Brief Overview of Respiratory Physiology
The Role of Surfactant
Pulmonary surfactant is a mixture of phospholipids produced by type 2 alveolar pneumocytes from around week 36 of gestational age. The primary function of surfactant is to lower surface tension in the alveolar air–liquid interface which helps to stabilise the expanded alveolus at the end of inspiration, prevent collapse at the end of expiration and increase compliance. There is a fluid film which coats the inner wall of the alveolus containing water molecules. Water molecules have a weak mutual physical attraction and the presence of surfactant reduces this physical attraction, preventing the walls of the alveoli collapsing in towards each other. In turn, it reduces the pressure required for subsequent re-inflation of the alveoli. Surfactant also contributes to the innate defence system and has been shown to possess anti-inflammatory properties.
Gas Exchange
All organ systems rely to a greater or lesser degree on the delivery of oxygen to maintain normal cellular metabolic function. The primary function of the respiratory system is to move O2 from the air to the blood and CO2 from the blood to the air.
This process of gas exchange involves three stages:
- Pulmonary ventilation – the movement of air between the atmosphere and the lungs which is dependent on the existence of a pressure gradient (Boyle’s law) and lung compliance.
- External respiration – the exchange of O2 and CO2 between the alveoli and the blood in the pulmonary capillaries and the conversion of deoxygenated blood to oxygenated blood. The exchange of gases occurs through diffusion and Fick’s law applies here. Several anatomical features assist with this process, including:
- the total thickness of the alveolar–capillary membrane, which is only 0.5 micrometres;
- multiple capillaries lying over each alveolus allow 100 ml of blood to participate in gas exchange at any one time;
- the structure of the pulmonary capillaries is designed to give maximum exposure to facilitate gas exchange.
- the total thickness of the alveolar–capillary membrane, which is only 0.5 micrometres;
- Internal respiration – oxygenated blood (transported by the circulatory system) leaves the lungs and is delivered to the tissue cells. The exchange of O2 and CO2 occurs again at this point through diffusion and the presence of a concentration gradient. At rest only 25%% of available O2 is extracted by the cells to meet metabolic demand.
Gas Transport
Partial Pressures of Gases
The total pressure of a gas is the sum of all the partial pressures of the gases within the mixture. Normal atmospheric air consists of two main gases – oxygen (O2) and nitrogen (N2) – with the remaining very small percentage being made up from carbon dioxide (CO2) along with argon and helium, and water. When considering respiratory physiology it is the two main gases that are significant. Normal atmospheric pressure is 760 mmHg (101 kPa). Of this, nitrogen provides the greatest quantity (78%), oxygen 21%, while carbon dioxide accounts for a mere 0.03%. Therefore, the partial pressures of the gases in dry air at sea level are:
- PN2 = 592.8 mmHg
- PO2 = 159.6 mmHg
- PCO2 = 0.2 mmHg
- P other = 7.4 mmHg
Almost all (≈ 98.5%) of the oxygen transported in systemic arterial blood is chemically bound to haemoglobin found in red blood cells with the remaining 1.5% being dissolved in plasma. The normal haemoglobin molecule is composed of four polypeptide chains: two alpha (α) globin chains and two beta (β) globin chains. Attached to each of the polypeptide chains is an iron-containing molecule called haem, therefore each haemoglobin molecule has four binding sites for oxygen to be transported around the body.
Because so much of the body’s oxygen is transported in this manner there are a number of factors which influence the ease with which it either binds to or dissociates from haemoglobin.
Partial Pressure of Oxygen
The most important factor influencing how much oxygen binds to haemoglobin is the partial pressure of oxygen (PO2). When the PO2 is high, haemoglobin binds with large amounts of oxygen and is almost 100% saturated. Therefore, in the pulmonary capillaries where the PO2 is high (because the PO2 in atmospheric air is higher) a lot of oxygen binds with haemoglobin. In the tissue capillaries where the PO2 is lower, haemoglobin does not hold as much oxygen; therefore oxygen is released for use by the tissues through diffusion. The relationship between the PO2 and haemoglobin binding is demonstrated by the oxyhaemoglobin dissociation curve (OHDC). This curve describes the relationship between the percentage saturation of O2 (shown on the y axis) to partial pressure of oxygen (shown on the x axis) (Figure 4.2). The curve can move to the left or the right depending on the environment and the influence of the factors noted in Table 4.1. At any given PO2 when the curve shifts to the right haemoglobin will be less well saturated with O2 (the Bohr effect), while if the curve shifts to the left haemoglobin will be more saturated with O2.
Figure 4.2 The oxyhaemoglobin dissociation curve.
From Dixon et al. (2009) Nursing the Highly Dependent Child or Infant: A Manual of Care. Reproduced with permission from John Wiley and Sons, Ltd.
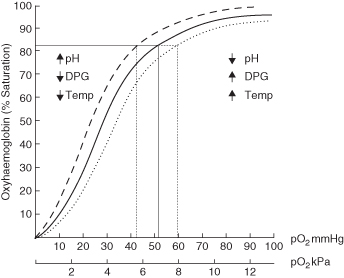
Table 4.1 Factors affecting haemoglobin and oxygen binding
| Factors | Effects |
| Acidity (blood pH) | Haemoglobin can act as a buffer for H+ ions in the blood to maintain blood pH within normal limits. In an acidosis, H+ can bind to the amino acids in haemoglobin causing a slight change in the structure of haemoglobin, decreasing its O2 carrying capacity. This causes a right shift on the curve and will facilitate increased oxygen release to the tissues in times of greater demand. |
| Carbon dioxide | This can affect the curve in two ways: 1. Most (80–90%) of the CO2 is transported as bicarbonate ions. The formation of a bicarbonate ion will release a proton into the plasma. Elevated CO2 levels create a respiratory acidosis and shift the oxygen–haemoglobin dissociation curve to the right as the CO2 accumulates. 2. Carbamino compounds are generated through chemical interactions, resulting in carbaminohaemoglobin. Low levels of carbamino compounds have the effect of shifting the curve to the right, while high levels cause a shift to the left. |
| Temperature | As body temperature increases the OHDC moves to the right and more oxygen is released to the tissues. This occurs because heat is a by-product of the metabolic activity in the cells and metabolically active cells require more O2 to maintain aerobic metabolism. In addition, a by-product of increased metabolism is the production of acids which in turn will decrease the pH. |
| BPG (2,3 bisphosphoglycerate) | Decreases the affinity of haemoglobin for O2 making it more available to the tissues. BPG is produced by the red cells when they metabolise glucose to produce energy in the form of ATP. Increased energy demands from cells cause an increase in BPG levels and so an increase in oxygen available for the tissues as the OHDC shifts to the right. (This is also referred to as 2,3 diphosphoglycerate or 2,3 DPG) |
Control of Respiration
Breathing is essentially an involuntary process which is controlled by the medulla and pons of the brain stem. The frequency of normal, involuntary breathing is controlled by three groups of neurons or brain stem centres: the medullary respiratory centre, the apneustic centre and the pneumotaxic centre. The cerebral cortex provides voluntary control of respiration, although this can be overridden by the responses generated by the chemoreceptors. An individual’s emotional state can effect changes in respiratory rate via the limbic system.
Central Chemoreceptors
These are located in the brainstem and are the most important for the minute-by-minute control of respiration. These chemoreceptors are located on the ventral surface of the medulla near the point of exit for the glossopharyngeal and vagus nerves and are only a short distance from the medullary inspiratory centre. Central chemoreceptors communicate directly with the inspiratory centre. The brain stem chemoreceptors are exquisitely sensitive to changes in the pH of cerebrospinal fluid (CSF). Decreases in the pH of CSF produce an increase in the respiratory rate, while an increase in the pH of CSF produce a decrease in the respiratory rate.
Peripheral Chemoreceptors
These are peripheral chemoreceptors for O2, CO2 and H+ in the carotid bodies, located at the bifurcation of the common carotid arteries and in the aortic bodies above and below the aortic arch. Information about PaO2/PaCO2 and pH is relayed to the medullary inspiratory centre, which orchestrates an appropriate change in respiratory rate. Decreases in PaO2 are the most important responsibility of the peripheral chemoreceptors but they are relatively insensitive to changes until PaO2 reaches 60 mmHg (8 kPa) or less. Once in this range the chemoreceptors are exceptionally sensitive to further changes. Decreases in arterial pH cause an increase in respiration mediated by the peripheral chemoreceptors based on their sensitivity to H+. This effect is independent of changes in the PaCO2 and is mediated only by the chemoreceptors in the carotid bodies, not by those in the aortic bodies. In a metabolic acidosis, therefore, where there is a decreased arterial pH but not an elevated PaCO2 level, the peripheral chemoreceptors are stimulated directly to increase the respiratory rate. The peripheral chemoreceptors also detect increases in PaCO2 but the effect is less important than their response to decreases in PaO2. Detection of changes in PaCO2 by the peripheral chemoreceptors is also less important than the detection of changes in PaCO2 by the central chemoreceptors.
Lung Stretch Receptors
Mechanoreceptors are present in the smooth muscle of the airways. When stimulated by distension of the lungs and airways, mechanoreceptors initiate a reflex decrease in the respiratory rate: this is called the Hering–Breuer reflex. The reflex decreases the respiratory rate by prolonging the expiratory time.
Joint and Muscle Receptors
Mechanoreceptors located in the joints and muscles detect the movement of limbs and instruct the inspiratory centre to increase the respiratory rate. Information from the joints and muscles is important in the early (anticipatory) ventilatory response to exercise.
Irritant Receptors
Receptors for noxious chemicals and particles are located between the epithelial cells lining the airways. Information from these receptors is relayed to the medulla and causes a reflex constriction of the bronchial smooth muscles in response with an associated increase in the respiratory rate.
J Receptors (Juxtacapillary Receptors)
These are found in the walls of each of the alveoli and, as the name suggests, are near the pulmonary capillaries. Increases in the presence of interstitial fluid volume may activate these receptors as well as increased pulmonary capillary blood flow. The response produced is an increase in respiratory rate.
Ventilation Perfusion Ratio and Mismatch
Gas exchange becomes optimal when both ventilation and pulmonary blood flow are equal. Even under normal conditions in fit healthy individuals, the ventilation/perfusion ratio (V/Q) is < 1.0. Gravitational forces create regional differences in intra-pleural and pulmonary pressures, which results in a mismatch between areas of the lung being ventilated and pulmonary blood flow. Conditions that affect either the ventilation component or the perfusion component will significantly increase the mismatch.
Intra-pulmonary shunting is a major cause of hypoxaemia. A shunt here refers to venous blood that travels from the right to left side of the circulation without coming into contact with ventilated lung. Shunts can be identified as anatomic or physiologic (capillary).
An anatomic shunt occurs when blood bypasses the lungs through an anatomic channel, such as from the right to the left ventricle through a ventricular septal defect or from a branch of the pulmonary artery directly to a pulmonary vein.
A physiologic (capillary) shunt occurs when a portion of the cardiac output goes through the regular pulmonary vasculature without coming into any contact with alveolar air for gas exchange. There is no abnormal connection between the blood vessels; rather, there is a redistribution of pulmonary blood flow. Physiologic shunting is often seen in pulmonary oedema, pneumonia and lobar atelectasis.
Venous blood passing non-functioning alveoli creates an admixture of venous and arterial blood which decreases the PaO2 and therefore increases the degree of hypoxaemia. This venous admixture can be described as the ratio of shunted blood (Qs) to total pulmonary blood flow (Qt). The normal Qs/Qt is 3–7% and changes of more than 5% are considered significant in terms of respiratory performance. From a clinical perspective, the work of breathing is markedly increased when the Qs/Qt > 15%, however measuring this in the clinical setting is extremely difficult.
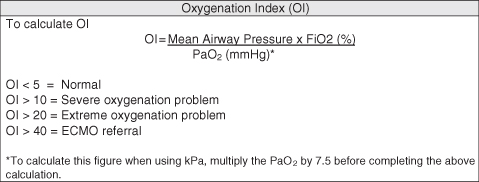
Calculating the Oxygenation Index (OI) (Figure 4.3) provides readily available information about the ability of the lungs to diffuse oxygen across the alveolar capillary membrane. It represents the ratio between the level of oxygen being delivered to the lungs and the amount diffusing into the blood and can be used as an assessment tool for the efficacy of interventions, such as the introduction of high frequency oscillation ventilation (HFOV) and/or nitric oxide therapy. It is also used as an index of the severity of underlying lung damage and as one of the referral criteria for the provision of extracorporeal membrane oxygenation (ECMO).
Pulmonary Volumes and Capacities
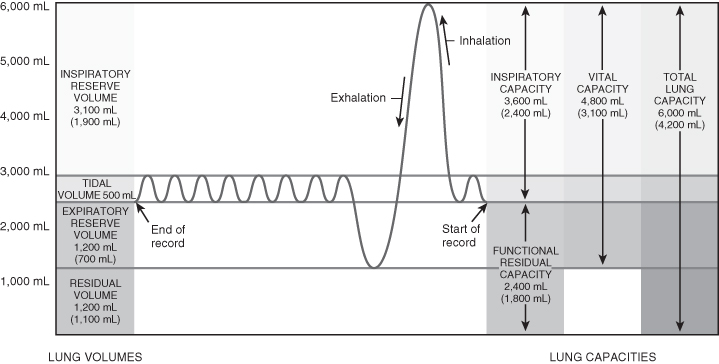
There are a number of important volumes or capacities which influence normal respiratory function. These are detailed in Table 4.2 and Figure 4.4.
Table 4.2 Summary of pulmonary volumes and capacities
| Definition and notes | |
| Tidal volume (TV or Vt) | The volume of air entering and leaving the lungs in a single breath in the resting state. Tidal volume is constant between 6 and 8 ml/kg throughout life. Infant of 3 kg: TV = 18–24 ml (6/8 × 3) Adult of 70 kg: TV = 420–560 ml (6/8 × 70) |
| Inspiratory reserve volume (IRV) | The amount of air that can be inspired over and above the resting tidal volume. |
| Expiratory reserve volume (ERV) | The volume of air remaining in the lungs at the end of normal expiration, which can be exhaled by active contraction of the expiratory muscles. |
| Residual volume (RV) | The amount of air remaining in the lungs after maximal expiration; the presence of this prevents the lungs from emptying completely. |
| Vital capacity (VC) | The sum of normal tidal volume, inspiratory reserve volume and expiratory reserve volume. Infants: 33–40 ml/kg Adults: 52 ml/kg |
| Functional residual capacity (FRC) | The amount of air remaining in the lungs at the end of normal expiration. In the presence of atelectasis FRC falls as the number of alveoli participating in gas exchange decreases. Any pulmonary disease which affects the relationship between tidal volume, FRC and closing capacity will contribute significantly to ventilation – perfusion mismatching and hypoxia such as chronic lung disease (infants), cystic fibrosis, asthma, bronchiolitis and pneumonia. |
| Closing volume/capacity | Airway closure (complete collapse) occurs in areas of the lungs that have low volumes – this is known as the closing volume or capacity:
|
| Dead space ventilation | Anatomic dead space – the volume of conducting air that fills the nose, mouth, pharynx, larynx, trachea, bronchi and distal bronchial branches which does not participate in gas exchange. Normal anatomic dead space is 2 ml/kg. Alveolar dead space – the volume of gas which fills alveoli, the perfusion of which is either reduced or absent. Contributing factors include hypotension, compression of the alveolar capillary bed and, rarely in children, pulmonary embolism. Physiologic dead space – the sum of both anatomic and alveolar dead space. Dead space ventilation – the amount of gas ventilating physiologic dead space per minute. It is expressed as a fraction of TV and the normal ratio is 0.3 (30%), which means that 30% of the volume of each breath does not participate in gas exchange. |
Figure 4.4 Lung volumes and capacities.
From Tortora, G.J. and Derrickson, B.H. (2009) Principles of Anatomy and Physiology, 12th edn. Reproduced with permission from John Wiley and Sons, Inc.
Respiratory Assessment
Within the critical care setting, respiratory assessment utilises a number of approaches to create a complete picture. Sound knowledge of anatomy and physiology, including the ability to identify thoracic landmarks (Figure 4.5 and Table 4.3), is the main underpinning requirement to an effective assessment. The key components of assessment include:
- General physical inspection, including palpation and percussion and assessment of respiratory pattern and rate.
- Auscultation.
- Chest X-ray (CXR) review.
- Blood gas analysis.
Figure 4.5 Anatomy of the respiratory system. (a) Anterior view showing the organs of respiration. (b) Branching of airways from the trachea.
From Tortora, G.J. and Derrickson, B.H. (2009) Principles of Anatomy and Physiology, 12th edn. Reproduced with permission from John Wiley and Sons, Inc.
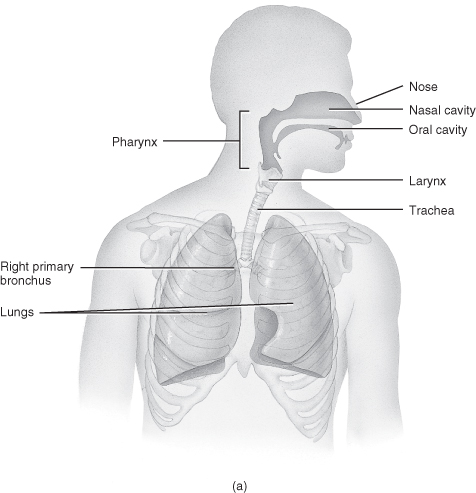
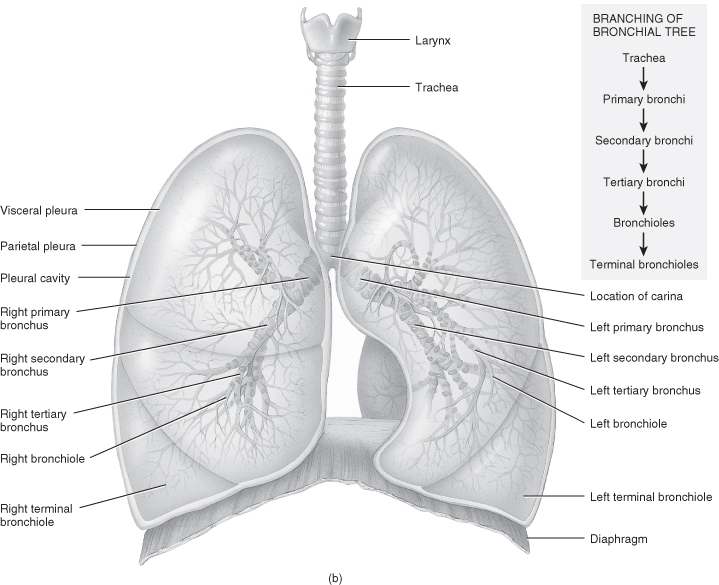
Table 4.3 Thoracic landmarks and their anatomical position for documentation of clinical assessment findings
| Landmark | Position |
| Midsternal (MS) line | Vertical down the midline of the sternum. |
| R/L midclavicular (MC) lines | Parallel to the MS line – begins at mid-clavicle. The inferior borders of lungs cross the 6th rib at the MC line. |
| R/L anterior axillary lines | Parallel to the MS line – begins at anterior axillary folds. |
| R/L mid-axillary (MA) lines | Parallel to the MS line – begins at mid-axilla. |
| R/L posterior axillary lines | Parallel to the MS line – begins at posterior axillary folds. |
| Vertebral line | Vertical down the spinal processes. |
| R/L scapular lines | Parallel to the vertebral line – through the inferior angle of the scapula if patient is sitting up. |
Physical Inspection
Good visual inspection before disturbing the child can provide the practitioner with a wealth of information about the child’s general wellbeing as well as previous and current medical history, which may or may not be relevant to the current illness episode. Areas to consider include:
- General nutritional status of the child – may be demonstrated by rib prominence or the presence of large amounts of fatty tissue.
- General colour of the child – presence of peripheral or central cyanosis. Look for signs of finger clubbing (indicative of chronic hypoxaemia) or prominent superficial venous patterns across the chest and abdominal walls, which can be a sign of increased right-sided heart pressure/vascular obstruction.
- The size and shape of the child’s chest and the presence of any skeletal abnormalities such as scoliosis, which may affect the child’s respiratory performance.
- The presence of scars from previous surgical interventions (e.g. sternotomy, thoracotomy or chest drain sites).
- The presence of new surgical incisions (front and back), chest drains and/or pacing wires in the postoperative cardiac patient.
Considerations for Practice – Newborns
Cyanosis of hands and feet (acrocyanosis) is common in the newborn and may persist for several days in a cool environment. Infants are obligate nose breathers (up to 6 months of age) and nasal flaring is a common finding without clinical significance in the absence of any other signs of respiratory distress. Infants born prematurely have a greater incidence of irregularity of respiratory pattern which is not clinically significant unless the infant is compromised by periods of apnoea. Coughing in newborns is rare and is normally pathological in origin, whereas sneezing is frequent and to be expected without underlying pathology. Hiccups in newborns are common, usually silent and associated with feeds. Frequent non-feed-associated hiccups maybe suggestive of seizures, drug withdrawal or encephalopathy in the newborn.
Cyanosis
Central cyanosis may be evident in a child with hypoxia, but may not be apparent in an anaemic child, even in the presence of profound hypoxia. The presence of cyanosis is therefore not a reliable or early indicator of hypoxia. If present, it should be considered a late and pre-terminal sign, unless the child has known underlying cyanotic heart disease. However, even in this scenario further investigation is necessary to rule out deterioration in the child’s condition from baseline.
Palpation
Palpation is useful to assess for:
- Pulsations.
- Areas of tenderness.
- Bulges.
- Depressions.
- Unusual movement of the chest wall during inspiration and/or expiration.
Normal palpation should reveal bilateral symmetry of movement of the chest wall, with a degree of elasticity of the rib cage. The sternum should be relatively inflexible, however consideration should be given to the child post-sternotomy and particular care must be taken in the child whose chest remains open post-cardiac surgery. The trachea should be positioned in the midline directly above the suprasternal notch (a very slight deviation to the right is a relatively common, non-symptomatic finding). Abnormal findings from palpation usually require further investigation and intervention.
Crepitus
This is a crackly/crinkly sensation which can be both palpated and heard, and is indicative of air in the subcutaneous tissues, either from an air leak or, much more rarely, from the presence of a gas-producing organism (Clostridium welchii). The cause will determine whether further intervention is necessary.
Pleural Friction Rub
This is a palpable, coarse, grating vibration felt on both inspiration and expiration, which may be compared to the feel of leather rubbing on leather. It may be indicative of a pleural friction rub caused by inflammation of the pleural surfaces. It is a rare finding in the under-5 age group.
Percussion
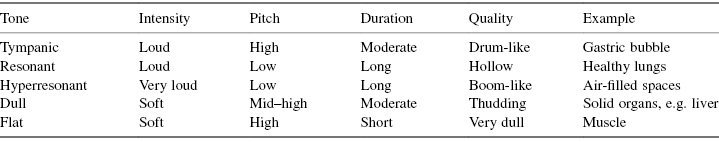
This is a valuable skill which enables the practitioner to assess whether underlying tissue is air-filled, fluid-filled or solid. Different percussive notes are gained accordingly and a description of the differences is detailed in Table 4.4. Percussion is underutilised in nursing assessment but the skill should be developed under the supervision of a member of the medical staff or a physiotherapist until practitioners feel confident in its use.
Table 4.4 Classification of percussive notes
Source: adapted from Mosby’s Guide to Clinical Examination Skills, 5th edition, 2003.
Considerations for Practice
The round shape of the infant’s chest gives rise to a hyperresonant percussive note, which is considered an abnormal finding in the older child. By the age of 6 years normal percussive notes should have a resonant pitch, therefore hyperresonance in this age group is indicative of underlying pathology such as pneumothorax.
The Respiratory Rate and Rhythm
The respiratory rate (Table 4.5) and pattern (Table 4.6) are important indicators of underlying respiratory pathology and also considerations for instituting or increasing respiratory support. The first step is to determine the rate and pattern appropriate for the age of the child, taking into consideration any pre-existing clinical conditions which may affect the child’s normal rate and pattern.
Table 4.5 Normal respiratory rates by age
| Age of child | Respiratory rate (range) |
| Newborn | <60 breaths/min |
| <1 year | 30–40 breaths/min |
| 1–2 years | 25–35 breaths/min |
| 2–5 years | 25–30 breaths/min |
| 5–12 years | 20–25 breaths/min |
| >12 years | 15–20 breaths/min |
Table 4.6 A summary of terminology and descriptors of abnormal respiratory rates and/or patterns
| Term used | Description |
| Tachypnoea | Rate is faster than the highest anticipated rate for age. |
| Bradypnoea | Rate is less than the lowest anticipated rate for age. |
| Apnoea | Absence of respirations. |
| Hyperpnoea | Rate is usually faster than anticipated for age and the breath taken is deeper. |
| Dyspnoea | Laboured or difficult respiration. |
| Orthopnoea | Dyspnoea at rest; difficulty in lying supine due to respiratory rate. |
| Hypoventilation | Slow, shallow breaths generating very small tidal volumes. |
| Sighing breaths | Frequently interspersed deeper breath. |
| Air trapping | Increasing difficulty in getting air out during expiration, usually associated with the presence of a wheeze. |
| Kussmaul respirations (also known as acidotic breathing) | Rapid, deep breaths with an additional effort at the end of expiration; this pattern is associated with the presence of a metabolic acidosis. |
| Biot respirations | Irregular, interspersed periods of apnoea in a disorganised sequence of breaths; usually indicative of pontine damage. |
| Ataxic respirations | Significant disorganisation with irregular and varying depth of respiration – an extreme form of biot respirations. |
| Cheyne–Stokes ‘periodic breathing’ | Varying periods of increasing depth of respiration interspersed with apnoea; this is often, although not always, a terminal respiratory pattern. |
Auscultation
This should be a symmetrical assessment allowing for side-by-side comparison, moving from top to bottom, anteriorly and posteriorly, remembering to auscultate into the axillary spaces on each side. The purpose of auscultation is to assess for:
- The presence and location of normal breath sounds.
- The presence of normal breath sounds in abnormal locations.
- The presence of adventitious breath sounds.
There are three types of normal breath sounds heard in the healthy infant and child:
- Bronchial – heard over the trachea, loud and high-pitched (large airways).
- Bronchovesicular – heard over the main bronchi, moderate in pitch and intensity.
- Vesicular – heard over smaller bronchi, bronchioles and healthy lung tissue, low in pitch and intensity.
Abnormal or adventitious breath sounds have a number of different descriptors in the literature and the terms used have changed over the years. Currently, the terms widely recognised are crackles, wheezes and stridors (Table 4.7).
Table 4.7 Descriptors of adventitious breath sounds
| Definition and notes | |
| Crackles | Discrete, non-continuous sounds, which can be divided into three types:
|
| Wheezes | ‘Musical’ sounds produced by the rapid or forced movement of air through narrowed airways. These sounds are common in the infant due to their narrower airways and are typically heard in expiration, although may also be present during inspiration. In normal, spontaneous ventilation, the intra-thoracic airways widen during inspiration and narrow on exhalation, while the opposite is true of the extra-thoracic airways. Maximum resistance to airflow occurs in expiration in the intra-thoracic airways and during inspiration in the extra-thoracic airways. Expiratory wheezes are indicative of a lower airway problem, while inspiratory wheezes indicate upper airway problems. The sudden disappearance of a wheeze in a child with asthma is usually indicative of impending respiratory failure. |
| Stridor | A high-pitched, piercing sound which is heard most often on inspiration. It is indicative of an obstruction in the upper respiratory tract and may be mechanical (e.g. due to tracheomalacia), due to infective processes (e.g. croup) or as a consequence of foreign body inhalation. |
Less common abnormal sounds found on auscultation are pleural rubs and mediastinal crunch (Hamman’s sign). As noted previously, a pleural rub is indicative of inflammation of the pleural membranes which may be heard in both inspiration and expiration. It is usually best heard over the lower lateral anterior surface of the chest wall. A mediastinal crunch is usually heard in the presence of mediastinal emphysema and is best described as a loud, clicking sound which is synchronous with the heart beat rather than with respiration, although it is sometimes louder towards the end of expiration.
Considerations for Practice
- Breath sounds from right middle and left lingular lobe are best heard in respective axillae, hence the need to auscultate into the axillary spaces.
- Breath sounds are usually louder in infants and young children due to their thinner chest wall.
- Bronchovesicular sounds may be heard right to lung edges.
- Referred breath sounds are common in infants and young children due to their small thoracic cavity. Therefore, even in the presence of significant pneumothorax breath sounds may be heard over collapsed areas.
The Chest X-ray
The chest X-ray remains a useful tool in assessing pulmonary health and function. A chest X-ray review should be approached in a logical, step-by-step manner to ensure that all areas are reviewed and small, sometime subtle changes are detected in a timely manner. A systematic approach is suggested in Table 4.8, however most practitioners will develop their own system.
Table 4.8 Systematic approach to CXR interpretation
| Review process | Comments |
| Name, date, time | Ensure the most recent film is being reviewed, plus previous film for comparison. |
| Film view | Check whether film is AP or PA. Consider changes in heart size accordingly. |
| Patient position | Check whether film is erect or supine view. |
| Exposure, penetration | Identify whether film is too light or too dark – good exposure and presentation will allow visualisation of the spinal processes and the disc spaces below the diaphragm. |
| Inspiratory/expiratory film | Inspiratory film – use anterior ribs and count down. A good film will have the 7th rib transecting the right hemi-diaphragm. Always use the right and not the left side (the right diaphragm sits higher than the left due to position of liver). Hyperinflation – expansion down to 9th anterior rib. |
| Rotation | The clavicle ends should be equidistant from the middle of the vertebral column. |
| Soft tissues and bones | Presence of chest wall oedema or fat. Signs of previous fractures (calcifications). Surgical emphysema – air striations in muscle fibres. |
| Additions | Endotracheal tube – check chin position halfway between clavicle and carina (remember – nose up tube up/nose down tube down).
|
| Borders – heart and diaphragm |
|
| Lung fields | Tracheal position – midline or deviated. Consider: Fluid – mediastinum moves to opposite side Collapse – loss of volume with mediastinal shift towards area. Consolidation – no loss of volume, no real mediastinal movement, presence of air bronchograms, usually non- segmental. R/LLL: loss of diaphragm. LUL: shadowing only (veil sign). RML: loss of heart border. RUL: movement of horizontal fissure upwards. L lingular: loss of heart border. Pneumo-pericardium – air right around and under heart. Pneumo-mediastinum – air pockets but not under heart. Pneumothorax – absence of visible lungs markings. Increased vascular markings – increased/excessive pulmonary blood flow. |
Blood Gas Analysis
Blood gas analysis is undertaken for two reasons: to monitor the child’s respiratory performance in terms of effective oxygenation and carbon dioxide removal; and, to provide information about the child’s blood acid base environment related to primary non-respiratory causes of imbalance (e.g. metabolic acidosis).
Blood gases may be taken from venous stabs/lines, arterial stabs/lines or from a capillary stab, however the choice of sample for assessment of respiratory performance is an arterial sample through established arterial access. The use of single arterial stabs is not common practice in paediatric intensive care.
Considerations for Practice
Arterial blood gases provide the most information from the measured parameters when assessing respiratory or metabolic function, while venous blood gases are more useful in providing an indication of metabolic function such as cardiac output and tissue perfusion rather than respiratory function per se. Capillary blood gases are useful for monitoring pH and PCO2 but are not particularly helpful for oxygenation levels. Additionally, a capillary sample needs to be obtained without applying too much squeeze to the area being sampled (if it is, the results will be inaccurate). Therefore, assessment of the child’s peripheral perfusion is essential before undertaking the procedure. The age of the child must also be considered when selecting the site to be used for sampling. As with any monitoring intervention, results should not be used in isolation; the goal is always to treat the child not the numbers.
Monitoring the acid base balance (an acid is a potential hydrogen (H+) ion donor while a base is a potential hydrogen ion acceptor) is important because the body systems will only operate effectively if the internal environment is conducive, which depends on an arterial pH of 7.35–7.45. Normal aerobic metabolism results in the production of acids mainly in the form of CO2, while anaerobic metabolism produces not only CO2 but other metabolic acids, such as lactic acid. The majority of acids produced are buffered and therefore not in free form. The measurement of those in free form (and therefore potentially immediately harmful) found in the extracellular fluid is quantified by the use of the term pH. The normal concentration of free H+ is very small, approximately 40 nanomilliequivalents (nmeq) per litre. The term pH is an expression of the negative logarithm of free H+ concentration and the relationship is inversely proportional. Therefore, as the free H+ concentration increases so the pH decreases, and vice versa.
The systemic effects of acid base imbalance are detailed in Table 4.9.
Table 4.9 The systemic effects of acid base imbalance
| Arterial pH < 7.35 (acidosis) | Arterial pH > 7.45 (alkalosis) |
| Increased pulmonary vascular resistance (PVR) Right shift of the oxyhaemoglobin dissociation curve Decreased insulin secretion/binding to receptors Decreased pulmonary macrophage function Lower threshold for ventricular fibrillation Decreased response to catecholamines Decreased immune responses Decreased mesenteric blood flow | Decreased vascular resistance and tone Left shift of the oxyhaemoglobin dissociation curve Increased response to catecholamines Decreased Krebs cycle oxidations in muscles and renal cortex Increased insulin glycolysis |
If pH is to be maintained within the normal range and prevent potentially harmful effects occurring, the level of these acids needs to be regulated. There are three discrete but interrelated pathways that work to maintain the acid base balance.
Buffering System
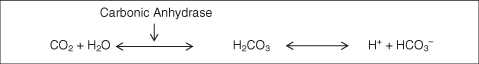
The buffering response is activated in seconds and is considered the first line of defence against changes in pH. Buffers in the blood include bicarbonate, proteins such as haemoglobin and other substances such as phosphate. The most important pair in this system is the bicarbonate–carbonic acid pairing, which is responsible for buffering the extracellular fluid (ECF). If the pH of the ECF is threatened by the presence of a strong acid, then the weak HC03− base becomes active, whereas if the ECF is threatened by the presence of a strong base, the weak H2CO3 acid becomes active (Figure 4.6).
As CO2 is formed and diffuses into the capillary blood it enters the erythrocytes and reacts with water to form carbonic acid (H2CO3). Carbonic acid dissociates to form H+ and H2O (this occurs rapidly in the presence of carbonic anhydrase, but less so in its absence). The H+ ions bind to reduced haemoglobin to form HHb. Bicarbonate ions (HCO3−) generated by this process pass back into the plasma in exchange for chloride ions (Cl−), a process known as chloride shift, which ensures there is no net loss or gain of negative ions by the red cells. In the lungs, this process is reversed – the H+ ions bound to haemoglobin in the form of HHb recombine with HCO3− to form carbonic acid, which in turn dissociates into CO2 and H2O. The CO2 diffuses into the alveoli to be excreted through ventilation. In addition, reduced HHb re-forms to return to the tissues carrying oxygen once more. This system does not work in isolation and the respiratory system plays a major role in maintaining pH balance.
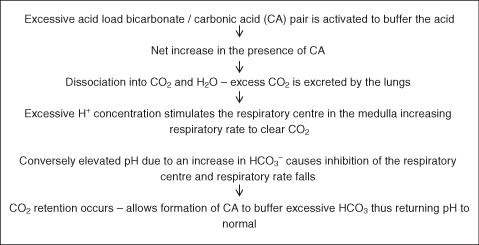
While the buffering system works within seconds, the respiratory system activates changes in pH within minutes. The balance is achieved through the conservation or elimination of CO2 as the respiratory system cannot achieve any loss or gain of hydrogen ions (Figure 4.7).
The respiratory system is able to compensate for changes in pH relating to metabolic disorders where there is a build-up of fixed or non-titratable acids (e.g. ketoacids in diabetic ketoacidosis or lactic acid in sepsis).
The final pathway in the regulation of the acid base environment is the renal system which is discussed in Chapter 6.
Compensatory Mechanisms
The body will seek ways in which it can compensate for acid base imbalances and for every acidosis or alkalosis will create the opposing acid base environment to maintain equilibrium.
| Primary disorder | Compensation |
| Respiratory acidosis | Metabolic alkalosis |
| Respiratory alkalosis | Metabolic acidosis |
| Metabolic acidosis | Respiratory alkalosis |
| Metabolic alkalosis | Respiratory acidosis |
Electrolytes and Acid Base Balance
Potassium interacts with hydrogen. When the H+ concentration is elevated in the ECF (e.g. metabolic acidosis), hydrogen moves into the cell and potassium moves out. This allows the H+ access to the intracellular protein buffers, which can minimise changes in pH. However, the shift in potassium from the intracellular to extracellular fluids can result in relative hyperkalaemia. When H+ concentration is reduced (e.g. metabolic alkalosis), H+ moves out of the cell and potassium moves in, which can result in relative hypokalaemia.
Calcium is vital for neuromuscular and cardiac function. When pH is normal, 45% of calcium is bound to protein (mostly albumin) and is biologically inert. Fifty per cent is free or ionised calcium and is metabolically active, while 5% is bound to other organic anions such as citrate. Changes in pH alter the split and an acidosis will produce a reduction in the free (ionised) calcium available to the body. The presence of NaHCO3− restores or maintains normal calcium reabsorption.
Normal Blood Gas Values
Some units use hydrogen ion values to determine acidity when reviewing blood gases, therefore it is essential that practitioners are familiar with their institution’s preferred method. Table 4.10 shows the blood gas values associated with standard pH measurements and a guide to hydrogen ions concentration matched to pH.
Table 4.10 Standard blood gas values
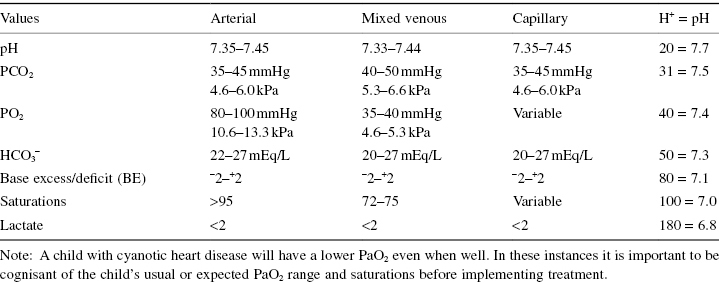
Blood Gas Interpretation
Developing a systematic approach to blood gas interpretation is essential to ensure that nothing is overlooked. There are different ways in which this can be done, which will depend on the individual practitioner. A commonly used approach using five main questions is outlined in Table 4.11.
Table 4.11 Systematic approach to blood gas interpretation
| Main question | Further questions |
| 1. What is the pH? | Is the primary disorder an acidosis? pH < 7.35 Is the primary disorder an alkalosis? pH > 7.45 |
| 2. What is PaCO2? | Is there agreement with the pH? Respiratory acidosis (PaCO2 > 45 mmHg/6.0 kPa) Respiratory alkalosis (PaCO2 < 35 mmHg/4/6 kPa) If there is no agreement, then the primary disorder is not respiratory in origin |
| 3. What is PaO2? | Is patient hypoxic? (PaO2 < 80 mmHg/10.6 kPa) |
| 4. What is HCO3−? | Is level raised? (HCO3− > 27 mEq/l) Is level low? (HCO3− < 22 mEq/l) Does this agree with pH? |
| 5. What is the base excess/deficit? | Is level raised/high? (BE > +2, e.g. +8 or a base excess) Is level low? BE < −2, e.g. −12 or a base deficit) Does this agree with HCO3− and pH? |
Finally, put all the answers together and it should be possible to describe the patient’s clinical status in terms of acid base balance and whether the result indicates a primary respiratory or metabolic acidosis/alkalosis or a mixed picture (i.e. the imbalance has both a respiratory and a metabolic component).
Lactate is an important indicator of tissue perfusion – high lactates are indicative of abnormal tissue metabolism secondary to inadequate delivery of the essential nutrients and oxygen required for normal metabolic processes. Rising lactate levels should always be investigated further.
There are two primary imbalances of the acid base environment which can be attributed to the respiratory system (Table 4.12). Primary metabolic imbalances are addressed in Chapter 5, although children may also present with a mixed imbalance, for example, in sepsis, children can often present with a primary respiratory and a primary metabolic acidosis from inadequate respiratory performance and inadequate tissue perfusion. In this instance the practitioner must address both problems as a matter of urgency.
Table 4.12 Primary respiratory acid base imbalances
| Respiratory acidosis | Respiratory alkalosis | |
| Definition | pH < 7.35 PCO2 > 45 mmHg/6.1 kPa HCO3− – normal or elevated | pH > 7.45 PCO2 < 35 mmHg/4.5 kPa |
| Causes | Results from an accumulation of CO2 due to inadequate respiration/ventilation
| Results from an excessive loss of CO2
|
| Management | 1. Respiratory support – invasive or non-invasive based on assessment of need 2. Adjustment to mechanical ventilation 3. Treat underlying cause | 1. Reduce ventilatory support by reducing rate/pressures 2. Encourage slow breathing and use a paper bag to allow for rebreathing of CO2 in a self-ventilating child 3. Assess and treat pain/anxiety |
Systemic Effects of a Respiratory Acidosis
Cardiovascular
- Tachycardia
- Increased cardiac output
- Increased blood pressure
Occurs due to sympathetic nervous system (SNS) stimulation and release of adrenaline from the adrenal medulla (the mechanism is stimulated by hypercapnia and low pH). While receptor response to inotropes/catecholamines is decreased in acidosis, this is overcome in the initial phase by an increase in the level of endogenous catecholamine released.
Pulmonary Vasculature
Increased PaCO2 can precipitate pulmonary vasospasm, which increases pulmonary vascular resistance (PVR) and decreases pulmonary blood flow. The underlying disorder responsible for the acidosis will be made worse by the increased PVR.
Cerebral Vasculature
The pH of CSF changes in relation to blood pH, but CSF contains far fewer buffers so that the pH of CSF will decrease markedly in response to a relatively small change in the blood pH. Increased PaCO2 leads to decreased cerebral vascular resistance and increased cerebral blood flow (CBF), which if unresolved may lead to cerebral oedema.
Peripheral Vasculature
There is a combination of vasoconstriction due to catecholamine release and vasodilation due to action of CO2 on smooth muscle.
Clinical Signs of a Respiratory Acidosis
- Altered respiratory pattern.
- Dyspnoea.
- Tachycardia.
- Headache.
- Fatigue or weakness.
- Decreased level of consciousness, with agitation and restlessness.
- Decreased reflexes.
- Seizures.
- Nausea and vomiting.
Assessment of The Non-Intubated Child or The Child with a Tracheostomy
- General appearance.
- Position adopted.
- Level of activity and responsiveness.
- Assessment of respiratory rate, pattern and mechanics (use of accessory muscles).
- Assessment of vital signs – heart rate, blood pressure and saturations.
- Auscultation of breath sounds.
- Chest X-ray review (if available).
- Blood gas analysis (as necessary).
In addition, for children with a tracheostomy:
- Cleanliness and security of tracheostomy tapes and dressings.
- Assessment of type of secretions yielded with suction.
- Integrity of the skin surrounding stoma and the neck.
Assessment of The Intubated Child or Child with a Tracheostomy Receiving Mechanical Ventilation
In addition to all the assessment points identified above, consideration must be given to the following when assessing a child who is intubated or child with a tracheostomy who is receiving mechanical ventilation:
- Assess for synchronicity with the ventilator.
- Is rate appropriate for age of infant/child?
- Note inspiratory/expiratory time.
- Tidal volumes of each breath.
- Adequate chest expansion with each breath.
- Appropriate alarm parameters set.
- Humidification provided.
- Assessment of level of sedation using an appropriate scoring tool (e.g. COMFORT score).
Respiratory Support and Airway Protection
Oxygen is required by all the cells of the body for effective cellular metabolism. The effects of even short episodes of inadequate oxygenation can be serious, while periods of prolonged hypoxia have potentially irreversible consequences.
The simplest form of respiratory support which can be delivered is oxygen therapy, however oxygen is a very potent medication and is not without complications in certain age groups or in children with chronic lung diseases or with single ventricle anatomy. Changes in the pulmonary parenchyma can occur as a consequence of the toxic effects of oxygen, such as damage to the capillary endothelium, destruction of type I alveolar cells and interstitial oedema. Preterm infants, in particular those born before 37 weeks of gestation, are deemed to be especially vulnerable to the harmful effects of supplementary oxygen therapy (Levene et al. 2008).
Consideration needs to be given to the age and size of the child, in addition to the amount of oxygen required, before deciding on the best method of delivery (Table 4.13).
Table 4.13 Methods of supplementary oxygen delivery
Source: Haines (2009) from Dixon et al. (2009) Nursing the Highly Dependent Child or Infant: A Manual of Care. Reproduced with permission from John Wiley and Sons, Ltd.
| Method | Age group | Oxygen available (approximate values) |
| Nasal cannula | Available in sizes for all age groups | 30–50% |
| Simple face mask | Available in sizes for all age ranges | 40–50% |
| Non-rebreathe mask | 1 year upwards | 98–100% |
| Head box | <8 months but need to consider size and weight of infant | 50–60% |
| Bucket mask | 3 years upwards | 30–60% |
| Vapotherm™ | Available in sizes for all age ranges | 21–100% |
All supplementary gases should be warmed and humidified prior to delivery to the child. Humidification prevents drying of the airways, potential cilial dysfunction and reduced airway defence (Fassassi et al. 2007), while warming supplementary gases reduces or prevents heat loss, which is especially important in the neonate, and increases the volume of the gas delivered to the lungs. According to Charles’ law, when a gas is heated, the molecules move faster and the force exerted by the molecules causes an expansion of the gas volume as the volume of a gas is directly proportional to temperature, assuming pressure is constant. In addition, it is thought that receptors in the nasal mucosa respond to cold and dry gas to elicit a protective bronchoconstrictor response (Fontanari et al. 1996).
Non-Invasive Respiratory Support – Continuous Positive Airway Pressure (CPAP)
CPAP is usually delivered non-invasively, although it can also be delivered to children with tracheostomies and those who are intubated. Like any intervention there are advantages and disadvantages (Table 4.14) with the therapy and it ultimately does not provide adequate respiratory support to the tiring infant or child. The use of nasal CPAP in infants with bronchiolitis has been shown to decrease respiratory muscle overload and improve symptoms of respiratory distress (Cambonie et al. 2008).
Table 4.14 Advantages and disadvantages of CPAP
| Advantages | Disadvantages |
| Non-invasive Splints the upper airway, therefore can help manage conditions which cause stridor Splints the lower airways and promotes smoother airflow through the bronchioles (e.g. bronchiolitis) Recruits collapsed alveoli and small airways Decreases intrapulmonary shunting Decreases PaCO2 and improves PaO2 Improves lung compliance and decreases work of breathing Sedative agents should not be required | Over-distension of alveoli if CPAP > 10 cm Barotrauma Increases ventilation–perfusion mismatching Increases work of breathing if CPAP moves the lung onto flat upper part of the compliance curve Possible increase in air trapping Increases ICP in children with intracranial hypertension Constant increased positive pressure in the thoracic cavity can decrease venous return Decreases cardiac output Fluid retention secondary to inappropriate anti-diuretic hormone (ADH) release |
There are a number of CPAP systems available and the therapy is usually delivered through the use of soft silicone nasal prongs or nasal mask in the infant or nasal or face mask in the older child. It is also possible to provide ‘long prong’ CPAP, making use of a shortened endotracheal tube, measured in the same way as measuring for a nasopharyngeal airway (measure from tip of nose to tragus of the ear) and a simple flow-driven, pressure-cycled ventilator.
Considerations for Practice
Careful positioning of the infant may reduce their work of breathing – ensure that the head of the bed is elevated to approximately 30° and the infant is well supported to maintain this position. This will reduce the pressure effects that the intra-abdominal contents may exert on the diaphragm, thus allowing better diaphragmatic expansion during inspiration. If there are no contraindications, then turning the infant prone will also reduce the effects of the compliant chest wall, again reducing the work of breathing. This positioning strategy also helps to reduce the effects of any gastro-oesophageal reflux and has been shown to reduce the infant’s energy expenditure (Pryor and Prasad 2002).
It is important to ensure that the calorific needs of the infant are augmented to meet their increased energy demands. Small frequent feeds (e.g. 2-hourly bolus feeds) help to reduce the splinting effects of a full stomach against the diaphragm and should enable the infant to carry on receiving enteral feeds via a naso/orogastric tube. Sometimes, smaller volume feeds are not adequate to satisfy the infant and 3-hourly feeds of larger volumes may be necessary. The decision about feeding strategy should always be based on clinical assessment prior to the infant receiving the feed, assessment of the infant’s tolerance during the feed and response at the end of the feed.
The use of sedative agents may be considered to facilitate the provision of CPAP, but their use should only be instituted when non-pharmacological methods, such as swaddling or the use of other comfort measures, have been tried. A small dose of an oral sedative agent such as chloral hydrate may be necessary to reduce distress or intolerance of the CPAP prongs/mask, however it is important that practitioners are familiar with the possible effects of any medications they are administering. Chloral hydrate is particularly effective in the younger patient population (<3 years of age), but its duration is unpredictable and it does have the potential to engender a degree of respiratory depression (Buck 2005).
Although the nasal prongs are made of a soft silicone material the constant pressure they exert can cause mild to severe trauma to the nares and nasal septum. While this is not a common finding in the infant population receiving short-term therapy (<48 hours), reddening of the skin and/or blanching may be seen even in this patient group if the mask/prongs are poorly fitting or inadequately applied (McCoskey 2008).
Airway Adjuncts
Oropharyngeal Airway
These are commonly known as a Guedel airway and can be used to maintain a patent airway in an emergency, prior to intubation and to facilitate effective bag–valve–mask ventilation after the administration of medications for intubation. As these are rigid plastic airways they should not be used for prolonged periods and should only be used in children or infants with absent gag reflexes. Correct sizing is determined by measuring from the centre of the incisors (or their anticipated position in infants lacking dentition) to the angle of the jaw. Care must be taken when inserting the airway as the rigid plastic structure can cause trauma to the soft oropharynx if significant force is used or the airway is inserted incorrectly.
Nasopharyngeal (NP) Airways
These can also be used to maintain a patent airway and are usually well tolerated by infants/children who are awake and have intact cough and gag reflexes. Shortened endotracheal tubes (ETT) or specialised soft silk tubes can be used and should be considered if the NP airway is to be used for a prolonged period. Correct length is determined by measuring from the tip of the nose to the tragus of the ear. If a shortened ETT is being used, then the correct size can be determined by calculating the size of ETT the child would normally require and then measuring as above for the length. Caution must be applied as the insertion of the airway may cause trauma to the friable tissues of the nasopharynx and consequent haemorrhage. Regular assessment of skin integrity around and under any securing tapes, along with inspection of the nares for signs of blanching/redness, as well as assessment of the patency of the airway must be undertaken. The clinical indication for use will determine how often the NP airway is changed. In infants and children with potential airway compromise, tube change should only be undertaken in the presence of a practitioner competent in advanced airway management in case the child decompensates after the indwelling NP airway has been removed.
Laryngeal Mask Airway
The laryngeal mask is a device for supporting and maintaining the airway without tracheal intubation. It can be used for the administration of inhalation anaesthesia, to help maintain the airway during difficult intubations or for the emergency management of the airway after unsuccessful intubation attempts. Sizing of the LMA is different from the conventional ETT sizing, but an approximate sizing guide can be used (Table 4.15).
Table 4.15 Size guide for laryngeal masks
| Calculated size of ETT | Laryngeal mask size |
| 3.5 | 1 |
| 4.5 | 2 |
| 5.0 | 3 |
| 6.0 (cuffed) | 4 |
| 7.5 (cuffed) | 5 |
Intubation
Endotracheal intubation may be undertaken for a number of reasons, including elective (e.g. for the provision of general anaesthesia during surgery), but in the PICU the majority are urgent or emergencies. Some of the clinical conditions seen in children necessitating intubation are detailed in Table 4.16.
Table 4.16 Clinical conditions in which intubation is indicated
| Physiological problem | Possible cause |
| Upper airway obstruction | Croup Epiglottitis Bacterial tracheitis Foreign body aspiration Inhalation injury – house fire/inhalation of chlorine gas Anaphylaxis (rare) |
| Respiratory failure | Pneumonia Bronchiolitis Asthma Acute respiratory distress syndrome (ARDS) |
| Respiratory depression | Seizures Neurological conditions, e.g. Guillain–Barré Poisoning Alcohol/drug intoxication |
| Systemic illness | Sepsis Multi-organ dysfunction Inborn errors of metabolism (often at initial presentation) |
| Trauma | Flail chest (rare) Penetrating lung injury (rare) Isolated head injury (common) |
| Cardiac | Severe cardiac failure Cardiogenic shock Cardioversion |
Clinical indications for intubation regardless of the underlying aetiology include:
- A silent chest.
- Respiratory exhaustion.
- Persistent or worsening acidosis with a pH < 7.2 (not usually indicated in diabetic ketoacidosis).
- Glasgow Coma Scale (GCS) score < 8.
- Poor cardiac output.
- Safe transportation.
Preparation for Intubation
Wherever possible, intubation should be undertaken in a controlled manner and if effective bag–valve–mask ventilation is being carried out, then a short period of time can be allocated to safe preparation. It is important to have two practitioners skilled in advanced airway management present where possible in case difficulties are encountered. The use of pre-intubation checklists is becoming more common to ensure that nothing is overlooked which may cause harm to the child during the procedure and to promote effective team working.
Oral intubation is the easier of the two methods and can usually be carried out rapidly with few complications. Disadvantages include the possibility of the child with teeth biting down on the tube, making ventilation and suctioning more difficult and excess oral secretions produced in response to the presence of the ETT make strapping and securing the tube more challenging.
Nasal intubation is advantageous as the tube tends to be more comfortable for the child and therefore better tolerated. Securing the tube is easier and the infant can also have a dummy or comforter as there is no risk of the tube being dislodged. However, nasal intubation is less straightforward and carries a greater risk of complications, such as traumatic haemorrhage due to the delicate nature of the tissue in the nasal passages, adenoidal trauma and, in long-term use, chronic sinusitis in the older child. Clinical contraindications for nasal intubation include:
- Basal skull fracture (confirmed or suspected).
- Presence of a cerebrospinal fluid (CSF) leak.
- Presence of clotting disorders, e.g. thrombocytopenia, disseminated intravascular coagulopathy (DIC).
- Nasal and/or mid-face deformities or trauma.
Rapid Sequence Induction (RSI)
Rapid sequence induction or intubation (both terms are used in the literature) is used when there is an unclear history from the patient or, more commonly in children, where there is a significant risk of gastric content aspiration due to a full stomach prior to intubation, e.g. when the child has been taking oral fluids or has received enteral nutrition prior to the need for intubation and there is insufficient time to ensure that gastric emptying has occurred. Aspiration of an NGT will facilitate gastric emptying but it is not a guarantee that the risk has been negated.
RSI involves pre-oxygenation of the child, the rapid administration of sedative and muscle relaxing agents, the application of cricoid pressure once the child is muscle-relaxed and oral intubation, without delivering positive pressure ventilation breaths before the ETT is inserted. The role of cricoid pressure in preventing regurgitation of gastric contents into the airway is crucial, therefore an experienced practitioner should be nominated for this task alone, and pressure should not be released until instructed to do so by the practitioner undertaking the intubation.
Fibreoptic Bronchoscope Intubation
The use of a flexible fibreoptic bronchoscope can assist with extremely difficult intubations where the bronchoscope is used to gain a view and then act as a guide over which the ETT can be passed. The bronchoscope is usually threaded through the ETT before the procedure begins and, once the scope has passed through the vocal cords, the ETT can be advanced into place. Accessibility to equipment (particularly out of hours) and the unpredictability of the need for such equipment matched to resource considerations mean that it is not an established routine or standard practice in the United Kingdom.
Intubation Risks
The ease or difficulty with which a patient can be intubated can be graded according to the view of the larynx offered to the practitioner under direct laryngoscopy (Cormack and Lehane 1984):
- Grade 1: entire aperture visible (full view of cords).
- Grade 2: posterior arytenoids visible, some of glottic aperture (partial view of cords).
- Grade 3: epiglottis visible (minimal or no view of the cords).
- Grade 4: no visible structures (can see the soft palate only).
Grades 3 and 4 predict difficult intubations.
Certain congenital clinical conditions are predictors of potentially difficult intubations as a consequence of the anatomical/structural defects linked to the condition (Table 4.17).
Table 4.17 Clinical conditions predictive of difficult intubations
Source: Gupta et al. 2005.
| Condition | Characteristics |
| Pierre–Robin syndrome | Micrognathia (small lower jaw). Macroglossia (large tongue). Cleft defect of the soft palate. |
| Treacher–Collins syndrome | Auricular and ocular defects. Malar (zygomatic bone) and mandibular hypoplasia. |
| Down’s syndrome | Poorly developed or absent bridge of the nose. Macroglossia. |
| Klippel–Feil syndrome | Congenital fusion of a variable number of cervical vertebrae, restriction of neck movement. |
Intubation Medications
The aim of intubation medication is for the child to have no pain or awareness of the procedure and to be fully muscle-relaxed, thus reducing the risk of laryngospasm.
Three drug groups are usually required for intubation:
- Analgesics.
- Sedatives.
- Muscle relaxants.
The choice of drug and method of administration will depend on the clinical condition of the child and practitioner preference. Some of the commonly used intravenous intubation drugs are listed in Table 4.18; however it is the responsibility of the practitioner administering them to ensure that the dose is correct for the child. Practitioners should refer to the British National Formulary for Children (BNFc) for further information.
Table 4.18 Commonly used intubation drugs in children
| Single dose | Considerations and notes | |
| Analgesic agents | ||
| Morphine sulphate | 100–200 mcg/kg | May cause respiratory depression and hypotension. Causes histamine release and therefore potential bronchospasm. |
| Fentanyl | 2–5 mcg/kg | May cause respiratory depression. Large doses given quickly may cause chest wall rigidity and bradycardia. |
| Sedatives/Anaesthetic agents | ||
| Midazolam | 100 mcg/kg | May cause respiratory depression and hypotension in large doses. |
| Ketamine | 2 mg/kg | Causes increased HR and BP secondary to catecholamine release. May cause increased intracranial pressure as a consequence, so caution required in patients with suspected RICP. |
| Propofol | 1 mg/kg | Excellent short-acting, general-purpose anaesthetic agent, not routinely used in children <3 years. May cause hypotension and bradycardia. Pain on administration. |
| Thiopentone | 5 mg/kg | Causes decrease in cerebral metabolic rate, cerebral blood flow and reduces intracranial pressure. Causes apnoea. Suppresses myocardial performance, which can lead to hypotension. |
| Muscle relaxants (neuromuscular blockade) | ||
| Suxamethonium | 1–2 mg/kg | A depolarising drug with very rapid onset and short duration of action. Causes the release of acetylcholine, which produces vagal responses, the most notable of which is bradycardia. To prevent bradycardia administration of atropine may be considered alongside the first dose and should always be given with a second dose. Its effects cannot be reversed. Contraindicated in burns, crush injuries and renal failure as it promotes the release of potassium, which may cause profound hyperkalaemia and cardiac dysrhythmias/arrhythmias. |
| Vecuronium | 100 mcg/kg | Non-depolarising agent. Longer duration of action; minimal cardiovascular effects. Metabolised by the liver. |
| Rocuronium | 0.6–1.2 mg/kg | Non-depolarising agent. Minimal cardiovascular effects. Has a rapid onset of action and has been used in place of suxamethonium for RSI. |
| Pancuronium | 100 mcg/kg | Non-depolarising agent. Increases HR as a consequence of vagolytic effects which may also increase BP. Metabolised by the liver and the kidneys, therefore should be used with caution in children with hepatic or renal dysfunction. |
| Atracurium | >2 years of age 0.4–0.5 mg/kg <2 years of age 0.3–0.4 mg/kg | Non-depolarising agent. Minimal cardiovascular effects. Not metabolised by the liver or kidneys. |
Depolarisation Versus Non-Depolarising Agents
Depolarising agents mimic the action of acetylcholine by binding to the post-synaptic membrane of the neuromuscular junction thus preventing acetylcholine from binding. This is a non-competitive binding. Unlike non-depolarising muscle relaxants they cannot be reversed and recovery is spontaneous (Rees 2005). Non-depolarising agents antagonise the neurotransmitter action of acetylcholine by binding competitively with cholinergic receptor sites on the motor end-plate. This antagonism is inhibited and neuromuscular block reversed by the administration of acetylcholinesterase inhibitors (e.g. neostigmine and pyridostigmine).
For children with primary airway problems such as croup, the use of inhalational anaesthetic agents is usually preferred as it is possible to maintain spontaneous breathing under its effects. Should intubation be unsuccessful, it is possible to lighten the child’s level of anaesthetic, allowing them to maintain a degree of respiratory function and gas exchange.
Intubation procedure
The equipment required for intubation is discussed in (Table 4.19). A summary of essential checkpoints prior to beginning intubation is detailed in Table 4.20. Historically, non-cuffed ETTs have been used in children <8 years of age to account for the anatomical differences in the child’s airway and the possibility of pressure-related mucosal trauma or post-extubation stridor. In recent years, low-pressure high-volume cuffed ETTs have become available for use in this age group. The evidence base for their use in the short term seems to suggest that the incidence of post-extubation stridor is comparable with children intubated with non-cuffed ETTs (Weiss et al. 2009), however there is little evidence relating to the incidence of long-term development of subglottic stenosis. It currently remains the choice of the medical team within the PICU whether to use cuffed ETTs in everyday practice or to reserve their use for selected cases where high airway pressures are anticipated during their intensive care stay, avoiding the need for reintubation because of air leak around the ETT.
Table 4.19 Equipment required for intubation
| Equipment | Notes |
| Oxygen source | Ideally this will be from a main supply source. If using cylinder supply, nominate one member of the team to monitor it. |
| Bagging circuit and face mask (ambu-bag/Ayres T piece) | Check face mask is appropriate size for the child. Choice of bagging circuit will depend on the individual practitioner however most PICU staff will use the Ayres T piece (open-ended anaesthetic-type circuit). |
| Laryngoscope | Check handle fit and the type of blade required:*
|
| Introducer/Stylette | Introducers may be inserted into the lumen of the ETT to assist with placement; however this must not protrude beyond the tip of the ETT as it will cause trauma to the airway. |
| Gum elastic bougie | A bougie is a straight, semi-rigid stylette-like device with a bent tip that can be used when intubation is known (or predicted) to be difficult. They are often helpful when the tracheal opening is anterior to the visual field. During laryngoscopy, the bougie is carefully advanced into the larynx and through the cords until the tip enters a main stem bronchus. The ETT can then be passed over the bougie and once in place, the bougie is removed. |
| Suction source Soft fine-bore suction catheters Yankauer | Ideally this will be from a main wall high pressure source. To calculate the required suction catheter size based on the size of the ETT required simply double the size of ETT, e.g. size 3.5 ETT = size 7Fr suction catheter. To clear secretions from the oropharynx. |
| Magill’s forceps | For nasal intubation. |
| Guedel airway | Ensure correct size available for the child. |
| ETTs | When using a cuffed ETT, check that the cuff inflates successfully prior to insertion. ETTs are sized according to their internal diameter. To estimate the size of ETT required, the following formula can be used for infants over 1 year of age** Size Age (in years) ÷ 4 + 4 = ETT size (Ensure that there is one size above and one size below the calculated size available) Length Oral – age (in years) ÷ 2 + 12 = length in cm Nasal – age (in years) ÷ 2 + 15 = length in cm |
| ETCO2 monitoring | Positive ETCO2 monitoring provides confirmation that the ETT has been correctly placed. Many units now use this method post-intubation and it is standard practice in theatres and emergency care situations. |
| Stethoscope | |
| Aqua gel | |
| Monitoring | The minimal standard of monitoring required is:
|
| Tape/ties | The chosen method of securing ETTs will depend on the individual unit’s preference. |
| Skin protector | |
| Nasogastric tube (NGT) | Gastric decompression is usually necessary after intubation; the insertion of a nasogastric tube will allow this. Always check the NGT position on chest X-ray before using for enteral feeding. |
* The choice of blade is ultimately that of the practitioner undertaking the procedure but is governed by the anatomy of the younger child. In infants < 1 year, the epiglottis is large and projects more posteriorly into the airway making the view more difficult. The straight blade is designed to pass over the whole of the epiglottis, moving it completely out of the way and therefore improving the view. Straight blades may be used up to the age of 5 years. As the child’s airway matures, the epiglottis becomes smaller in comparison and assumes the same position as that found in adult anatomy. The curved blade used for these patients is designed to sit in the vallecula (just short of the epiglottis) and the upward lift of the blade moves the epiglottis out of view.
** Tube sizes in neonates are based on weight. Below 2 kg is not identified here; refer to local neonatal intensive care guidelines and protocols (Kattwinkel et al. 2010).
2 kg Size 3.0 ETT 7–8 cm at the lips
3 kg Size 3.5 ETT 8–9 cm at the lips
>3 kg Size 3.5–4.0 ETT 9–10 cm at the lips
Table 4.20 Key checkpoints prior to intubation.
|
Intubation attempts should take a maximum of 30 seconds and, if unsuccessful, the practitioner should withdraw the ETT and recommence bag–valve–mask ventilation and oxygenation. After successful intubation, hand ventilation should recommence while tube placement is confirmed through the following methods:
- Observe the chest for equal, bilateral movement.
- Attach ETCO2 monitoring and assess waveform and reading.
- Auscultate for bilateral equal breath sounds.
- Chest X-ray.
Once the position is confirmed, secure the ETT at the calculated desired length according to unit policy. Many units use Elastoplast™ or similar adhesive fabric tape to secure ETTs in infants and young children, while in the older child/adolescent white twill/cotton tape ties may be utilised. There are also a number of commercially available fixation devices, but again their use will be determined by local policy. Care should be taken to protect the skin to reduce the risk of epidermal stripping through contact with strong adhesives.
The complications associated with intubation may occur at any time during the intubation event and while many strategies have been put in place to minimise the risk to the child, there are occasions where adverse events will occur. These include blunt airway trauma, damage to dentition, hypoxia, oesophageal intubation, right/left main-stem bronchus intubation, vocal cord damage and exacerbation of cervical spine injury.
Tracheostomies
Some children require the formation of a tracheostomy to maintain a patent airway and help facilitate long-term ventilation. Most tracheostomies in children are elective surgical procedures involving an incision made below the cricoid cartilage through which a tube is inserted into the trachea through the 2nd and 4th tracheal rings. There is a variety of tracheostomy tubes available so it important to be aware of the differences between manufacturers’ sizing, particularly in terms of tube length. Customised tracheostomy tubes can be ordered from manufacturers according to the individual child’s needs. As with ETTs, tracheostomy tubes may be uncuffed or cuffed.
It is usual practice for the first tracheostomy tube to remain insitu for a week postoperatively, after which it is electively changed, normally by the ENT team responsible for the care of the child. The purpose of this delay is to allow time to promote healing of the surgical incision and for the stoma to form completely. ‘Stay sutures’ (stitches brought out from the sides of the wound and taped to the child’s chest) are usually used to hold the edges of the stoma apart, maintaining stoma patency, and these are removed at the time of first tube change.
Consideration for Practice
Securing the tracheostomy is usually done using either white twill cotton ties or Velcro™ fixation devices. Each unit will have its own policy and preferences for the type of material used. In the immediate postoperative period, cotton ties are used. With due care and attention these can be changed before the first tube change if they are blood-stained or soiled, however this should be done under medical or senior nursing staff supervision if possible. It is important that the tracheostomy tube is well secured to prevent accidental displacement, but the tapes should not be too tight as this has the potential to cause chafing and eventual breakdown of the skin.
Assessment of skin integrity should be undertaken at least twice a day, however this may need to be increased if there are any concerns about the skin surrounding the stoma or underneath the tapes/ties used to secure the tube.
The type of dressings used will depend on local unit policy or preference, for example, Lyofoam™ absorbent dressings designed to protect the skin underneath the tracheostomy tube phalanges from undue pressure and to keep the skin around the stoma dry. Dressings should be changed at least daily, and more often if wet or soiled.
When suctioning the child’s tracheostomy tube, it is important to suction to a maximum of 0.5 cm beyond the length of the tube to avoid causing suction-related trauma to the airway beyond this.
Principles of Ventilation
Children in PICU undergo multiple invasive interventions, such as line insertion, renal replacement therapy and placement of invasive monitoring lines, all of which are geared towards supporting the child until organ function or recovery occurs rather than curing the disease itself. Similarly, intubation and mechanical ventilation do not cure illness but facilitate the provision of intensive care and/or support the respiratory system while lung tissue and gas exchange recover. Mechanical ventilation is not without risk and the potential side-effects on the lungs include acute lung injury secondary to forces exerted by positive pressure ventilation, oxygen toxicity and the development of nosocomial infections (ventilator-associated or acquired pneumonia – VAP). In addition, adverse events (e.g. equipment malfunction) add to the potential harm a child may suffer secondary to this intervention.
Types of Ventilation
Ventilation can be either positive or negative pressure, invasive or non-invasive. Determinants of ventilation will depend on the individual child and their underlying disease pathology or indication for ventilation. The practitioner is required to make a number of decisions when determining a ventilatory strategy for the child, based on the current clinical assessment and expected normal values for the age of the child. Ventilators vary in their methods of accessing the settings; the basic underlying settings of any ventilatory mode are highlighted in Table 4.21. It is important to achieve synchronicity or a smooth interaction between the ventilator and the child and wherever possible to maintain the child’s own respiratory effort, if the child’s clinical condition allows this.
Table 4.21 Basic ventilation parameters
| Setting | Notes and considerations |
| Inspiratory time | Spontaneous inspiratory time is determined by lung compliance, airway resistance and flow rate. Settings for the inspiratory time on the ventilator mimic those anticipated in the well child – shortest in the neonate, gradually increasing with age:
|
| Peak inspiratory pressure (PIP) | The main factors that determine PIP are lung compliance, airway resistance, inspiratory time and tidal volume. Minimising PIP to avoid volutrauma is part of a lung protective strategy. |
| Positive end expiratory pressure (PEEP) | PEEP is applied at the end of expiration to maintain FRC and therefore maintain alveolar recruitment preventing areas of collapse developing in the lungs. |
| Tidal volume (Vt) | Tidal volumes should reflect expected tidal volumes for the spontaneously breathing child. As previously noted, tidal volume is constant at 6–8 ml/kg throughout life and the ventilator settings should aim to achieve this. In certain modes the Vt can be set, e.g. volume control (VC) or pressure-regulated volume control (PRVC). Increasing Vt over normal values will lead to hyperinflation and may cause a pneumothorax. |
| Inspiratory flow | In a spontaneously breathing child, flow rates and patterns are more variable than in the older child/adolescent in whom a more constant flow is typical. It is possible to adjust flow rate and pattern settings on most ventilators to optimise alveolar ventilation and improve patient comfort. Types of flow pattern include sinusoidal, decelerating and constant. Sinusoidal is the flow pattern seen in patients receiving CPAP. Decelerating flow patterns are seen in pressure-targeted ventilation – the majority of the flow is delivered on initiation of the breath with the remainder being delivered more slowly during the rest of the inspiratory phase which enhances oxygen distribution to alveoli. |
| Mean airway pressure (MAP) | MAP is the average airway pressure measured at the proximal airway from one inspiration to the beginning of the next. This value will vary according to the mode of ventilation used, e.g. in a pressure-controlled mode it will be an essentially static value, whereas in a pressure-regulated or volume-controlled mode it will be more variable. Factors that influence MAP include tidal volume, PIP, flow rate, respiratory rate and end expiratory pressure. |
Negative-Pressure Ventilation
This is not widely used in the paediatric critical care setting as its delivery can be problematic, although some units do have experience of its use. Historically, negative-pressure ventilation is associated with the ‘iron lung’, first used in the management of polio in children in 1928 in the United States at the Children’s Hospital, Boston. During the worldwide epidemics of the 1950s these basic respiratory support devices were widely used in all age groups and continue to be used by some adults to this day. Smaller, more portable devices, such as the Hayek Cuirass™, have been available for a number of years and as a therapy it has been used successfully in patients with neuromuscular degenerative disorders (e.g. Duchenne’s muscular dystrophy or spinal muscular atrophy – SMA) as well as in children post-cardiac surgery in whom the increased intrathoracic pressures seen with positive-pressure ventilation can significantly alter blood flow to the pulmonary circuit (e.g. post-Fontan procedure). Today this mode of ventilation has developed further and is known as biphasic cuirass ventilation rather than negative-pressure ventilation; however, it does mimic the action of normal respiration rather than exerting a positive pressure while assisting with respiratory effort.
Positive-Pressure Ventilation (PPV)
This can be delivered as a non-invasive support or as an invasive procedure following endotracheal intubation or formation of tracheostomy. Positive-pressure ventilation works on a simple basis – the ventilator causes alveolar ventilation by generating a positive pressure at the proximal airway which exceeds the alveolar pressure, thus creating a pressure gradient. This gradient forces gas to flow into the lungs. Expiration is essentially a passive process that occurs when the ventilator stops exerting the positive pressure (end of inspiration).
There is an increased risk of iatrogenic lung damage with positive-pressure mechanical ventilation because of children’s developmental and anatomical differences:
- Increased chest wall compliance provides less protection against ventilator induced lung injury (VILI), especially if pressure-cycled ventilation leads to large tidal volumes.
- Concentrations of lung elastin (which correlates with the elastic recoil of the lung) are lower in children than in adults.
- Concentrations of lung collagen (which is associated with structural integrity of the lung) are lower in children compared to adults.
- An inverse relationship of chest wall compliance and the risk of microvascular damage with mechanical ventilation may lead to increased pulmonary oedema in the younger child/infant with higher chest wall compliance.
Each brand of ventilator has slightly different modes/terms used and it is the responsibility of the practitioner to become familiar with the ventilators used in their unit.
Non-Invasive PPV (NIPPV)
As the name suggests, this is advantageous as it avoids the need for intubation with its associated risks. As a therapy it can be used in both the short and longer term and can be used to support children for whom intubation significantly increases their mortality and morbidity risk (Najaf-Zadeh and Leclerc 2011). In addition, NIPPV can be used very effectively as a weaning tool following a period of invasive ventilation, particularly in children with muscle weakness or those with acquired or congenital immune deficiency.
NIPPV provides two levels of support by delivering both inspiratory and expiratory positive pressure. The most commonly used mode of NIPPV in the acute setting is Bi-phasic Positive Airway Pressure (BiPAP) which is a form of CPAP that alternates between high and low positive airway pressures, permitting inspiration (and expiration) throughout. It reduces the work of breathing, improves alveolar ventilation and splints the alveoli open during the expiratory phase.
Characteristics of NIPPV Ventilation
- Triggered or timed pressure-limited ventilation, combined with PEEP/CPAP.
- Permits spontaneous breathing during inspiration and expiration maintaining constant pressures.
- PIP, PEEP, rate, trigger sensitivity and inspiratory flow are set by operator.
- NIPPV ventilators can compensate for large leaks associated with the masks used for delivery of therapy.
One of the most important features of successful NIPPV is a well-fitting mask for effective delivery. Different types of mask are available, from small nasal masks, masks covering the nose and mouth, to full face masks and helmets, however finding a mask that fits well can be challenging in the infant/toddler age group. The consequences of a poorly fitting mask can be significant, causing pressure sores, especially on the bridge of the nose, and in long-term use this pressure can lead to mid-third facial deformities (Faroux et al. 2005).
Contraindications to NIPPV
- Severe hypoxaemia.
- Inadequate airway, e.g. croup/burns.
- Cardiovascular instability.
- Presence of a pneumothorax.
- Recent upper gastrointestinal surgery.
Considerations for Practice – Management of the Child Receiving NIPPV
The child’s mask should be released every two hours to avoid the risk of pressure sores developing. Particular attention should be paid to the areas such as the bridge of the nose and the forehead as these are prone to pressure ulceration. In addition, the child’s face should be washed and dried at the time of mask removal as constant contact with the mask can make the skin sweat.
Frequent eye care is required to reduce potential irritation from the leak around the mask and careful attention to oral hygiene is also necessary as the mucous membranes of the mouth can become dry and easily irritated.
The nature of NIPPV means that the child is at risk of increased of gastric distension therefore an oro-/nasogastric tube should be sited and aspirated every 2 hours along with mask care. Alternatively, if the child has a gastrostomy, this should be aspirated 2-hourly to minimise diaphragmatic splinting.
Invasive Positive-Pressure Ventilation
Ventilation modes may be defined by the type of breath (mandatory or synchronised) based on patient effort and the amount of support delivered with any spontaneous breaths initiated (Table 4.22).
Table 4.22 Ventilation modes in IPPV
| Mode | Notes |
Controlled or mandatory
| Suitable for the child who is apnoeic, e.g. use of muscle relaxants. All the breaths are ventilator breaths and the cycling is usually time-regulated. Can be pressure or volume-limited. All settings are controlled by the operator. Most suitable if deficiency in gas exchange is so severe that other modes are unable to achieve adequate ventilation and/or oxygenation. |
| Assist control | The ventilator delivers pre-set breaths in coordination with the respiratory effort of the patient. With each inspiratory effort, the ventilator delivers a full assisted tidal volume. Spontaneous breathing independent of the ventilator between the A/C breaths however is not enabled. |
Synchronised
| The ventilator delivers a set number of breaths per minute but, unlike mandatory modes, these are synchronised breaths triggered by child’s inspiratory effort. If the child makes no respiratory effort, the ventilator will continue to deliver the set breaths per minute. The size of breath (PIP or Vt), inspiration time, trigger sensitivity and rate are set by the operator. Inspiration is initiated when the child generates a small inspiratory gas flow or a small negative pressure in the ventilator tubing. This mode is often used in combination with pressure support which is a peak pressure above PEEP delivered to support breaths taken over the set breath rate. This level of support can be adjusted to increase or decrease the child’s respiratory effort according to their clinical condition. |
Pressure support
| The ventilator supports every spontaneous breath with positive pressure during inspiration. The operator determines the level of support delivered with each breath. In the PS mode, there may be no back-up rate on the ventilator if the child tires, therefore it is often used in conjunction with SIMV. This mode decreases the work of breathing but allows the child to exercise inspiratory muscles. The combination of PS and CPAP ensures that the child receives end expiratory pressure in addition to inspiratory pressure support. |
Improving oxygenation without increasing FiO2
| |
High Frequency Oscillatory (Oscillation) Ventilation (HFOV)
This ventilatory support is utilised when the child is unresponsive to conventional ventilation strategies. Historically, it was used as a treatment of last resort but it has in recent years gained a place in everyday practice. It is a useful alternative which may be used in combination with nitric oxide therapy for children who reach the referral criteria for extracorporeal membrane oxygenation (ECMO).
Indications for use in PIC include:
- Acute lung injury secondary to aspiration.
- Acute lung injury secondary to pulmonary infection.
- Severe sepsis.
- Smoke inhalation injury.
- Acute respiratory distress syndrome.
- Near-drowning.
- Prevention of barotrauma in children with severe restrictive lung disease.
Principles of HFOV (Table 4.23)
The ventilator uses a diaphragm or piston to deliver oscillating pressure to the airway and drive a volume of gas into the lungs at a frequency of 60–3600/min (1–60 Hz). Exhalation is active as a consequence of the piston/diaphragm oscillations rather than passive and this has two benefits: first, it reduces the risk of air trapping, and second, it facilitates CO2 clearance. The Vt delivered during each oscillation is ≤ anatomic dead-space and is approximately 1–3 ml.
Table 4.23 HFOV parameter settings
| Parameter | Notes |
| Mean airway pressure (MAP) Initial setting – 3–5 times higher than MAP on conventional ventilator, then reduce as able to avoid over-expansion | The MAP determines the degree of lung expansion and FRC. As such it is the principal mechanism for regulating oxygenation. It is important to avoid over-expansion, which will increase the resistance to gas flow, or under-expansion, which will cause areas of atelectasis. This must therefore be reviewed with a chest X-ray. |
| Power amplitude (ΔP) Initial setting – 35–45 | This regulates the tidal volume by determining the amplitude (the amount of movement with each oscillation) of the piston. There is no magic formula that predicts the correct ΔP but initial settings are usually based on a clinical impression of the adequacy of chest ‘wobble’ or ‘bounce’ achieved with the set ΔP. Usual settings vary between 30 and 80 although higher settings may well be required based on blood gas analysis. |
Frequency
| The frequency (in Hz) is similar to setting a rate on the conventional ventilator but it also directly influences Vt. Along with the ΔP, CO2 clearance is determined by the frequency setting. Unlike conventional ventilation, to increase CO2 clearance the frequency should be reduced or the ΔP increased. |
| Inspiratory time | As the oscillator can deliver up to 600 oscillations/min it is not possible to set an inspiratory time as used in conventional ventilation. An inspiratory time of 33% is most commonly used as setting higher than this (e.g. 50%) would not allow sufficient time for the expiration phase and air trapping would occur. |
Considerations for Practice
In essence there are very few differences between the care of the child receiving conventional ventilation and the child receiving HFOV. A couple of points worthy of mention are:
- Suctioning – The use of a closed or in-line suction system is advocated to prevent alveolar derecruitment for patients with higher MAPs. Some units will use the in-line suction system with all their patients, while others reserve them for patients on HFOV. Some units routinely take the child off the oscillator once every 24 hours, hand ventilate and use that opportunity to provide deep suction using the open suction technique, however this practice is not supported by evidence and therefore cannot be advocated as regular practice. Effective humidification and the well-judged use of 0.9% sodium chloride through the instillation port on the suction system may have less of a detrimental effect on arterial oxygenation than the derecruitment associated with disconnection from the oscillator.
- Tubing management – The inflexible nature of the oscillator tubing makes repositioning the child receiving HFOV slightly more challenging than other children. It is prudent to have one practitioner allocated specifically to managing the child’s tube to prevent accidental extubation during position changes. In addition, the child is at greater risk of pressure being exerted on the nares (if nasally intubated) or the corner of the mouth (if orally intubated) as it is more difficult to position the tubing of the oscillator to maintain a straight line between the machine and the child’s ETT.
The best form of respiratory support, therefore, is one that gives the greatest chance of survival with the least risk of long-term damage to other organs (Table 4.24). In order to facilitate this, targets for parameters, such as blood carbon dioxide levels, may be altered to prevent secondary lung damage (e.g. adopting a ‘permissive hypercapnia’ ventilation strategy). Examples of target parameters are detailed in Table 4.25.
Table 4.24 Clinical implications of positive pressure ventilation (PPV)
| Body system | Effects |
| Cardiovascular | PPV causes a drop in cardiac output because:
|
| Neurological | PPV can exert significant effects on cerebral perfusion:
|
| Renal | The effects of PPV on the renal system are complicated and linked to the effects exerted on the cardiovascular system:
|
| Gastrointestinal |
|
Table 4.25 Parameter targets for lung protective ventilation
| Normal | Targets to minimise lung damage | |
| pH | 7.35–7.45 | >7.25 |
| PaCO2 | 35–45 mmHg 4.5–5.5 kPa | <80 mmHg <10.5 kPa |
| PaO2 | >80 mmHg 10.5 kPa | FiO2 < 50% |
| PIP | <35 cmH2O | |
| PEEP | 5–15 cmH2O |
Studies continue into the use of a permissive hypercapnia strategy, with discussion focusing on the possible detrimental effects, as well as its protective role in lung injury (Ijland et al. 2010; Laffey et al. 2009) and it is clear from the published literature, most of which is in the adult field, that it should not be used in isolation but should form part of a general lung protective strategy, including the use of reduced tidal volumes, effective lung recruitment and appropriate use of PEEP to prevent alveolar collapse at end expiration (Albuali et al. 2007; Matthay and Calfee 2005; Petrucci and Iacovelli 2007).
Acute lung injury or ventilator-induced lung injury (VILI) secondary to mechanical ventilation can occur in one of two phases of each mechanical breath delivered (Figure 4.8). The difficulty encountered when deciding on a ventilation strategy is finding the ‘sweet spot’, or the point at which all atelectatic alveoli are open and able to participate in ventilation without overextending the alveoli to the point at which compliance decreases and gas exchange is compromised (Froese 1997; Matthews and Noviski 2001). These two points are referred to in the literature as the lower inflection point (LIP) and the upper inflection point (UIP).
Figure 4.8 Ventilation curve with lower and upper inflection points.
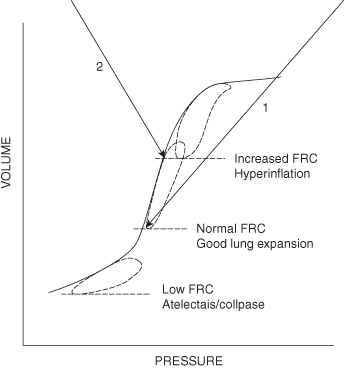
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree