Chapter 37 Care of Patients with Cardiac Problems
Safe and Effective Care Environment
1. Evaluate the status of patients with end-stage heart disease regarding advance directives.
2. Provide the patient with heart failure (HF) and the family information on discharge to home, hospice, or other community-based setting.
3. Collaborate with the interdisciplinary team when providing care to patients with cardiac problems.
Health Promotion and Maintenance
4. Identify community resources for patients with cardiac problems and their families.
5. Provide special care needs of older adults with heart failure.
6. Teach patients about actions to maintain health and prevent worsening HF.
8. Explain the pathophysiology of HF.
9. Compare and contrast left-sided and right-sided HF.
10. Identify priority problems for patients with HF.
11. Perform a comprehensive assessment of patients experiencing cardiac problems.
12. Explain how common drug therapies improve cardiac output and prevent worsening of HF.
13. Assess patients for adverse effects of drug therapy for cardiac problems.
14. Monitor the laboratory values for patients with cardiac problems.
15. Plan nursing interventions to improve the patient’s cardiovascular status when needed.
16. Provide emergency care for patients experiencing life-threatening complications, such as cardiac tamponade and pulmonary edema.
17. Identify the four Heart Failure Core Measures required by The Joint Commission.
18. Describe essential focused assessments used by the home care nurse for patients with heart failure.
19. Compare and contrast common valvular disorders.
20. Describe surgical management for patients with valvular disease.
21. Develop a teaching/learning plan for patients with valvular disease.
22. Differentiate between common cardiac inflammations and infections—endocarditis, pericarditis, and rheumatic carditis.
23. Provide postoperative care for patients having a heart transplant.
24. Identify clinical assessment findings for patients with cardiomyopathy.
http://evolve.elsevier.com/Iggy/
Animation: Congestive Heart Failure
Animation: Pericardial Tamponade
Answers to NCLEX Examination Challenges and Decision-Making Challenges
Review Questions for the NCLEX® Examination
This chapter focuses on heart failure and its common causes in the adult population; coronary artery disease is discussed in Chapter 40. Heart failure is the most common reason for hospital stays in patients older than 65 years in the United States. When the heart is diseased, it cannot effectively pump an adequate amount of arterial blood to the rest of the body. Arterial blood carries oxygen and nutrients to vital organs, such as the kidneys and brain, and peripheral tissues. When these organs and other body tissues are not adequately perfused, they may not function properly.
Heart Failure
Pathophysiology
Types of Heart Failure
The major types of heart failure are:
Systolic heart failure (systolic ventricular dysfunction) results when the heart cannot contract forcefully enough during systole to eject adequate amounts of blood into the circulation. Preload increases with decreased contractility, and afterload increases as a result of increased peripheral resistance (e.g., hypertension) (McCance et al., 2010). The ejection fraction (the percentage of blood ejected from the heart during systole) drops from a normal of 50% to 70% to below 40% with ventricular dilation. As it decreases, tissue perfusion diminishes and blood accumulates in the pulmonary vessels. Manifestations of systolic dysfunction may include symptoms of inadequate tissue perfusion or pulmonary and systemic congestion. Systolic heart failure is often called “forward failure” because cardiac output is decreased and fluid backs up into the pulmonary system. Because these patients are at high risk for sudden cardiac death, patients with an ejection fraction of less than 30% are considered candidates for an implantable cardioverter/defibrillator (ICD; also known as an internal cardioverter/defibrillator) (see Chapter 36).
In contrast, diastolic heart failure (heart failure with preserved left ventricular function) occurs when the left ventricle cannot relax adequately during diastole. Inadequate relaxation or “stiffening” prevents the ventricle from filling with sufficient blood to ensure an adequate cardiac output. Although ejection fraction is more than 40%, the ventricle becomes less compliant over time because more pressure is needed to move the same amount of volume as compared with a healthy heart. Diastolic failure represents about 20% to 40% of all heart failure, primarily in older adults and in women who have chronic hypertension and undetected coronary artery disease. Clinical manifestations and management of diastolic failure are similar to those of systolic dysfunction (McCance et al., 2010).
Classification and Staging of Heart Failure
The American College of Cardiology (ACC) and American Heart Association (AHA) have developed evidence-based guidelines for staging and managing heart failure as a chronic, progressive disease. These guidelines do not replace the New York Heart Association (NYHA) functional classification system, which is used to describe symptoms a patient may exhibit (see Table 35-2 in Chapter 35).
The ACC/AHA staging system when compared with the NYHA system categorizes patients as:
A. Patients at high risk for developing heart failure (class I NYHA)
B. Patients with cardiac structural abnormalities or remodeling who have not developed HF symptoms (class I NYHA)
C. Patients with current or prior symptoms of heart failure (class II or III NYHA)
D. Patients with refractory end-stage heart failure (class IV NYHA)
Another method for staging HF is the Killip classification system, which is based on the heart’s hemodynamic ability. Table 40-3 in Chapter 40 outlines this system.
Compensatory Mechanisms
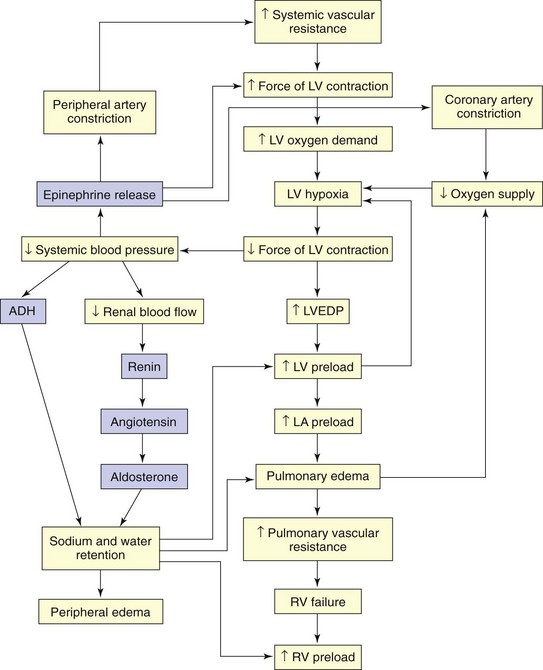
When cardiac output is insufficient to meet the demands of the body, compensatory mechanisms work to improve cardiac output (Fig. 37-1). Although these mechanisms may initially increase cardiac output, they eventually have a damaging effect on pump function. Major compensatory mechanisms include:
Renin-Angiotensin System Activation
Reduced blood flow to the kidneys, a common occurrence in low-output states, results in activation of the renin-angiotensin system (RAS). Vasoconstriction becomes more pronounced in response to angiotensin II, and aldosterone secretion causes sodium and water retention. Preload and afterload increase. Angiotensin II contributes to ventricular remodeling resulting in progressive myocyte (myocardial cell) contractile dysfunction over time (McCance et al., 2010).
Other Chemical Responses
Natriuretic peptides are neurohormones that work to promote vasodilation and diuresis through sodium loss in the renal tubules. The B-type natriuretic peptide (BNP) is produced and released by the ventricles when the patient has fluid overload as a result of HF. It increases with age and has a greater concentration in women (Jessup et al., 2009). People who are obese have lower BNP levels compared with those who are not (Noveanu et al., 2009).
Etiology
Heart failure (HF) is caused by systemic hypertension in most cases. About a third of patients experiencing myocardial infarction (MI, “heart attack”) also develop HF. The next most common cause is structural heart changes, such as valvular dysfunction, particularly pulmonic or aortic stenosis, which leads to pressure or volume overload on the heart. Common direct causes and risk factors for HF are listed in Table 37-1.
TABLE 37-1 COMMON CAUSES AND RISK FACTORS FOR HEART FAILURE
Incidence/Prevalence
Considerations for Older Adults
Heart failure has been referred to as a U.S. epidemic, although it is a major problem worldwide (Joseph et al., 2009). One of the proposed U.S. Healthy People 2020 objectives is to reduce the number of hospitalizations of older adults with HF as the principal diagnosis. Patient and family education can help meet this objective (Table 37-2). As the “baby boomer” population reaches 65 years of age, the numbers of hospital stays and deaths from HF are likely to increase dramatically.
TABLE 37-2 MEETING PROPOSED HEALTHY PEOPLE 2020 OBJECTIVES
Patient-Centered Collaborative Care
Assessment
Physical Assessment/Clinical Manifestations
Manifestations of HF depend on the type of failure, the ventricle involved, and the underlying cause. Impaired tissue perfusion, pulmonary congestion, and edema are associated with left ventricular failure (Chart 37-1). Conversely, systemic venous congestion and peripheral edema are associated with right ventricular failure (Chart 37-2).
Left-Sided Heart Failure
| DECREASED CARDIAC OUTPUT | PULMONARY CONGESTION |
|---|---|
Left-Sided Heart Failure
The pulse may be tachycardic, or it may alternate in strength (pulsus alternans). Take the apical pulse for a full minute, noting any irregularity in heart rhythm. An irregular heart rhythm resulting from premature atrial contractions (PACs), premature ventricular contractions (PVCs), or atrial fibrillation (AF) is common in HF (see Chapter 36). The sudden development of an irregular rhythm may further compromise CO. Carefully monitor the patient’s respiratory rate, rhythm, and character, as well as oxygen saturation. The respiratory rate typically exceeds 20 breaths/min.
Psychosocial Assessment
Patients with HF, especially those with advanced disease, are at high risk for depression. It is not certain whether the functional impairments contribute to the depression or depression affects functional ability. Older hospitalized patients may be depressed, particularly those who have been readmitted for an acute episode of HF. Lifestyle changes and quality-of-life issues can also cause depression many months after the initial diagnosis of HF (Thomas et al., 2008).
Laboratory Assessment
B-type natriuretic peptide (BNP) is used for diagnosing HF (in particular, diastolic HF) in patients with acute dyspnea. As discussed earlier, it is part of the body’s response to decreased cardiac output from either left or right ventricular dysfunction. An increase in BNP, in conjunction with history and physical, best differentiates between the dyspnea of HF and that associated with lung dysfunction (Wexler et al., 2009). However, patients with renal disease may also have elevated BNP levels (Chen et al., 2010).
Imaging Assessment
Radionuclide studies (thallium imaging or technetium pyrophosphate scanning) can also indicate the presence and cause of HF. Multigated angiographic (MUGA) scans provide information about left ventricular ejection fraction and velocity, which are typically low in patients with HF. These tests are discussed in Chapter 35.
Other Diagnostic Assessment
Invasive hemodynamic monitoring allows the direct assessment of cardiac function and volume status in acutely ill patients. These measurements can confirm the diagnosis and guide the management of HF. Right atrial pressure may be normal or elevated in left ventricular failure and is elevated in right ventricular failure. Pulmonary artery pressure (PAP) and pulmonary artery wedge pressure (PAWP) are elevated in left-sided HF because volumes and pressures are increased in the left ventricle. (See Chapter 35 for a more detailed description of hemodynamic monitoring.)
Improving Cardiac Output
Interventions
Nonsurgical Management
Nonsurgical management relies primarily on a variety of drugs (Table 37-3). If drug therapy is ineffective, other nonsurgical options are available.
TABLE 37-3 COMMONLY USED DRUG CLASSIFICATIONS FOR PATIENTS WITH SYSTOLIC HEART FAILURE
Drugs to improve stroke volume include those that reduce afterload, reduce preload, and improve cardiac muscle contractility. A major role of the nurse is to give medications as prescribed, monitor for their therapeutic and adverse effects, and teach the patient and family about drug therapy. A variety of classes of drugs that reduce afterload and preload are used to manage heart failure (see Table 37-3).
Angiotensin-Converting Enzyme Inhibitors (ACEIs) and Angiotensin-Receptor Blockers (ARBs)
Drug Alert
Assess for orthostatic hypotension, acute confusion, poor peripheral perfusion, and reduced urine output in patients with low systolic blood pressure. Monitor serum potassium and creatinine levels to determine renal dysfunction. Additional nursing implications for selected ACE inhibitor/ARB drugs are described in Chapter 38 on p. 783 in the Drug Therapy section.
Interventions That Reduce Preload
Considerations for Older Adults
Loop diuretics continue to work even after excess fluid is removed. As a result, some patients, especially older adults, can become dehydrated. Observe for manifestations of dehydration in the older adult, especially acute confusion, decreased urinary output, and dizziness. Provide evidence-based interventions to reduce the risk of falls, as discussed in Chapter 3.
• Returning venous vasculature to a more normal capacity
• Decreasing the volume of blood returning to the heart
Unfortunately, tolerance to the vasodilating effects develops when nitrates are given around-the-clock. To prevent this tolerance, the health care provider may prescribe at least one 12-hour nitrate-free period out of every 24 hours (usually overnight). Nitrates such as isosorbide (Imdur, ISMO) are prescribed to provide nitrate-free periods and reduce the problem of tolerance. Chapter 40 discusses nitrates in more detail.
Physiological Integrity
Drugs That Enhance Contractility
The potential benefits of digoxin include:
• Slowing of conduction through the atrioventricular node
• Inhibition of sympathetic activity while enhancing parasympathetic activity
Drug Alert
Levosimendan (Simdax) is a calcium-sensitizing medication and a positive inotropic drug. It appears to bind to troponin C in the heart muscle and therefore increases the contraction of the heart. Simdax is used most often in patients who have had or are at high risk for myocardial infarction. Chapter 40 discusses inotropic drugs in more detail.
Physiological Integrity
A. Call the ED physician immediately.
B. Draw a serum digoxin level.
C. Assess for signs of hypokalemia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree