findings for patients with CRC.
12. Explain the role of the nurse in managing the patient with CRC.
13. Develop a perioperative plan of care for a patient undergoing a colon resection and colostomy.
14. Explain the differences between small-bowel and large-bowel obstructions.
15. Develop a plan of care for a patient with an intestinal obstruction to promote elimination.
16. Describe the postoperative care for a patient having a hemorrhoid surgical procedure.
http://evolve.elsevier.com/Iggy/
Animation: Nasogastric Tube Placement
Answer Key for NCLEX Examination Challenges and Decision-Making Challenges
Review Questions for the NCLEX® Examination
Irritable Bowel Syndrome
Pathophysiology
Irritable bowel syndrome (IBS) is a functional GI disorder that causes chronic or recurrent diarrhea, constipation, and/or abdominal pain and bloating. It is sometimes referred to as spastic colon, mucous colon, or nervous colon (Fig. 59-1). IBS is the most common digestive disorder seen in clinical practice and may affect as many as one in five people in the United States.
The etiology of IBS remains unclear. Recent research suggests that a combination of environmental, immunologic, genetic, hormonal, and stress factors play a role in the development and course of the disease. Examples of environmental factors include foods and fluids like caffeinated or carbonated beverages and dairy products. Infectious agents have also been identified. Several recent studies have found that patients with IBS often have small-bowel bacterial overgrowth, which causes bloating and abdominal distention. Multiple normal flora and pathogenic agents have been identified, including Pseudomonas aeruginosa (Kerckhoffs et al., 2011). Other researchers believe that these agents are less causative and serve as measurable biomarkers for the disease (Malinen et al., 2010).
Immunologic and genetic factors have also been associated with IBS, especially cytokine genes, including pro-inflammatory interleukins (IL) such as IL-6 and tumor necrosis factor (TNF)–alpha (Barkhordari et al., 2010). These findings may provide the basis of targeted drug therapy for the disease.
In the United States, women are two times more likely to have IBS than men. This difference may be the result of hormonal differences. However, in other areas of the world, this distribution pattern may not occur. For example, researchers found that there is not a female predominance for the disease in Asian countries (Gwee et al., 2010).
Considerable evidence relates the role of stress and mental or behavioral illness, especially anxiety and depression, to IBS. Many patients diagnosed with IBS meet the criteria for at least one primary mental health disorder. Some researchers suggest that psychosocial problems may be a cause for IBS (Nicholl et al., 2008). However, the pain and other chronic symptoms of the disease may lead to secondary mental health disorders. For example, when diarrhea is predominant, patients fear that there will be no bathroom facilities available and can become very anxious. The long-term nature of dealing with a chronic disease for which there is no cure can lead to secondary depression in some patients.
Patient-Centered Collaborative Care
Assessment
Routine laboratory values (including a complete blood count [CBC], serum albumin, erythrocyte sedimentation rate [ESR], and stools for occult blood) are normal in IBS. Some health care providers request a hydrogen breath test (Lindberg, 2009). When small-intestinal bacterial overgrowth or malabsorption of nutrients is present, excess hydrogen is produced. Some of this hydrogen is absorbed into the bloodstream and travels to the lungs where it is exhaled. Patients with IBS often have an increased amount of hydrogen during exhalation.
Teach the patient that he or she will need to be NPO (may have water) for at least 12 hours before the hydrogen breath test. At the beginning of the test, the patient blows into a hydrogen analyzer. Then, small amounts of test sugar are ingested, depending on the purpose of the test, and additional breath samples are taken every 15 minutes for 1 hour or longer. If lactose tolerance is evaluated, lactose is ingested. If bacterial overgrowth is tested, lactulose is given (Pagana & Pagana, 2010).
Interventions
The patient with IBS is usually cared for in an ambulatory care setting and learns self-management strategies. Interventions include health teaching, drug therapy, and stress reduction. Some patients also use complementary and alternative therapies. A holistic approach to patient care is essential for positive outcomes (Bengtsson et al., 2010).
Drug Alert
Many patients with IBS who have bloating and abdominal distention without constipation have success with rifaximin (Xifaxan), an antibiotic that works locally with little systemic absorption (Pimental et al., 2011). Although the drug has been approved for “traveler’s diarrhea” and other illnesses, the U.S. Food and Drug Administration (FDA) has not yet approved its use for patients with IBS.
Complementary and Alternative Therapies
For patients with increased intestinal bacterial overgrowth, recommend daily probiotic supplements. Probiotics have been shown to be effective for reducing bacteria and successfully alleviating GI symptoms of IBS (Lyra et al., 2010). There is also evidence that peppermint oil capsules may be effective in reducing symptoms for patients with IBS (Pirotta, 2009).
Acupuncture and moxibustion (Acu-Moxa) treatment has helped some patients by reducing flatulence and bloating and improving stool consistency (Anastasi et al., 2009). Moxibustion is the use of herbs to facilitate healing. Encourage patients to try these therapies, especially if they can be reimbursed by insurance companies. Third-party payers are becoming more sensitive to the use of these proven therapies as adjuncts in holistic disease management.
Herniation
Pathophysiology
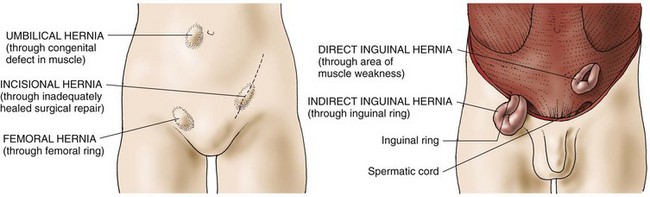
The most common types of abdominal hernias (Fig. 59-2) are indirect, direct, femoral, umbilical, and incisional.
• An indirect inguinal hernia is a sac formed from the peritoneum that contains a portion of the intestine or omentum. The hernia pushes downward at an angle into the inguinal canal. In males, indirect inguinal hernias can become large and often descend into the scrotum.
• Direct inguinal hernias, in contrast, pass through a weak point in the abdominal wall (Fig. 59-3).
• Femoral hernias protrude through the femoral ring. A plug of fat in the femoral canal enlarges and eventually pulls the peritoneum and often the urinary bladder into the sac.
• Umbilical hernias are congenital or acquired. Congenital umbilical hernias appear in infancy. Acquired umbilical hernias directly result from increased intra-abdominal pressure. They are most commonly seen in obese people.
• Incisional, or ventral, hernias occur at the site of a previous surgical incision. These hernias result from inadequate healing of the incision, which is usually caused by postoperative wound infections, inadequate nutrition, and obesity.
Patient-Centered Collaborative Care
Surgical Management
In addition to patient education about the procedure, the most important preoperative preparation is to teach the patient to remain NPO for the number of hours before surgery that the surgeon specifies. If same-day surgery is planned, remind the patient to arrange for someone to take him or her home and be available for the rest of the day at home. For patients having an open surgical approach, provide general preoperative care as described in Chapter 16.
General postoperative care of patients having a hernia repair is the same as that described in Chapter 18 except that they should avoid coughing. To promote lung expansion, encourage deep breathing and ambulation. With repair of an indirect inguinal hernia, the physician may suggest a scrotal support and ice bags applied to the scrotum to prevent swelling, which often contributes to pain. Elevation of the scrotum with a soft pillow helps prevent and control swelling.
Physiological Integrity
Colorectal Cancer
Pathophysiology
Most CRCs are adenocarcinomas, which are tumors that arise from the glandular epithelial tissue of the colon. They develop as a multi-step process, resulting in a number of molecular changes, such as loss of key tumor suppressor genes and activation of certain oncogenes that alter colonic mucosa cell division. The increased proliferation of the colonic mucosa forms polyps that can transform into malignant tumors. Most CRCs are believed to arise from adenomatous polyps that present as a visible protrusion from the mucosal surface of the bowel (McCance et al., 2010).
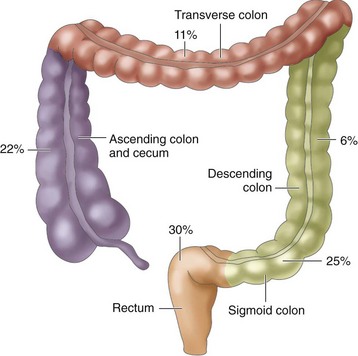
Tumors occur in different areas of the colon, with about two thirds occurring within the rectosigmoid region. The percentages in Fig. 59-4 indicate an increased incidence of cancer in the proximal sections of the large intestine over the past 35 years.
Complications related to the increasing growth of the tumor locally or through metastatic spread include bowel obstruction or perforation with resultant peritonitis, abscess formation, and fistula formation to the urinary bladder or the vagina. The tumor may invade neighboring blood vessels and cause frank bleeding. Tumors growing into the bowel lumen can gradually obstruct the intestine and eventually block it completely. Those extending beyond the bowel wall may place pressure on neighboring organs (uterus, urinary bladder, and ureters) and cause symptoms that mask those of the cancer. Chapter 23 discusses cancer pathophysiology in more detail.
Etiology and Genetic Risk
The major risk factors for the development of colorectal cancer (CRC) include being older than 50 years, genetic predisposition, personal or family history of cancer, and/or diseases that predispose the patient to cancer such as familial adenomatous polyposis (FAP), Crohn’s disease, and ulcerative colitis (McCance et al., 2010). Only a small percentage of colorectal cancers are familial and transmitted genetically.
Genetic/Genomic Considerations
People with a first-degree relative (sister, sibling, or child) diagnosed with colorectal cancer (CRC) have three to four times the risk for developing the disease. An autosomal dominant inherited genetic disorder known as familial adenomatous polyposis (FAP) accounts for 1% of CRCs. FAP is the result of one or more mutations in the adenomatous polyposis coli (APC) gene (McCance et al., 2010). In these very young patients, thousands of adenomatous polyps develop over the course of 10 to 15 years and have nearly a 100% chance of becoming malignant. By 20 years of age, most patients require surgical intervention, usually a colectomy with ileostomy or ileoanal pull-through, to prevent cancer. Chemotherapy may also be used for cancer prevention.
Hereditary nonpolyposis colorectal cancer (HNPCC) is another autosomal dominant disorder and accounts for a small percentage of all colorectal cancers. HNPCC is also caused by gene mutations, including MLH1 and MLH2. People with these mutations have an 80% chance of developing CRC at an average of 45 years of age. They also tend to have a higher incidence of endometrial, ovarian, stomach, and ureteral cancers (Nussbaum et al., 2007). Genetic testing is available for both of these familial CRC syndromes. Refer patients for genetic counseling and possible testing if the patient prefers.
There is also strong evidence that long-term smoking, increased body fat, physical inactivity, and heavy alcohol consumption are risk factors for colorectal cancer (American Cancer Society [ACS], 2010). A high-fat diet, particularly animal fat from red meats, increases bile acid secretion and anaerobic bacteria, which are thought to be carcinogenic within the bowel. Diets with large amounts of refined carbohydrates that lack fiber decrease bowel transit time.
Incidence/Prevalence
Colorectal cancer (CRC) is the third most common cause of cancer death in the United States (ACS, 2010). It is not common before 40 years of age, but the incidence in younger adults is slowly increasing, most likely due to increases in HPV infections (Stubenrauch, 2010). The overall incidence of CRC has decreased over the past 10 years, most likely as a result of increased cancer screenings. The disease is most common in African Americans, and their survival rate is lower than that of Euro-Americans (Caucasians). The possible reasons for this difference include less use of diagnostic testing (especially colonoscopy), decreased access to health care, cultural beliefs, and lack of education about the need for early cancer detection (Good et al., 2010; Hamlyn, 2008).
Health Promotion and Maintenance
Teach people about the need for diagnostic screening. When an adult turns 40 years of age, he or she should discuss with the health care provider about the need for colon cancer screening. The interval depends on level of risk. People of average risk who are 50 years of age and older, without a family history, should undergo regular CRC screening. The screening includes fecal occult blood testing (FOBT) and colonoscopy every 10 years or double-contrast barium enema every 5 years. People who have a personal or family history of the disease should begin screening earlier and more frequently (National Comprehensive Cancer Network, 2008). Teach all patients to follow the American Cancer Society recommendations for CRC screening listed in Chart 59-1.
Chart 59-1 Best Practice for Patient Safety & Quality Care
Screening Recommendations for Men and Women Ages 50 Years and Older at Average Risk for Colorectal Cancer
| PROCEDURE: CHOICE OF ONE OF THE FOLLOWING | INTERVAL AFTER SCREENING INITIATED AT AGE 50 YEARS | COMMENTS |
|---|---|---|
| FOBT and sigmoidoscopy | Every 5 years | FOBT procedure: two or three samples from three consecutive bowel movements obtained at home; tested by physician or nurse |
| OR | ||
| Double-contrast barium enema | Every 5 years | |
| OR | ||
| Colonoscopy | Every 10 years |
FOBT, Fecal occult blood testing.
Patient-Centered Collaborative Care
Assessment
Physical Assessment/Clinical Manifestations
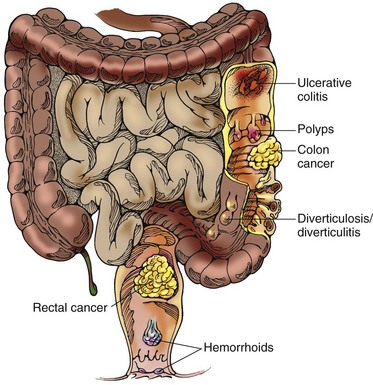
The clinical manifestations of CRC depend on the location of the tumor. However, the most common signs are rectal bleeding, anemia, and a change in stool consistency or shape. Stools may contain microscopic amounts of blood that are not noticeably visible, or the patient may have mahogany (dark)-colored or bright red stools (Fig. 59-5). Gross blood is not usually detected with tumors of the right side of the colon but is common (but not massive) with tumors of the left side of the colon and the rectum.
Laboratory Assessment
Carcinoembryonic antigen (CEA), an oncofetal antigen, is elevated in many people with CRC. The normal value is less that 5 ng/mL or 5 mcg/L (SI units) (Pagana & Pagana, 2010). This protein is not specifically associated with the colorectal cancer, and it may be elevated in the presence of other benign or malignant diseases and in smokers. CEA is often used to monitor the effectiveness of treatment and to identify disease recurrence.
Other Diagnostic Assessment
A sigmoidoscopy provides visualization of the lower colon using a fiberoptic scope. Polyps can be visualized, and tissue samples can be taken for biopsy. Polyps are usually removed during the procedure. A colonoscopy provides views of the entire large bowel from the rectum to the ileocecal valve. As with sigmoidoscopy, polyps can be seen and removed, and tissue samples can be taken for biopsy. Colonoscopy is the definitive test for the diagnosis of colorectal cancer. These procedures and associated nursing care are discussed in Chapter 55.
Preventing or Controlling Metastasis
Interventions
Nonsurgical Management
• Stage I—Tumor invades up to muscle layer
• Stage II—Tumor invades up to other organs or perforates peritoneum
• Stage III—Any level of tumor invasion and up to 4 regional lymph nodes
• Stage IV—Any level of tumor invasion; many lymph nodes affected with distant metastases
Radiation Therapy
The administration of preoperative radiation therapy has not improved overall survival rates for colon cancer, but it has been effective in providing local or regional control of the disease. Postoperative radiation has not demonstrated any consistent improvement in survival or recurrence. However, as a palliative measure, radiation therapy may be used to control pain, hemorrhage, bowel obstruction, or metastasis to the lung in advanced disease. For rectal cancer, unlike colon cancer, radiation therapy is almost always a part of the treatment plan. Reinforce information about the radiation therapy procedure to the patient and family, and monitor for possible side effects (e.g., diarrhea, fatigue). Chapter 24 describes the general care of patients undergoing radiation therapy.
Surgical Management
Surgical removal of the tumor with margins free of disease is the best method of ensuring removal of CRC. The size of the tumor, its location, the extent of metastasis, the integrity of the bowel, and the condition of the patient determine which surgical procedure is performed for colorectal cancer (Table 59-1). Many regional lymph nodes are removed and examined for presence of cancer. The number of lymph nodes that contain cancer is a strong predictor of prognosis. The most common surgeries performed are colon resection (removal of the tumor and regional lymph nodes) with reanastomosis, colectomy (colon removal) with colostomy (temporary or permanent) or ileostomy/ileoanal pull-through, and abdominoperineal (AP) resection. A colostomy is the surgical creation of an opening of the colon onto the surface of the abdomen. An AP resection is performed when rectal tumors are present. The surgeon removes the sigmoid colon, rectum, and anus through combined abdominal and perineal incisions.
TABLE 59-1 SURGICAL PROCEDURES FOR COLORECTAL CANCERS IN VARIOUS LOCATIONS
| Right-Sided Colon Tumors |
| Left-Sided Colon Tumors |
| Sigmoid Colon Tumors |
| Rectal Tumors |
Preoperative Care
Before evaluating the tumor and colon during surgery, the surgeon may not be able to determine whether a colostomy (or less commonly, an ileostomy) will be necessary. The patient is told that a colostomy is a possibility. If a colostomy is planned, the surgeon consults a certified wound, ostomy, continence nurse (CWOCN) or an enterostomal therapist (ET) (ostomy nurse) to recommend optimal placement of the ostomy. He or she teaches the patient about the rationale and general principles of ostomy care. In many settings, the CWOCN marks the patient’s abdomen to indicate a potential ostomy site that will decrease the risk for complications such as interference of the undergarments or a prosthesis with the ostomy appliance. Table 59-2 describes the role of the CWOCN or ET.
TABLE 59-2 PREOPERATIVE ASSESSMENT BY THE CWOCN OR ET NURSE
| Key Points of Psychosocial Assessment |
• Patient’s and family’s level of knowledge of disease and ostomy care • Patient’s physical limitations (particularly sensory) • Support available to patient • Patient’s type of employment • Patient’s involvement in activities such as hobbies Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|