Chapter 71 Care of Patients with Acute Kidney Injury and Chronic Kidney Disease
Safe and Effective Care Environment
1. Evaluate patient risk for dehydration, shock, and acute kidney injury.
2. Collaborate with members of the health care team to reduce patient exposure to nephrotoxins in the acute care setting.
3. Prevent injury in the patient who has bone density loss or skin changes from kidney disease.
4. Apply principles of infection control to prevent infection in patients receiving immunosuppressive therapy.
Health Promotion and Maintenance
5. Teach everyone to drink fluids to prevent dehydration during hot weather and when engaging in heavy work or exercise.
6. Assess intake and output for anyone at risk for or with hypovolemia.
7. Teach transplant recipients and their families about the importance of adhering to anti-rejection therapy.
8. Encourage patients and families to express any concerns about the risk for death and the disruption of lifestyle as a result of treatment for kidney dysfunction.
9. Assess the patient for depression and nonacceptance of the diagnosis or treatment plan.
10. Refer patients to community resources and support groups.
11. Compare the pathophysiology and causes of acute kidney injury (AKI) with those of chronic kidney disease (CKD).
12. Use laboratory data and clinical assessment to determine the effectiveness of therapy for kidney dysfunction.
13. Discuss interventions to prevent AKI.
14. Discuss the mechanisms of peritoneal dialysis (PD) and hemodialysis (HD) as renal replacement therapies.
15. Coordinate nursing care for the patient with severe CKD or end-stage kidney disease (ESKD).
16. Plan prevention strategies for the complications of PD.
17. Coordinate nursing care for the patient during the first 24 hours after kidney transplantation.
http://evolve.elsevier.com/Iggy/
Animation: Renal and Urinary Disorders
Answer Key for NCLEX Examination Challenges and Decision-Making Challenges
Concept Map: End-Stage Kidney Disease
Review Questions for the NCLEX® Examination
Severe kidney disease leading to renal failure is common in North America. Acute kidney injury (AKI) is most common in the acute care setting, and chronic kidney disease (CKD), which may take years to develop, is more common in the community. Both types of kidney dysfunction cause problems by interfering with urinary elimination and disrupting homeostasis of fluid volume, blood pressure, electrolytes, wastes, and acid-base balance (see Fig. 70-1 in Chapter 70). These problems can reduce general function, shorten life, and decrease quality of life. Diabetes, hypertension, and cardiovascular disease are much more common in people with CKD, and their incidence increases as the stage of CKD worsens (U.S. Renal Data Systems [USRDS], 2010). Between 2003 and 2006, the number of patients with stage 3 CKD rose by 1.8% (USRDS, 2010). Thus overall, the incidence has increased only slightly and is most likely related to the use of kidney protective therapy with antihypertensive drugs such as angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blocker (ARB) drugs, as well as the benefits associated with use of beta-blocker drugs in the treatment of heart failure. The incidence is expected to continue to rise as a result of the aging population and the huge increase in the incidence of type 2 diabetes. In the United States, about half a million people with end-stage-kidney disease (ESKD) are treated yearly with dialysis or kidney transplantation (Okusa et al., 2009). Kidney dysfunction has many causes. CKD is most commonly caused by hypertension (HTN) and diabetes.
As described in Chapter 68, kidney functions include excretion of waste, water and salt balance, acid-base balance, and hormone secretion. When kidney function declines gradually, as occurs most often with CKD (also known as chronic renal failure [CRF]), 90% to 95% of the nephrons must be destroyed before kidney dysfunction is obvious. The patient may have many years of reduced kidney function (renal insufficiency) before the uremia of ESKD develops. During this time of decreased kidney function, the patient is at increased risk for acute kidney injury because of the stress on remaining nephrons.
When kidney decline is sudden, the functioning nephrons are overworked and kidney dysfunction may develop with the loss of only 50% of functioning nephrons. Acute kidney injury and chronic kidney disease are compared in Table 71-1. Acute kidney injury affects many body systems. Chronic kidney disease affects every body system. The problems that occur with loss of kidney function are related to fluid overload, electrolyte and acid-base abnormalities, buildup of nitrogen-based wastes, and loss of kidney hormone function.
TABLE 71-1 CHARACTERISTICS OF ACUTE KIDNEY INJURY AND CHRONIC KIDNEY DISEASE
| CHARACTERISTIC | ACUTE KIDNEY INJURY | CHRONIC KIDNEY DISEASE |
|---|---|---|
| Onset | Sudden (hours to days) | Gradual (months to years) |
| % of nephron involvement | ≈50% | 90%-95% |
| Duration | 2-4 wks; less than 3 months | Permanent |
| Prognosis | Good for return of kidney function with supportive care; high mortality in some situations | Fatal without a renal replacement therapy such as dialysis or transplantation |
Acute Kidney Injury
Pathophysiology
With shock or other problems causing an acute reduction in blood flow to the kidney (hypoperfusion), the kidney compensates by constricting renal blood vessels, activating the renin-angiotensin-aldosterone pathway, and releasing antidiuretic hormone (ADH). These responses increase blood volume and improve kidney perfusion. However, these same responses reduce urine volume, resulting in oliguria (urine output less than 400 mL/day) and azotemia (the retention and buildup of nitrogenous wastes in the blood). Nephron cell injury is more likely to occur from the lack of oxygen (ischemia) related to reduced blood flow (McCance et al., 2010). Toxins can cause blood vessel constriction in the kidney, leading to reduced kidney blood flow and kidney ischemia.
Classification of Acute Kidney Injury Risk
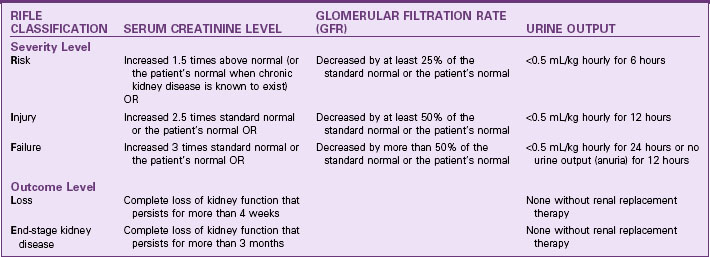
The recent change in terminology from acute renal failure to acute kidney injury resulted in the development of standardized criteria to recognize the changes associated with the problem earlier, when interventions are more likely to reverse the process and prevent permanent kidney damage (Dirkes, 2011; Martin, 2010). This change was aimed at preventing kidney damage both in people who had healthy kidneys before a specific precipitating event and in those who already had some degree of chronic kidney disease (CKD). (An acute kidney injury occurring in a person with CKD can greatly increase the rate of progression to end-stage kidney disease.)
Table 71-2 lists the criteria for classification of acute kidney injury based on the five RIFLE categories of changes in glomerular filtration, serum creatinine levels, and reduction of hourly urine output. (RIFLE stands for Risk, Injury, Failure, Loss, and End-stage kidney disease.) These criteria apply to patients with no known kidney problems and to patients with CKD. The first three criteria (R,I,F), are injury severity levels. Identification of AKI at these levels and proper management can prevent progression to more serious injury that may not be reversible. The last two criteria (L,E) indicate serious injury that requires renal replacement therapy at least on a temporary basis. Even at level L, some people have return of kidney function. It is the responsibility of all health care professionals to be alert to the possibility of AKI, implement prevention strategies, and identify the need to implement appropriate interventions to prevent permanent impairment of kidney function.
Types of Acute Kidney Injury
The types of AKI are described by their causes. These include prerenal azotemia, intrarenal (intrinsic) AKI, and postrenal azotemia. Table 71-3 lists causes of AKI.
TABLE 71-3 CAUSES OF THE THREE TYPES OF ACUTE KIDNEY INJURY
| Prerenal Acute Kidney Injury |
| Intrarenal (Intrinsic) Acute Kidney Injury |
| Postrenal Acute Kidney Injury |
Prerenal azotemia is kidney injury caused by poor blood flow to the kidneys. The most common problems leading to AKI are hypovolemic shock and heart failure (Ali & Gray-Vickrey, 2011). Early AKI (levels R and I) often can be reversed by correcting blood volume, increasing blood pressure, and improving cardiac output. When the reduced blood flow is prolonged, the kidneys are severely damaged and intrarenal kidney injury results.
When kidney function declines, the oliguric phases of AKI begin (Table 71-4). Some patients have a nonoliguric form of AKI in which urine output remains near normal but serum creatinine levels rise. Ideally, interventions to restore circulating volume, improve cardiac output, and increase blood pressure prevent progression to a more severe level of kidney injury.
TABLE 71-4 THE PHASES OF OLIGURIC ACUTE KIDNEY INJURY
| PHASE | DESCRIPTION | CHARACTERISTICS |
|---|---|---|
| Onset phase | Begins with the precipitating event and continues until oliguria develops. Lasts hours to days. | The gradual accumulation of nitrogenous wastes, such as increasing serum creatinine and BUN, may be noted. |
| Oliguric phase | Characterized by a urine output of 100-400 mL/24 hr that does not respond to fluid challenges or diuretics. Lasts 1-3 weeks. | Laboratory data include increasing serum creatinine and BUN levels, hyperkalemia, bicarbonate deficit (metabolic acidosis), hyperphosphatemia, hypocalcemia, and hypermagnesemia. |
| Sodium retention occurs, but this is masked by the dilutional effects of water retention. | ||
| Urinary indices are typically low and fixed; regulation of water balance by the kidneys is impaired, so urine specific gravity and urine osmolarity do not vary as plasma osmolarity changes. | ||
| Diuretic phase (high-output phase) | Often has a sudden onset within 2-6 wk after oliguric stage. Urine flow increases rapidly over a period of several days. The diuresis can result in an output of up to 10 L/day of dilute urine. | Electrolyte losses typically precede clearance of nitrogenous wastes. |
| Later in the diuretic phase, the BUN level starts to fall and continues to fall until the level reaches normal limits or reaches a plateau. | ||
| Normal kidney tubular function is re-established during this phase. | ||
| Recovery phase (convalescent phase) | In this phase, the patient begins to return to normal levels of activity. Complete recovery may take up to 12 months. | The patient functions at a lower energy level and has less stamina than before the illness. |
| Residual kidney dysfunction may be noted through regular monitoring of kidney function. | ||
| Kidney function may never return to pre-illness levels, but function sufficient for a long and healthy life is likely. |
BUN, Blood urea nitrogen.
Etiology
Many types of problems can reduce kidney function. Severe hypotension from shock or dehydration reduces kidney blood flow and can lead to prerenal AKI. Cardiac disease or heart failure also can reduce kidney blood flow. The patient may be oliguric or even anuric (less than 100 mL/24 hr) if kidney blood flow reduction is severe. Conditions causing AKI are listed in Table 71-3.
Incidence/Prevalence
Health care–acquired AKI occurs in as many as 4% of hospital admissions and 20% of critical care admissions (Peacock & Sinert, 2010). Most AKI episodes are due to events that lead to hypotension with poor kidney perfusion and worsening of chronic kidney problems. For patients who survive the precipitating event, the chance for return of kidney function is good. However, complications during the course of AKI can greatly increase the risk for death. Bloodstream infections from IV line contamination are frequent complications that lead to death. However, the highest death rate occurs with trauma (70%) and surgery. AKI caused by nephrotoxic (kidney damaging) substances (Table 71-5) has the lowest rates of recovery. The prognosis for AKI caused by obstruction or glomerulonephritis is much better.
TABLE 71-5 SOME POTENTIALLY NEPHROTOXIC SUBSTANCES
| Drugs | |
Nonsteroidal Anti-inflammatory Drugs (NSAIDs) | |
| Other Substances | |
Health Promotion and Maintenance
Critical Rescue
Be aware of nephrotoxic substances that the patient may ingest or be exposed to (see Table 71-5). Question any prescription for potentially nephrotoxic drugs, and validate the dose before the patient receives the drug. Antibiotics are common drugs that have nephrotoxic side effects. NSAIDs can cause or increase the risk for AKI. Combining two or more nephrotoxic drugs dramatically increases the risk for AKI. If a patient must receive a known nephrotoxic drug, closely monitor laboratory values, including BUN, creatinine, and drug peak and trough levels, for indications of reduced kidney function.
Patient-Centered Collaborative Care
Assessment
Physical Assessment/Clinical Manifestations
The manifestations of AKI are related to the buildup of nitrogenous wastes (azotemia), as well as to as the underlying cause (Chart 71-1). Manifestations of prerenal azotemia are hypotension, tachycardia, decreased urine output, decreased cardiac output, decreased central venous pressure (CVP), and lethargy. The appearance of a patient with prerenal azotemia is similar to that of a patient with heart failure or dehydration, depending on the cause of the poor kidney blood flow.
Acute Kidney Injury
Laboratory Assessment
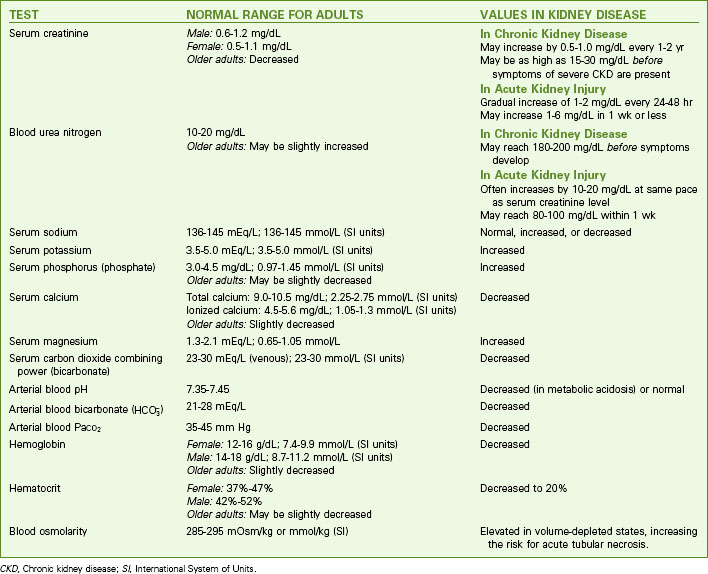
The many changes in laboratory values in the patient with AKI are similar to those occurring in chronic kidney disease (CKD) (Chart 71-2; see also p. 1552 in the discussion of Laboratory Assessment in the Chronic Kidney Disease section). Expect to see rising BUN and serum creatinine levels and abnormal blood electrolyte values. Patients with AKI, however, usually do not have the anemia associated with CKD unless there is hemorrhagic blood loss or unless blood urea levels are high enough to break (lyse) red blood cells.
Kidney Disease
| TEST | NORMAL RANGE FOR ADULTS | VALUES IN KIDNEY DISEASE |
|---|---|---|
| Serum creatinine | Male: 0.6-1.2 mg/dL | May increase by 0.5-1.0 mg/dL every 1-2 yr May be as high as 15-30 mg/dL before symptoms of severe CKD are present |
| Female: 0.5-1.1 mg/dL | ||
| Older adults: Decreased | ||
| Blood urea nitrogen | 10-20 mg/dL | |
| Older adults: May be slightly increased | ||
| Serum sodium | 136-145 mEq/L; 136-145 mmol/L (SI units) | Normal, increased, or decreased |
| Serum potassium | 3.5-5.0 mEq/L; 3.5-5.0 mmol/L (SI units) | Increased |
| Serum phosphorus (phosphate) | 3.0-4.5 mg/dL; 0.97-1.45 mmol/L (SI units) | Increased |
| Older adults: May be slightly decreased | ||
| Serum calcium | Total calcium: 9.0-10.5 mg/dL; 2.25-2.75 mmol/L (SI units) | Decreased |
| Ionized calcium: 4.5-5.6 mg/dL; 1.05-1.3 mmol/L (SI units) | ||
| Older adults: Slightly decreased | ||
| Serum magnesium | 1.3-2.1 mEq/L; 0.65-1.05 mmol/L | Increased |
| Serum carbon dioxide combining power (bicarbonate) | 23-30 mEq/L (venous); 23-30 mmol/L (SI units) | Decreased |
| Arterial blood pH | 7.35-7.45 | Decreased (in metabolic acidosis) or normal |
Arterial blood bicarbonate ( ) ) | 21-28 mEq/L | Decreased |
| Arterial blood PaCO2 | 35-45 mm Hg | Decreased |
| Hemoglobin | Female: 12-16 g/dL; 7.4-9.9 mmol/L (SI units) | Decreased |
| Male: 14-18 g/dL; 8.7-11.2 mmol/L (SI units) | ||
| Older adults: Slightly decreased | ||
| Hematocrit | Female: 37%-47% | Decreased to 20% |
| Male: 42%-52% | ||
| Older adults: May be slightly decreased | ||
| Blood osmolarity | 285-295 mOsm/kg or mmol/kg (SI) | Elevated in volume-depleted states, increasing the risk for acute tubular necrosis. |
CKD, Chronic kidney disease; SI, International System of Units.
Other Diagnostic Assessment
Kidney biopsy is performed if the cause of AKI is uncertain, an immunologic disease is suspected, or the reversibility of the kidney dysfunction needs to be determined after AKI has persisted for an extended period. Prepare the patient before the test, and provide follow-up care. Be aware of all test results and understand how they might affect the treatment regimen. (See Chapter 68 for a detailed discussion of renal diagnostic tests.)
Safe and Effective Care Environment
Interventions
Drug Therapy
Patients with AKI receive many drugs. As kidney function changes, drug dosages are changed. It is important to be knowledgeable about the site of drug metabolism and especially careful when giving drugs. Constantly monitor for possible side effects and interactions of the drugs that the patient with AKI is receiving (Chart 71-3; see also the discussion of drug therapy on pp. 1553, 1556, and 1557 in the Chronic Kidney Disease section). Diuretics may be used to increase urine output.
Patient-Centered Care; Evidence-Based Practice; Quality Improvement
1. What additional assessment data should you obtain? Provide a rationale for your choices.
2. What risk factors does she have for acute kidney injury?
3. Is her risk greatest for prerenal acute kidney injury, intrarenal azotemia, or postrenal acute kidney injury? Explain your answer.
4. What questions will you ask her about her urine output?
5. What resources or changes in policy could have helped prevent this problem and future problems?
Nutrition Therapy
If the patient with AKI has a good dietary intake (see discussion of Enhancing Nutrition on pp. 1555-1556 in the Chronic Kidney Disease section), nutritional support may not be needed. A dietary consult with a dietitian, who will calculate the patient’s caloric needs, may be prescribed. Work with the dietitian to provide a diet with specified amounts of protein, sodium, and fluids. For the patient who does not require dialysis, 0.6 g/kg of body weight or 40 g/day of protein is usually prescribed. For patients who do require dialysis, the protein level needed will range from 1 to 1.5 g/kg. The amount of dietary sodium ranges from 60 to 90 mEq. If high blood potassium levels are present, dietary potassium is restricted to 60 to 70 mEq. The amount of fluid permitted is generally calculated to be equal to the urine volume plus 500 mL. Assess food intake every shift to ensure that caloric intake is adequate.
Dialysis Therapies
The subclavian vein is used, when possible, instead of the femoral site because the catheter can be left in place between dialysis treatments. However, the longer the catheter is left in this place, the greater the chance for infection. The subclavian dialysis catheter (Fig. 71-1) is inserted at the bedside. A physician or nurse practitioner performs the sterile procedure, and then the catheter is covered with a sterile dressing. Monitor for manifestations of procedure complications such as pneumothorax (reduced breath sounds, tracheal deviation away from midline, prominence and poor movement of one side of the chest) or subcutaneous emphysema (crackling and swelling of tissue around the site). Catheter placement is checked by chest x-ray before its use.
Peritoneal dialysis (PD) may also be used in the treatment of AKI, although some patients, such as those being mechanically ventilated, may not be able to tolerate the abdominal distention that occurs with PD. PD uses the peritoneum as the dialyzing membrane. The dialysate is infused through a catheter implanted in the peritoneum. A complete discussion of PD is provided in the Chronic Kidney Disease section, pp. 1564-1567.
Posthospital Care
The care for a patient with AKI after discharge from the hospital varies, depending on the status of the disorder when the patient is discharged. The course of AKI varies, with recovery lasting up to several months. If the kidney injury is resolving, follow-up care may be provided by a nephrologist or by the family physician in consultation with the nephrologist. However, AKI may result in permanent kidney damage and CKD with the need for chronic dialysis or even transplantation. In these cases, follow-up care is similar to that needed for patients with chronic kidney disease (see Community-Based Care, pp. 1571-1572).
Health Promotion and Maintenance
Chronic Kidney Disease
Pathophysiology
Unlike acute kidney injury (AKI), chronic kidney disease (CKD) is a progressive, irreversible disorder and kidney function does not recover. When kidney function is too poor to sustain life, CKD becomes end-stage kidney disease (ESKD). Terms used with kidney dysfunction include azotemia (buildup of nitrogen-based wastes in the blood), uremia (azotemia with clinical symptoms [Chart 71-4]), and uremic syndrome. Table 71-1 compares AKI and CKD.
Uremia
Stages of Chronic Kidney Disease
The kidneys lose function (fail) in an organized fashion involving five stages based on estimated glomerular filtration rate (GFR). Progression toward ESKD usually starts with a gradual decrease in GFR (Table 71-6). In this first stage, the person may have a normal GFR (greater than 90 mL/min) with normal kidney function and no obvious kidney disease. However, there may be a reduced renal reserve in which reduced kidney function occurs without buildup of wastes in the blood because the unaffected nephrons overwork to compensate for the diseased nephrons. Although no manifestations of kidney dysfunction are usually present at this stage, if the patient is stressed with infection, fluid overload, pregnancy, or dehydration, kidney function at this stage can appear reduced.
TABLE 71-6 PROGRESSION OF CHRONIC KIDNEY DISEASE
| STAGE OF CHRONIC KIDNEY DISEASE (CKD) | ESTIMATED GLOMERULAR FILTRATION RATE | INTERVENTION |
|---|---|---|
| Stage 1 | >90 mL/min | Screening for risk factors |
| At risk; normal kidney function (early kidney disease may or may not be present) | ||
| Stage 2 | 60-89 mL/min | Focus on reduction of risk factors |
| Mild CKD | ||
| Stage 3 | 30-59 mL/min | Implement strategies to slow disease progression |
| Moderate CKD | ||
| Stage 4 | 15-29 mL/min | Manage complications, and prepare for eventual renal replacement therapy |
| Severe CKD | ||
| Stage 5 | <15 mL/min | Implement renal replacement therapy/kidney transplantation |
| End-stage kidney disease (ESKD) |
Action Alert
Over time, patients progress to severe CKD (the fourth stage) and end-stage kidney disease (ESKD) (the fifth stage). Excessive amounts of urea and creatinine build up in the blood, and the kidneys cannot maintain homeostasis. Severe fluid, electrolyte, and acid-base imbalances occur (Pradeep & Verrelli, 2010). Without renal replacement therapy, fatal complications occur.
Metabolic Changes
In the later stages of CKD, kidney excretion of sodium is reduced as urine production decreases. Then sodium retention and high serum sodium levels (hypernatremia) can occur with only modest increases in dietary sodium intake. This problem leads to severe fluid and electrolyte imbalances (see Chapter 13). Sodium retention causes hypertension and edema.
Even with sodium retention, the serum sodium level may appear normal because plasma water is retained at the same time. If fluid retention occurs at a greater rate than sodium retention, the serum sodium level is falsely low because of dilution (see Chart 71-2).
Potassium excretion occurs mainly through the kidney. Any increase in potassium load during the later stages of CKD can lead to hyperkalemia (high serum potassium levels). Normal serum potassium levels of 3.5 to 5 mEq/L are maintained until the 24-hour urine output falls below 500 mL. High potassium levels then develop quickly, reaching 7 to 8 mEq/L or greater. Severe ECG changes result from this elevation, and fatal dysrhythmias can occur. Other factors contribute to high potassium levels in CKD, including the ingestion of potassium in drugs, failure to restrict dietary potassium, tissue breakdown, blood transfusions, and bleeding or hemorrhage. (See Chapter 13 for discussion of potassium problems.)
Acid-base balance is affected by CKD. In the early stages, blood pH changes little because the remaining healthy nephrons increase their rate of acid excretion. As more nephrons are lost, acid excretion is reduced and metabolic acidosis results (see Chapter 14).
Health Promotion and Maintenance
Calcium and phosphorus balance is disrupted by CKD. A complex, balanced normal relationship exists between calcium and phosphate and is influenced by vitamin D (see Chapter 13). The kidney produces calcitriol, the active form of vitamin D, which then enhances intestinal absorption of calcium.
In CKD, phosphate retention and a deficiency of active vitamin D disrupt the calcium and phosphate balance. Normally, excessive dietary phosphate is excreted by the kidneys in the urine. Parathyroid hormone (PTH) controls the amount of phosphate in the blood by causing tubular excretion of phosphate when there is an excess. An early effect of CKD is reduced phosphate excretion (Fig. 71-2). As plasma phosphate levels increase (hyperphosphatemia), calcium levels decrease (hypocalcemia). Chronic low blood calcium levels stimulate the parathyroid glands to release more PTH. Under the influence of additional PTH, calcium is released from storage areas in bones (bone resorption), which results in bone density loss. The extra calcium from the bone is needed to balance the excess plasma phosphate level. The problem of low blood calcium levels is made worse with severe CKD because kidney cell damage also reduces production of active vitamin D. Thus less calcium is absorbed through the intestinal tract in the absence of sufficient vitamin D.
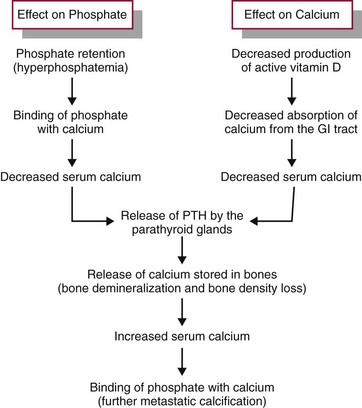
FIG. 71-2 The effects of kidney dysfunction on phosphate and calcium balance. PTH, Parathyroid hormone.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree