Between days 21 and 28 the primitive heart tube elongates, thickens and twists to the right. This is referred to as dextral or D looping and results in the correct anatomical positioning of the two ventricles. If the heart loops to the left at this stage (levo or L looping) then ventricular inversion occurs. By day 28 there is blood flow through the four identifiable heart chambers.
Atrial septation begins at day 28. The septum primum grows from a fold in the upper portion of the atria down towards the endocardial cushion, closing the opening known as the ostium primum. Fenestrations then appear in the upper section of the septum primum and these become the ostium secundum.
Next the septum secundum grows from the upper portion of the atria and this, in conjunction with the septum primum, forms the flap-like structure of the foramen ovale which permits blood to flow from the right to the left atrium when right atrial pressures are high, as they are in utero.
Ventricular septation occurs between weeks 4 and 8. A muscular fold appears at about day 30 and grows from the anterior wall and floor of the developing ventricles towards the endocardial cushion. The ventricles continue to grow downwards on either side of the evolving septum and septation is completed by growth of the bulbar ridges and the endocardial cushion at the end of week 7.
The developing ventricles initially share a single outflow tract, known as the truncus arteriosus. By day 40 the base begins to rotate clockwise, placing the evolving aorta anteriorly and the pulmonary artery posteriorly. Truncoconal swellings form from the truncal endocardium and these grow and rotate inwards before joining and separating the aorta and pulmonary artery.
Fusion of the endocardial cushions at week 6 divides the atrioventricular canal into two channels. The septal leaflets of the mitral and tricuspid valves develop from the endocardial cushion tissue while the mural leaflets develop from the myocardial wall (Kirby 2007).
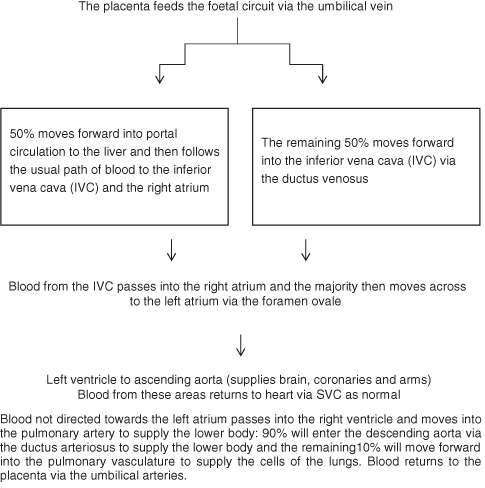
Foetal Circulation
The foetal circuit (Figure 5.2) consists of two arteries and one vein and three key openings or passages, the ductus venosus, foramen ovale and ductus arteriosus, which move blood around the foetus, bypassing the pulmonary circulation.
The foetal circuit is relatively hypoxic as it is passively fed from the maternal circulation and the pulmonary vascular resistance is high due to hypoxia-induced vasoconstriction and fluid rather than air-filled alveoli.
Changes in pulmonary vascular resistance (PVR) secondary to the reversal of hypoxia-induced vasoconstriction in the lungs at birth cause pressures in the right side of the heart to fall. Cord clamping and removal of low-resistance placental circulation cause closure of the ductus venosus and produces an increase in systemic vascular resistance (SVR) and increased pressure in the left ventricle. The combination of the fall in RA pressure and rise in LA pressure produces functional closure of the foramen ovale, however obstructive lesions on the right side of the heart may lead to delayed closure. Constriction of ductal smooth muscle leads to gradual closure of the ductus arteriosus over the first 7–10 days of life. During the first 2–9 weeks of life, in a structurally normal heart, there is a gradual thinning of the medial smooth muscle layer of the pulmonary arteries, which leads to further reduction in PVR. At 3 months, therefore, in a normal healthy term infant PVR is equal to that found in adults.
Brief Overview of Cardiac Anatomy and Physiology (Figure 5.3)
External Structure of the Heart
The heart is surrounded by the pericardium, a loose-fitting, inextensible sac, which consists of two layers:
- Fibrous pericardium – a tough, loose-fitting and inelastic sac around the heart.
- Serous pericardium– consists of two layers:
- The parietal layer is the lining of the fibrous pericardium.
- The visceral layer adheres to the outside of the heart.
- The parietal layer is the lining of the fibrous pericardium.
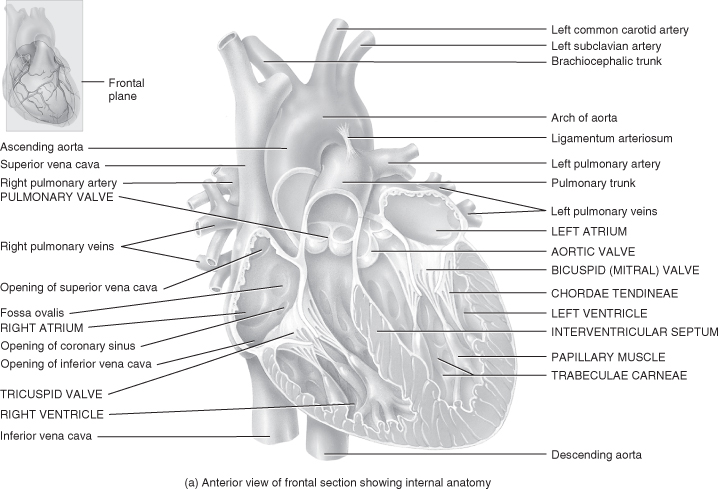
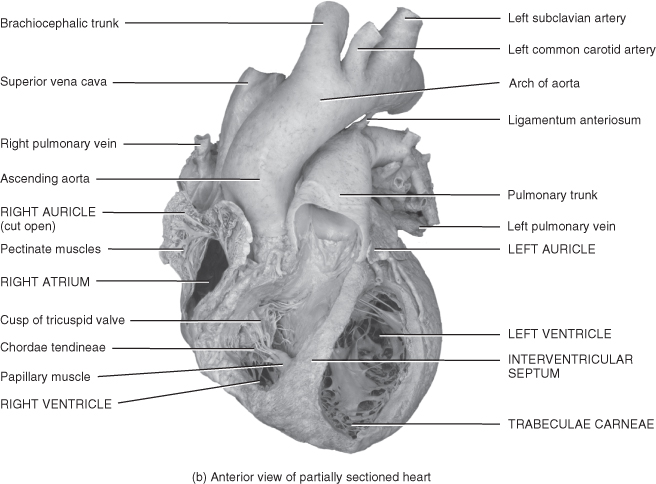
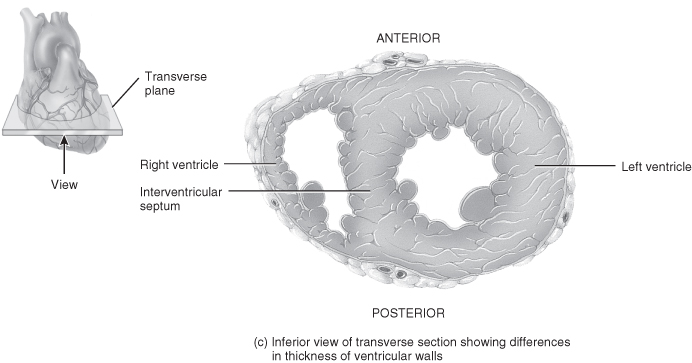
Figure 5.3 Gross anatomy of the heart.
From Tortora, G.J. and Derrickson, B.H. (2009) Principles of Anatomy and Physiology, 12th edn. Reproduced with permission from John Wiley and Sons, Inc.
The gap between these layers is known as the pericardial space and contains a small amount of pericardial fluid whose purpose is to reduce surface friction between the two layers of the serous pericardium. Any condition that leads to fluid accumulating in this sac will cause restriction of cardiac output and cardiac tamponade if it is not recognised and managed. The heart wall is made up of three layers of tissue: epicardium, myocardium and endocardium.
Epicardium
This is the outer layer of the heart wall and is the visceral layer of the serous pericardium; therefore, these two layers are one and the same.
Myocardium
This is the middle layer of the heart wall and is a thick, contractile layer of specially constructed and arranged cardiac muscle cells.
Endocardium
This is the lining of the interior of the myocardial wall and is a delicate layer of endothelial tissue. The endocardium covers projections of myocardial tissue from the ventricular walls known as trabeculae. Specialised folds or pockets formed by the endocardium make up the functional components of the intra-cardiac valves.
Intracardiac Valves
There are four sets of valves in the heart which ensure that blood flows in one direction only, preventing an increase in the pressures within the atria and or ventricles which may cause damage to the structure or function of the myocardium.
- Atrioventricular valves (also called cuspid valves).
- Tricuspid valve (RA–RV) is a three-leaflet valve.
- Bicuspid valve (LA–LV) is a two-leaflet valve (the mitral valve).
These valves are held in place by the papillary muscle and additionally in the right ventricle by structures known as the chordae tendineae.
- Tricuspid valve (RA–RV) is a three-leaflet valve.
- Semilunar valves – consist of half-moon-shaped flaps growing out of the lining of the pulmonary artery and the aorta. The purpose of these valves is to prevent blood flowing back into the right or left ventricle from the pulmonary artery or aorta respectively at the end of ventricular systole.
Myocardial Blood Supply (Figure 5.4)
Myocardial cells receive blood to meet their metabolic demands from two small vessels known as the coronary arteries. The coronary arteries are found in the aorta behind the flaps of the aortic semilunar valves. The location of the coronary arteries has significance in terms of myocardial performance in the presence of a sustained tachycardia or low diastolic blood pressure.
Figure 5.4 Coronary circulation.
From Tortora, G.J. and Derrickson, B.H. (2009) Principles of Anatomy and Physiology, 12th edn. Reproduced with permission from John Wiley and Sons, Inc.
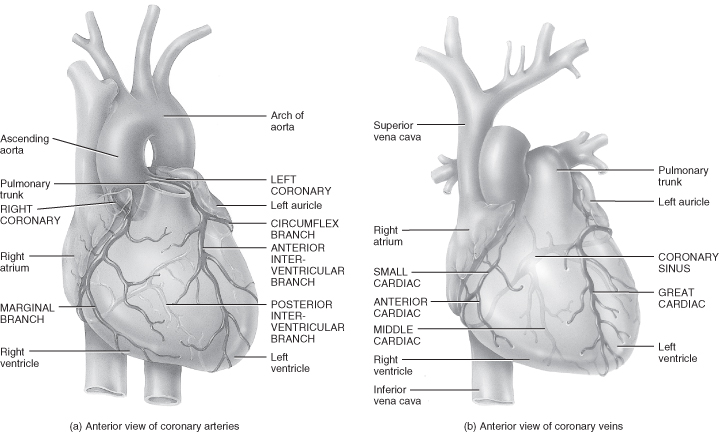
Both coronary arteries have two main branches, but while the ventricles receive their blood supply from branches of both of the coronary arteries, the atria by contrast receive blood from a small branch of the corresponding coronary artery.
Venous Return
Once blood has passed through the capillary beds in the myocardium it enters a series of cardiac veins before draining into the right atrium through a common venous channel known as the coronary sinus. (Several veins which collect blood from a small area of the right ventricle do not end in the coronary sinus but drain directly into the right atrium.)
The Cardiac Cycle
Innervation of the Heart
Both divisions of the autonomic nervous system send fibres to the heart:
- Sympathetic fibres are contained in the middle, superior and inferior cardiac nerves.
- Parasympathetic fibres are contained in branches of the vagus nerve.
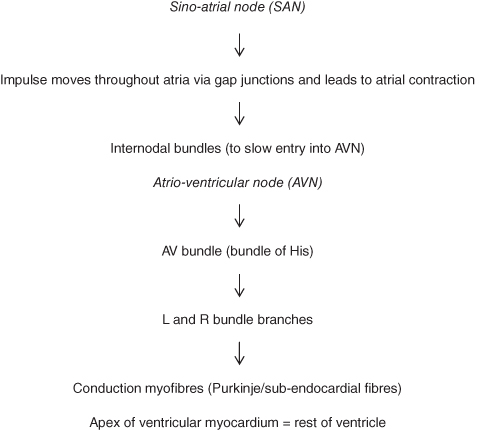
These fibres combine to form the cardiac plexuses located close to the arch of the aorta. From the cardiac plexuses fibres accompany the coronary arteries to enter the heart, where most will terminate in the sino-atrial node (SAN), some in the atrio-ventricular node (AVN) and some in the atrial myocardium. From the SAN the impulse is transmitted through the conduction pathways of the atria and ventricles to generate contraction of both sets of chambers (Figure 5.5).
Structure and Function of Cardiac Muscle Fibres
The structure and properties of cardiac muscle are essentially a cross between that of the smooth muscle layers of the lungs, blood vessels and the gut and that of the striated muscle in the musculoskeletal system. While the basic contractile machinery is similar there are several important morphological and functional differences between cardiac muscle and skeletal muscle:
- Smooth (cardiac) muscle contracts and relaxes slowly, can initiate contraction and does not tire, whereas striated (skeletal) muscle can contract and relax more rapidly but cannot initiate contraction and tires quickly.
- Cardiac muscle fibres are shorter than skeletal muscle fibres, but they have a larger diameter.
- Cardiac muscle has limited intracellular reserves of Ca++compared to skeletal muscle.
The cells in the heart are known as myocytes (myocardial cells). The ends of individual myocytes connect to neighbouring cells through irregular transverse thickenings of the cell membrane, known as intercalated discs. These discs contain desmosomes, which hold the fibres together, and gap junctions, which allow action potentials to spread from one muscle fibre to another; consequently the walls/septum of the atria and the walls/septum of the ventricles form functional networks. This ensures that the impulse moves smoothly from the top of the atria downwards and from the apex of the ventricle upwards. Myocytes contain a large number of mitochondria and are surrounded by a strong capillary network to ensure that the oxygen demands of the cells are constantly addressed.
Each myocyte is made up of myofibrils, which in turn are made up of structures known as sarcomeres, which are the basic contractile units. The myofibrils are surrounded by the sarcoplasmic reticulum, the function of which is to act as a reservoir for calcium stores within the cell. Coordinated shortening of all the sarcomeres within each cell is the process through which contraction occurs.
The sarcomere is made up of filaments which interlace with each other. Some of the filaments are thick and contain the protein myosin while the remainder are thin and contain the protein actin. Heads on the myosin filaments stick out and are attracted to binding sites on the actin filaments. When activated, the myosin head attaches to the binding site (called a cross-bridge) on the actin filament. Each myosin head has two actin binding sites and two enzymatic sites which can change ATP to ADP and inorganic phosphate (involved in chemomechanical transduction, which is the molecular basis of energy transformation in all muscles). The interactions between the individual cross-bridges and the actin filaments is dependent on the presence of ATP, but is inadequate to generate enough force to affect muscle contraction. However, millions of cross-bridges cycling asynchronously can combine to generate considerable force, which contributes to the overall muscle cell shortening and contraction.
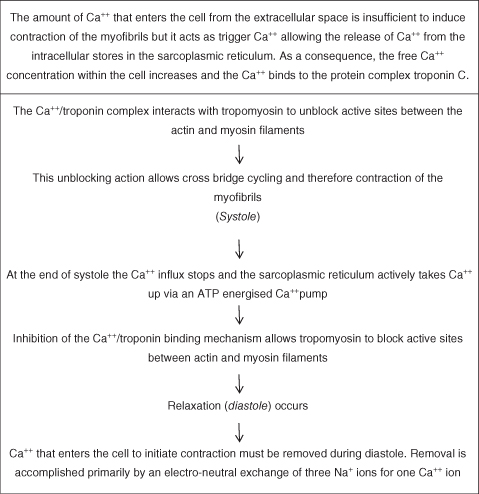
At rest actin and myosin are prevented from contacting each other by two other proteins: tropomyosin and the Ca++ binding protein troponin. Troponin is a complex protein which has three parts: troponin C, which binds calcium, troponin I; which prevents the interaction between actin and myosin; and troponin T, which binds the troponin molecule to tropomyosin. Upon stimulation, Ca++ is released from internal stores and binds to troponin C which induces a conformational change of tropomyosin and allows actin–myosin interaction. This process is outlined in Figure 5.6.
Considerations for Practice
- Removal of Ca++ from the extracellular fluid decreases contractile force and will eventually cause arrest in diastole.
- Increases in the concentration of extracellular Ca++ enhance contractile force but very high Ca++ concentrations will eventually induce cardiac arrest in systole (rigor).
Cardiac Action Potentials
There are two general types of cardiac action potentials:
- Non-pacemaker action potentials (also called fast response action potentials because of their rapid depolarisation) are found throughout the heart, except for the pacemaker cells.
- The pacemaker cells generate spontaneous action potentials (also termed slow response action potentials because of their slower rate of depolarisation). These are found in the SA and AV nodes.
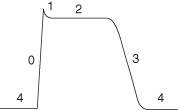
The action potential generated in the cardiac cells is different from that generated in the other types of cell as it contains a plateau phase which is essential to prevent myocardial tetany. The phases of a cardiac cell action potential are detailed in Figure 5.7.
- Cell is ready to generate an action potential
- Sodium moves into cell via the fast channels
- Cell becomes +ve charged
- Slow channels open allowing movement of calcium into the cell
- Results from closure of fast channels
- Plateau in AP caused by continued diffusion of calcium into the cell and delays repolarisation
- Slow calcium channels close and potassium channels open
- Membrane permeability to potassium is restored
- Significant potassium efflux to the outside of the cell
- Cell is ready to generate another action potential
Regulation of Cardiac Action Potentials
To maintain a normal rhythm it is vital that the cardiac muscle cells cannot generate a second action potential until completion of the previous cycle. The regulation of action potentials within the heart is achieved through the division of the action potential into four periods which control whether the cell can generate an action potential:
- Actual refractory period (ARP) –no matter how large a stimulus (i.e. inward current), the cell is unable to generate a second action potential during the absolute refractory period (ARP), because most of the Na+ channels are closed. The absolute refractory period includes the upstroke, the entire plateau and a portion of the repolarisation. This period concludes when the cell has repolarised to approximately −50 mV.
- Effective refractory period (ERP) –includes and is slightly longer than the absolute refractory period. At the end of the effective refractory period, the Na+ channels start to recover (i.e. become available to carry inward current).
(The distinction between the absolute and effective refractory periods is that absolute means absolutely no stimulus is large enough to generate another action potential; effective means that a while an action potential may be generated it will not be a conducted action potential as there is not enough inward current to conduct it forward to the next site.)
- Relative refractory period (RRP) –begins at the end of the effective refractory period and continues until the cell membrane has repolarised to about −70 mV. During the relative refractory period even more Na+ channels have recovered and it is possible to generate a second action potential, although a greater than normal stimulus is required. If a second action potential is generated during the relative refractory period, it will have an abnormal configuration and a shortened plateau phase.
- Supranormal period (SNP) –follows the relative refractory period. It begins when the membrane potential is −70 mV and continues until the membrane is fully repolarised to −85 mV. As the name suggests, the cell is more excitable than normal during this period and therefore less inward current is required to depolarise the cell to the threshold potential. The physiological explanation for this increased excitability is that the Na+ channels are recovered (i.e. the inactivation gates are open again), and because the membrane potential is closer to threshold than it is at rest, it is easier to fire an action potential than when the cell membrane is at the resting membrane potential.
Disorders of electrolyte balance, particularly potassium and calcium, as well as hypoxia and acidosis all have significant implications for cardiac rhythm (Table 5.1).
Table 5.1 Clinical implications of electrolyte imbalance, hypoxia and acidosis
| Clinical state | Effects |
| Hypokalaemia (decreased extracellular potassium) | Affects the resting membrane potential and causes cardiac cells to become irritable; delays the repolarisation which leads to tachycardias and irregular beats (ectopics). |
| Hyperkalaemia (increased extracellular potassium) | Hypopolarises cardiac myocytes and causes a decrease and lengthening of depolarisation and repolarisation; heart block/arrhythmias common with fibrillation at higher serum K levels. |
| Hypocalcaemia (decreased extracellular calcium) | Reduces the strength of cardiac contraction by affecting the intracellular release of calcium necessary for actin–myosin binding, which reduces cardiac output and may also produce ectopic foci. |
| Hypercalcaemia (increased extracellular calcium) | Causes premature repolarisation and may produce spasmodic contractions of the cardiac muscle. |
| Hypoxia | Affects the function of the sodium–potassium pump which is ATP-dependent. This reduces the ability of the cells to maintain ionic balance and may lead to cell death. |
| Acidosis | Alters the function of the sodium and calcium channels reducing their specific conductance. This will lead to reduced cardiac contractility and cardiac output. |
Each part of the cardiac cycle is associated with the movement of blood through the heart (Figure 5.8).
Figure 5.8 The cardiac cycle.
From Tortora, G.J. and Derrickson, B.H. (2009) Principles of Anatomy and Physiology, 12th edn. Reproduced with permission from John Wiley and Sons, Inc.
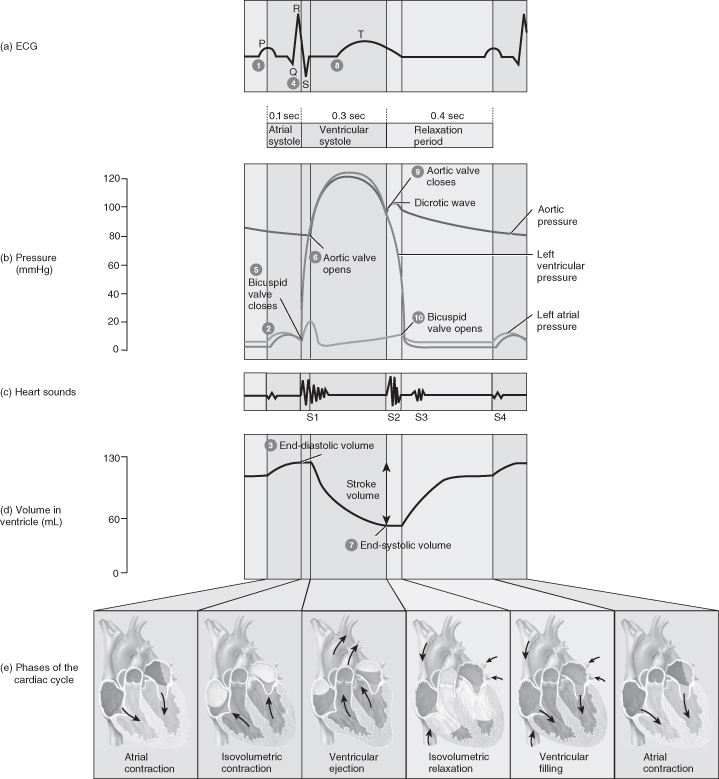
Atrial Systole (Ventricular Diastole)
Onset of atrial systole occurs soon after the beginning of the P-wave on the ECG (curve of atrial depolarisation). Transfer of blood from the atrium to the ventricle is accomplished by a peristaltic-like wave of atrial contraction. The atrial pressure barely exceeds the ventricular pressure and the resistance of the pathway via the AV valves is normally very low.
Isovolumetric Contraction (First Heart Sound)
The onset of ventricular contraction coincides with the peak of R-wave on the ECG and is the earliest rise in ventricular pressure after atrial contraction. The interval between the start of ventricular systole and the opening of the semilunar valves (when ventricular pressures rises sharply) is known as isovolumetric contraction because ventricular volume is constant during this period.
Ejection (R–S–T)
The opening of semilunar valves marks onset of the ejection phase which can be divided into two phases – rapid and reduced:
Rapid:
- Sharp rise in ventricular and aortic/pulmonary pressure.
- Decrease in ventricular volume.
- Large forward aortic/pulmonary blood flow.
Reduced:
- Decline in ventricular and aortic/pulmonary pressure.
- Reduced forward blood flow to the aorta/pulmonary artery.
- Stored energy in the arterial walls continues to move blood forward.
At the end of ejection a volume of blood which equals the volume of blood ejected in systole remains in the ventricle and is known as the residual volume. Closure of semilunar valves gives rises to the second heart sound.
- If outflow resistance decreases = reduced residual volume
- If outflow resistance increases = increased residual volume
Isovolumetric Relaxation (End of T-Wave to Beginning of P-Wave)
The gap between the closure of the semilunar valves and the opening of the AV valves is called isovolumetric relaxation. There is a large drop in ventricular pressure without a corresponding change in the ventricular volume.
Rapid Filling Phase (End of T-Wave to Beginning of P-Wave)
Most ventricular filling occurs immediately after the AV valves open when blood which has returned to the atria during ventricular systole is released into the relaxing ventricles. The atrial and ventricular pressures both drop despite increases in ventricular volume because the relaxing ventricles exert less force on the blood.
Diastasis (Slow Filling Phase)
Blood returning from the peripheries flows into the right ventricle from the right atria and blood from the pulmonary circulation into the left ventricle. Small slow additions to ventricular filling are indicated by gradual increases in atrial, ventricular and venous pressures.
Cardiac output
Cardiac output is defined as the volume of blood ejected from the heart in one minute and is determined by heart rate and stroke volume (amount of blood ejected from the ventricle per beat/cardiac cycle).
Heart Rate
When the heart rate is normal for the individual’s age the following applies:
- Systolic component = 30% of each cardiac cycle.
- Diastolic component = 70% of each cardiac cycle.
If the heart rate increases, the systolic component remains relatively static but the diastolic component is reduced. This has two significant consequences. First, the period for ventricular filling is reduced, and second, myocardial oxygen supply decreases because the coronary arteries are filled and the myocardium is perfused during diastole. A persistent tachycardia may result in reduced cardiac output because of suboptimal myocardial performance, while in infants and children in particular bradycardia will reduce cardiac output because the stroke volume is relatively limited by the size of the heart and the fewer contractile elements in the immature heart. To maintain cardiac output, therefore, infants and young children need to increase their heart rate.
Factors affecting heart rate include:
- Chemicals such as adrenaline and noradrenaline.
- Electrolytes – mainly K+, Na+, Ca++ and magnesium.
- Age and gender.
- Body temperature – hyperthermia gives rise to tachycardia, hypothermia to bradycardia.
- Emotions.
In addition, there are a number of baroreceptors located in the carotid sinus, the aortic arch and the right atrium which work with the autonomic nervous system (ANS) to regulate the heart rate. Structures of the ANS include the cardioacceleratory and cardioinhibitory centres in the medulla, the vagal nerve and the SA and AV nodes.
Stroke Volume
This is determined by preload, afterload, contractility and compliance.
Preload – Key Points
This is the distending force or stretch exerted on the myocardial muscle fibre just prior to electrical stimulation and ventricular contraction. The force of myocardial muscle contraction is directly related to the initial length of the muscle fibres.
Preload determines the force and efficiency of ventricular contraction because it:
- Regulates the resting sarcomeres’ length.
- Determines the number of actin–myosin cross bridges formed during systole.
Preload is dependent on venous return. A decrease in venous return will cause a decrease in cardiac output. The relationship between preload and its effects on stroke volume is described by Starling’s law of the heart. The greater the diastolic volume or fibre stretch at end diastole, the greater the force of the next contraction during systole. However, once maximum stretch is achieved any further volume loading will reduce rather than increase stroke volume.
Afterload – Key Points
The amount of resistance to ventricular ejection and stroke volume is inversely proportional to afterload, therefore the greater the afterload the smaller the stroke volume. Influences on afterload include:
- Ventricular size.
- Ventricular shape.
- Aortic/pulmonary artery impedance.
- Systemic and/or pulmonary vascular resistance.
Compliance – Key Points
This is defined in terms of the relationship between end diastolic pressure (EDP) and end diastolic volume (EDV) and is the ability of the ventricle to relax and fill during diastole. In a normal mature heart compliance is high, allowing it to accept large increases in EDV without a significant increase in ventricular pressure. At birth infants have a greater proportion of non-contractile myocardial fibres, consequently their ventricular compliance is less. Postoperative myocardial wall oedema or either unilateral or bilateral ventricular hypertrophy will reduce compliance and will manifest as reduced diastolic function.
Considerations for Practice
- Volume loading an infant who is not hypovolaemic will reduce cardiac performance because the immature ventricle cannot tolerate increased EDV without significantly increasing EDP.
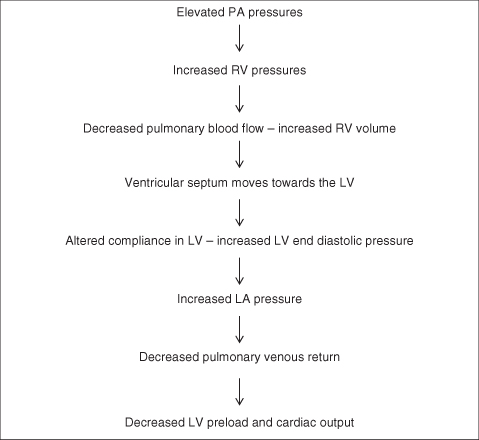
- Right ventricular volume overload will displace the ventricular septum towards the left ventricle. This will inhibit the filling ability of the left ventricle, leading to obstruction of the left ventricular outflow tract and therefore reduce cardiac output.
Contractility – Key Points
This is the ability of the heart to modify its contractile performance independently of fibre length and therefore independent of volume loading. Sympathetic stimulation increases contractility as a result of the direct release of noradrenaline into the cardiac tissues (from intra-cardiac stores).
Other influences on contractility independent of fibre length are K+, Na+, Ca++, hypoxia and acidosis.
Neonatal hearts function at near-capacity with minimal reserves as a consequence of both structural and functional immaturity, the key points of which are:
- Myocardial cells in infants are smaller and have more non-contractile elements.
- The degree and velocity of fibre shortening are less in the newborn heart, therefore less force is generated.
- Increased afterload decreases functional capacity far quicker in neonates than in the mature heart.
- Newborn myocardium lacks complete development of sympathetic innervation but parasympathetic innervation is complete at birth.
- Cardiac stores of noradrenaline in the ventricular myocardium are low, therefore there is a limited response in the neonate to alter contractility.
Cardiac Assessment
A thorough cardiovascular assessment is essential to ensure the child’s condition and any changes to that condition are quickly identified. It is therefore vital to have a good knowledge of normal cardiovascular parameters and the ability to link any variants with underlying cardiac pathophysiology.
- At birth the heart is classed as transverse and is large in proportion to the diameter of the thoracic cavity.
- In the infant, the heart is 1/130 of the total body weight, whereas in the adult it is 1/300.
- While myocardial performance reaches maturity around the age of 8 years, the heart continues to develop in terms of size and shape up to the age of 25.
- The size of the heart is normally roughly that of the individual’s closed fist.
- In adulthood the heart’s shape tends to resemble the shape of the chest – in tall thin individuals it is often elongated, in short stocky individuals it is transverse.
Practitioners also need to be aware of the maturational changes in cardiovascular performance.
Some key points to consider:
- Ventricular size – at birth both the right and left ventricle are similar in size. Over the next few months, as pulmonary vascular resistance decreases and systemic vascular resistance increases, the left ventricular (LV) wall thickens while the right ventricular (RV) wall remains the same size (Blackburn 2007). This change in size and power of the ventricles is reflected on a 12-lead ECG which demonstrates RV dominance up to around 4 months and then subsequently LV dominance. ECGs that demonstrate RV dominance in older infants and children merit further investigation. While the changes in PVR are usually complete by the age of 3–4 months the reciprocal changes in SVR occur throughout childhood. SVR is low in utero and rises immediately after birth, continuing to increase until adulthood is reached.
- Stroke volume – infants have a small stroke volume of about 1.5ml/kg at birth and this will increase as the ventricular size develops. In infants the stroke volume is also relatively fixed; to increase cardiac output their heart rate will need to increase and tachycardia in infants always reflects some degree of cardiovascular compromise. By the age of 2 years myocardial function is similar to that of an adult, although their reserves remain limited in comparison.
- Circulating volume – an infant’s circulating blood volume is approximately 80–90ml/kg which is higher per kg body weight than that of an adult. However, the much smaller actual volumes make the infant more vulnerable to the changes in fluid distribution associated with blood loss from trauma or surgery or the increased capillary permeability associated with sepsis, for example.
Physical Inspection
As time allows, a detailed history should be taken of prenatal, perinatal and family health, feeding patterns, fatigue and psychosocial history. Where appropriate, in an awake child the physical assessment should employ developmentally appropriate techniques to engage or distract the child as distress will affect the values gained from the examination. In the PICU the effects of critical illness such as the response to fever or the hormonal/neural compensatory mechanisms in the shock process need to be taken into account. During the physical examination, although focusing on cardiovascular assessment, other systems should not be ignored and any scars from previous surgery, respiratory distress or hepatomegaly in particular should be noted.
Physical assessment should begin with general observation of the child, paying attention to any dysmorphic or unusual features which may suggest a chromosomal abnormality. A number of chromosomal abnormalities such as Trisomy 21 (Down’s syndrome), DiGeorge syndrome (micro-deletion of 22q11.2) or Marfan syndrome are associated with a higher incidence of cardiac defects (Park 2010).
Assessment of the child’s state of growth and nutrition is a key part of cardiovascular assessment. Infants with a cardiac problem often present with or have clinical signs of tachypnoea and reduced cardiac output, indicated by increased respiratory effort and tachycardia, which mean they will tire easily during feeding and have difficulty coordinating their breathing with feeding and swallowing. This inability to maintain adequate calorific intake can result in faltering growth (Nydegger and Bines 2006; Steltzer et al. 2005).
Signs of pallor, mottling, diaphoresis (excessive sweats where the skin is cool to touch, thought to be triggered by continued stimulation of the sympathetic nervous system) or cyanosis should be noted. The presence and severity of clubbing of the fingers and toes, which is indicative of chronic hypoxaemia, may be observed in any child with a long-standing arterial desaturation (usually of more than 6 months). Clubbing is bulbous enlargement of soft parts of the terminal phalanges with both transverse and longitudinal curving of the nails. This occurs due to interstitial oedema and dilation of the arterioles and capillaries. The exact mechanism for the development of clubbing is uncertain but it does appear to be a consequence of hypoxia.
Palpation should be undertaken by an experienced practitioner or under the direct supervision of one. A hyperactive precordium is a clinical finding in cardiac conditions where there is high volume overload (e.g. left-to-right shunts or severe valvular regurgitation).
Auscultation
The cardiovascular examination itself often begins with auscultation of the apex beat and of the heart sounds. In the newborn the apex beat may be auscultated with a stethoscope placed over the 4th–5th intercostal space in the mid-clavicular line. Experienced practitioners may choose to auscultate and listen for any deviation from the normal heart sounds heard as a result of the closure of the cardiac valves. Murmurs are caused by turbulent blood flow through abnormal connections or an obstruction to flow. Approximately 30% of children with murmurs have structurally normal hearts; these murmurs are referred to as innocent or ejection murmurs. Murmurs are graded 1–6 according to how loud they are and if there is a thrill present (Table 5.2).
Table 5.2 Grading of cardiac murmurs
Source: adapted from Park 2010.
| Grade | |
| 1 | Barely audible with auscultation |
| 2 | Soft sound but easily audible with auscultation |
| 3 | Moderately loud but not accompanied by a thrill |
| 4 | Loud and associated with a thrill |
| 5 | Audible with the stethoscope barely on the chest |
| 6 | Audible with the stethoscope off the chest |
A thrill is a palpable murmur and is always abnormal. The location of the thrill can be indicative of certain conditions, for example, a thrill in the upper left sternal border may indicate pulmonary stenosis. Fever or anxiety will cause tachycardia and this should be considered when evaluating if the rate is appropriate for the child’s age. A pulse which is faster on inspiration and slower on expiration (sinus arrhythmia) is also common in children and not an indication of cardiovascular compromise.
Palpation
Palpation of the major pulses will enable the practitioner to assess cardiac output through the volume of the pulse and perfusion. The brachial pulse is usually the first choice to palpate in infants and the radial pulse in older children. The pulse should be palpated for 1 minute using the second and third fingers, taking care not to apply excess pressure which may occlude the artery. The carotid, femoral, dorsalis pedis and posterior tibia should also be assessed, noting the volume, which may vary from bounding to diminished or absent, as well as the rate and regularity. The pulses should be compared in the upper and lower extremities and from right to left.
The infant/child should be examined for any signs of oedema, including the presence of hepatomegaly, which is symptomatic of cardiac failure. The oedema may be central, generalised, gravitationally dependent or restricted to the peripheries. In infants who are predominantly in the supine position oedema may be periorbital. In addition, it may be worse at certain times of the day and may indent and leave the imprint of fingers if touched (pitting oedema).
A central capillary refill time is a good reflection of perfusion and can be assessed by pressing a finger on the sternum for 5 seconds, releasing and then timing how long it takes for the blanched area of skin to return to normal colour.
Comparing core and peripheral temperatures will generate similar data providing the child has not been in a cold environment. These can either be measured with a temperature probe in the nasopharynx and one attached to the child’s foot, or simply by feeling the child’s head and feet and comparing the difference. This is not a reliable measure and should be used with caution, in conjunction with other components of the assessment.
Blood Pressure Assessment
Non-invasive blood pressure (NIBP) is usually measured from one of the child’s arms, but the NIBP in all four limbs should be checked at the initial assessment, especially in newborns, as four limb blood pressure recordings are used as one of the diagnostic criteria for coarctation of the aorta. Ideally, the child should be sitting quietly with the midpoint of their arm at the level of their heart but this is clearly dependent not only on the age of the child but also their clinical condition. The cuff should cover two-thirds of the length of the upper arm and the bladder within the cuff should encircle 80–100% of the child’s arm.
Additional Assessment Points
Urine output can be a good indicator of renal perfusion, which in turn reflects the adequacy or otherwise of cardiac output. Normal urine output values are age-dependent, and cumulative values of less than the anticipated volume based on the expected norms may indicate inadequate cardiac output and/or renal dysfunction. It is common practice to calculate the expected urine output over a 4-hour period and then match this to actual output:
- Newborn and infants up to 1 year: 1.5–2 ml/kg/hr.
- Toddler: 1.5 ml/kg/hr.
- Older child: 1 ml/kg/hr during adolescence.
- Adult: 1ml/min.
If the child has central venous access, a central venous pressure (CVP) can be transduced and this value will reflect right ventricular preload and right ventricular function, which are important determinants of cardiac output. Similarly, if the child has pulmonary artery or left atrial lines in situ, information can be gained about right ventricular afterload, left ventricular preload and left ventricular function.
The child’s respiratory condition should be noted as cardiac function and respiratory function are strongly linked. Any compromise in cardiac output will result in an increased respiratory rate to in an attempt to maintain oxygen delivery to the tissues. Left ventricular failure and the associated high pulmonary venous pressures will increase pulmonary interstitial fluid causing the lungs to be less compliant, resulting in increased respiratory effort and rate.
Congenital Heart Disease
Congenital heart disease occurs in 5–8/1000 live births and is the most common congenital condition in newborns (Billet et al. 2008; Knowles et al. 2005). A chromosome abnormality is present in 5–8% of babies with congenital heart disease (www.ipch.org/DiseaseHealthInfo/HealthLibrary). It has been suggested that 40–50% of children with Trisomy 21 have a congenital heart lesion and children with DiGeorge syndrome have a higher incidence of outflow tract anomalies such as tetralogy of Fallot and interrupted aortic arch. Approximately 50% of children with an interrupted aortic arch will have DiGeorge syndrome (www.emedicine.medscape.com/article/896979-overview) and the presence of a heart lesion should prompt genetic screening as this can be significant in the child’s initial management (e.g. the need for irradiated blood products) as well as ongoing care. Other influencing factors clearly exist as only one of monozygotic twins may have a congenital heart lesion.
As children with congenital heart lesions reach adult age and go on to become parents themselves it is becoming evident that there is a higher incidence of congenital heart disease in children of parents who themselves have a congenital heart lesion. The incidence is slightly higher in children born to mothers with a congenital heart lesion at 2.5–18%, than children born to fathers with a congenital heart lesion, at 1.5–3% (Park 2010).
There are also established links between maternal health and the incidence of congenital heart disease, with a higher incidence associated with maternal rubella, diabetes and phenylketonuria. Children born to mothers who have rubella during the first 8 weeks of pregnancy have a higher incidence of pulmonary stenosis and maternal diabetes causes an increased incidence of between 10 and 20% for lesions such as a ventricular septal defect (VSD) and transposition of the great arteries (TGA), as well as cardiomyopathy (Park 2010). Some anti-epileptic medications, smoking, alcohol and illegal drugs are also known to adversely affect foetal cardiac development.
Diagnosis of Congenital Heart Disease
With advances in foetal screening programmes, the antenatal diagnosis of congenital heart disease is improving, although it only remains around 50% of the total number of infants receiving a diagnosis and requiring intervention in the first year of life (Central Cardiac Audit Database 2010).
For infants who have not received an antenatal diagnosis, postnatal diagnosis is based primarily on clinical presentation, chest X-ray (CXR) and echocardiography (ECHO). Advances in the technical quality of ECHO machines (e.g. colour flow mapping and Doppler echocardiography) have made accurate diagnosis, and therefore treatment planning, more effective.
Hyperoxia Test
The hyperoxia or nitrogen washout test is helpful in trying to distinguish between cardiac and respiratory causes of cyanosis in the newborn when there is no access to other diagnostic tools such as ECHO. It works on the assumption that if there is right-to-left shunting, as in cyanotic heart disease, no amount of oxygenation in the pulmonary circulation will alter the desaturating effect of the shunt. However, if there is a pulmonary defect causing cyanosis, this may be corrected by increasing the inspired oxygen. The test is carried out by placing the infant in 100% oxygen for 10 minutes. If the infant remains cyanotic after this period, the cyanosis is said to be secondary to cyanotic heart disease. This test is not a guarantee of diagnosis and there are exceptions – severe respiratory disease may result in persistent cyanosis even in 100% inspired oxygen. Furthermore, the test is not without risk as placing an infant with a duct-dependent lesion in 100% oxygen may cause a degree of ductal closure and this may be harmful to the infant.
Pulse Oximetry
The use of pulse oximetry as a diagnostic tool is currently under evaluation in a trial in UK maternity units. Results of the completed trial are expected in 2012 but preliminary results suggest that it may be a useful routine additional tool in newborn assessment; however, this is also related to the increased detection of diseases other than congenital heart disease and is therefore not advocated as a single assessment tool (Ewer et al. 2011).
Sequential Segmental Cardiac Analysis
Segmental cardiac analysis describes cardiac anatomy by identifying how the various structures relate to each other and the characteristics of those relationships. The technique was first used by Shinebourne et al. (1976) and has since been refined, notably by Anderson and colleagues (1984) and Anderson and Girish (2009).
The heart is described in a sequential manner focusing on:
- The arrangement of the atrial chambers.
- The junction between the atria and the attached ventricle.
- The atrioventricular (AV) valve.
- The ventricular topology.
- The ventricular–arterial junction and the morphology of the valves.
- The arterial relations.
Finally, a description of any abnormalities can be made. The terms used in sequential analysis are detailed in Table 5.3.
Table 5.3 Terms used in sequential analysis
| Term | Meaning |
| Morphology | Structure of an organism. |
| Situs | Place or position: Solitus – in the usual or normal position. Inversus – opposite to usual position. |
| Ambiguous | Not clearly related to one side or the other. |
| Inversus | Mirror image. |
| Concordance | In harmony or agreement. |
| Discordance | Not in agreement, disharmony. |
| Dextrocardia | Heart is right-sided. |
| Levocardia | Heart is left-sided. |
| Mesocardia | Heart is in the midline position. |
Atrial Morphology
The right and left atria are structurally different and it is these morphological variants that determine which chamber is a morphological right atria and which is a morphological left atria rather than their position on the right or left side of the heart. The main difference is the size and shape of the atrial appendage: the right atrial appendage is large, has a wide connection to the atria and is a broad-based triangle in shape; in contrast the left atrial appendage is small and tubular, with a narrow connection to the atria. In situs solitus the morphological right atrium is to the right of the heart and the morphological left atrium is to the left. Alternatively, the atria may be transposed into a mirror image of the situs solitus arrangement with the morphological right atrium on the left of the heart and the morphological left atrium on the right of the heart. Finally, the child may have right atrial isomerism where both atria are morphological right atria, or left atrial isomerism where both atria are morphological left atria.
Atrioventricular (AV) Connections
There are three possible variables when describing AV connections.
Each atria connects to an underlying ventricle: This is the most common type of AV relationship and will result in a right-sided and a left-sided AV connection. If a morphological right atrium connects to a morphological right ventricle and a morphological left atrium connects to a morphological left ventricle, then this is described as AV concordance. This can be true with either a situs solitus or a mirror image atrial arrangement.
If a morphological right atrium connects with a morphological left ventricle and vice versa, then this is described as AV discordance. In solitude this statement does not clarify whether it is the atrial or the ventricular arrangement which is abnormal and further explanation of the ventricular topology is required. Description of ventricular topology is also required if the child has atrial isomerism.
The AV valve may or may not be normal but this does not affect the description of the AV connection.
There is a univentricular AV connection: The right- and left-sided atrial chambers may be connected to the same ventricle; this is known as a double-inlet ventricle. There may be two distinct AV valves or one common one but, as before, this does not affect the description of the connection.
Either the right- or the left-sided AV connection is absent: There is an absence of any potential communication between the atrial chamber and the underlying ventricle. This is known as an absent atrioventricular connection and is distinguished from an atretic AV valve.
The Atrioventricular (AV) Valve
Only if there are completely separate right- and left-sided AV connections with completely separate right- and left-sided AV valves should the terms tricuspid and mitral valve be used. Then the valve associated with the right ventricle can be referred to as the tricuspid valve and the valve associated with the left ventricle as the mitral valve.
Valves may be patent, imperforate, straddling, overriding or one common structure instead of two separate structures.
Either the right- or left-sided AV valve may be imperforate, which means that the valve structure is present but not patent, or it may be that the valve leaflets are fused, but the important factor is that there is the potential for communication between the atria and the ventricles. Straddling and overriding valves are also structurally different. A straddling valve is defined as one in which the papillary muscles and chordae tendineae (the tension apparatus) arise from the ventricular myocardium on both sides of the septum, whereas with an overriding valve it is the annulus which is situated over both sides of the septum. A common valve is one that controls both right and left AV connections and it may straddle and/or override.
Ventricular Topology
There are three main components to the structure of each ventricle: the inlet, which extends from the AV junction to the distal tension apparatus; the apical trabecular component; and the outlet. The most obvious structural difference is seen in the apical trabeculations which are coarse within the right ventricle but fine within the left. Additionally, within the morphological right ventricle the AV valve has chordal attachments to the septum, whereas within the morphological left ventricle the AV valve has no septal attachments.
The relationship between the ventricles can be further defined as being of right or left-handed topology. A structurally normal heart has right-hand ventricular topology and this describes the way in which the palmar surface of the right hand could be placed on the septal surface of the morphological right ventricle with the thumb positioned above the inlet component (tricuspid valve) and the fingers above the outlet component (pulmonary artery) and the wrist covering the apical area. The left hand would then fit in comparable fashion on the left ventricle. In situations of AV discordance the additional description of ventricular topology can clarify whether it is the atria or the ventricles which are abnormally situated.
The Ventricular–Arterial (VA) Junction and the Morphology of the Valve
The connection between the ventricle and the artery that arises from it should be described before comment is made about the presence, absence or integrity of any valve.
Four possible types of VA connection may be found:
- The pulmonary artery arises from a morphological right ventricle and the aorta from a morphological left ventricle. This is described as a concordant VA connection.
- The pulmonary artery arises from a morphological left ventricle and the aorta from a morphological right ventricle. This is described as a discordant VA connection.
- Both arteries arise from the same ventricle. This is described as a double-outlet connection or ventricle.
- There is only one artery arising from a ventricle or overriding both ventricles. This is described as a single outlet and it may be that there is a common arterial trunk (persistent truncus arteriosus) which gives rise to the coronary arteries, the pulmonary arteries and the aorta and has a single truncal valve. Both the vessel and the valve override the right and left ventricles. Alternatively, there may be a pulmonary artery with aortic atresia or an aorta with pulmonary atresia. In both cases there is no potential exit from the opposing ventricle in distinction from there being an imperforate pulmonary or aortic valve.
The valve may be patent or imperforate. There can only be a common valve if there is a single common artery. VA valves cannot be described as straddling as they have no distinct tension apparatus, but they can be described as overriding. This would be when the valve annulus is situated over both sides of the septum and the vessel receives blood from both ventricles.
The Arterial Relations
The positioning of the pulmonary artery and aorta is described in relation to the positioning of the pulmonary and aortic valve. This is a ‘stable base’ from which the vessels should spiral around each other, usually reflecting normal concordant VA connections. If the vessels are in parallel, then the VA connection is usually discordant or double-outlet. The terms used to describe the valve positions are right or left, anterior or posterior and side by side.
The structure of the normal heart can be described as:
- Situs solitus.
- A–V concordance.
- Correct ventricular architecture.
- V–A concordance.
- Posterior aorta.
- Levocardia.
- No malformations/malformations present.
(Abdulla et al. 2004; Carvalho et al. 2005; Craatz et al. 2002).
Categorising Cardiac Lesions
Congenital heart lesions are difficult to classify due to the many complicated variations that are seen even within one diagnostic term, however it is common for them to be classified according to the area of the heart affected and the direction of any shunting of blood caused by the defect. Blood will always flow or shunt from areas of high pressure to areas of low pressure. A defect of the ventricular septum will therefore result in a left-to-right shunt as pressures in the left ventricle are higher than those in the right. To generate a right-to-left shunt and cyanosis there has to be either persistent high pulmonary vascular resistance or a right-sided obstruction or stenosis of the tricuspid valve, the right ventricular outflow tract or the pulmonary artery causing the right-sided pressures to be abnormally high.
Accordingly, broad diagnostic classifications are acyanotic or cyanotic heart lesions. This is determined by whether any shunt is predominantly left to right (acyanotic), with oxygenated blood from the left side of the heart being shunted back to the right side, or right to left (cyanotic), with desaturated blood from the right side of the heart shunting to the left side of the heart and perfusing the systemic circulation. Subdivisions can then be generated according to any changes in pulmonary blood flow, which may be increased, decreased or variable, and any obstructions to outflow. In addition, there are lesions that affect the heart generally, such as cardiomyopathy or congenital arrhythmias or obstructive lesions. Lesions in this group will increase the work of the heart, especially the left ventricle, leading to left ventricular hypertrophy, for coarctation of the aorta or aortic stenosis and right ventricular hypertrophy for pulmonary stenosis. As there are no abnormal connections between systemic and pulmonary circulations there is no shunting of blood.
An understanding of normal intracardiac pressures and saturations, as listed in Table 5.4, helps to clarify the direction of shunting when a cardiac lesion is present.
Table 5.4 Normal intracardiac pressures and saturations
| Chamber/Vessel | Pressure (mmHg) | Saturation (per cent) |
| Right atrium | Mean 2–8 | 65–75 |
| Right ventricle | Systolic 15–20 Diastolic 2–8 | 65–75 |
| Pulmonary artery | Systolic 15–20 Diastolic 5–10 Mean 15 | 65–75 |
| Left atrium | Mean 5–10 | 95–100 |
| Left ventricle* | Systolic 100–140 Diastolic 5–10 | 95–100 |
| Aorta* | Systolic 100–140 Diastolic 60–80 | 95–100 |
* Denotes a value that varies according to age and figures given relate to adult values.
Common Management Strategies
Prostaglandin
Prostaglandin is a potent arterial vasodilator which as well as dilating the ductus arteriosus causes general vasodilation. This may lead to hypotension so close monitoring of the infant’s cardiovascular status is required. Prostaglandin has also been associated with apnoeas and respiratory depression and if they occur, these side-effects may require respiratory support with CPAP or intubation and ventilation.
There are two types of prostaglandin currently used in the United Kingdom: Prostaglandin E1 (Alprostadil) and Prostaglandin E2 (Dinoprostone). Both are detailed in the British National Formulary for Children (2011–12) and use is determined by local policy and practice. Prostaglandin should be given as a continuous IV infusion and the delivery of the drug should not be interrupted. Additional IV access will therefore be required for maintenance fluids and any other IV bolus drugs the child is prescribed.
Modified Blalock–Taussig (BT) Shunt
A modified BT shunt is performed through a thoracotomy incision and is a closed procedure. Synthetic or allograft tissue is used to create a conduit between the subclavian artery and a branch of the pulmonary artery. If the aorta is left-sided, the conduit is usually inserted between the right subclavian artery and the right pulmonary artery. The diameter of the shunt will be determined by the size of the child, the volume of blood required to flow through the shunt and the length of time the shunt needs to stay in place before being upgraded or removed.
Following a modified BT shunt there is the potential of shunt blockage during the immediate postoperative period. A heparin infusion is usually maintained until enteral feeds are established when regular oral aspirin should be commenced to sustain mild anti-coagulation and promote patency of the conduit. Volume boluses may also be required to support the child’s blood pressure and maintain adequate flow through the conduit. The infant’s arterial saturations will usually be in the region of 70–80%. Close monitoring of the infant’s oxygenation is important as poor shunt flow will be indicated by decreasing saturations and an increasing metabolic acidosis. Patency of the conduit can be confirmed by auscultation when a shunt murmur should be heard, or by cardiac ECHO examination.
Glenn Procedure
A Glenn shunt involves removing the distal end of the superior vena cava (SVC) from the right atrium and anastomosing it to the right pulmonary artery. If the right pulmonary artery is still attached to the main and left pulmonary artery, blood from the SVC will perfuse both the right and the left lungs. This is termed a bi-directional Glenn and is the most common type of Glenn surgery. Alternatively, if the right pulmonary artery is resected from the main and left pulmonary artery and the resected end is over-sown, blood from the SVC will only perfuse the right lung. This is a classic Glenn.
For the Glenn shunt to provide effective pulmonary blood flow it is important that the child has low pulmonary vascular resistance as the driving force for pulmonary blood flow is now the SVC pressure. To achieve optimum Glenn flow the child should ideally be extubated within the first few hours postoperatively as spontaneous breathing generates lower pulmonary pressures than positive pressure ventilation. Volume boluses may be required to maintain a high preload, which will result in a high central venous pressure and good systemic venous return from the head and upper body, the source of the pulmonary blood flow. The child will be nursed in a head raised or sitting position to optimise venous return from the head and upper body and maximise flow through the Glenn. A heparin infusion will be commenced in the early postoperative period until enteral feeds are established, when oral aspirin will be commenced to reduce the risk of the shunt clotting. The child’s arterial saturations will usually be in the region of 70–80%. Close monitoring of the child’s oxygenation is important as poor shunt flow will be indicated by decreasing saturations and an increasing metabolic acidosis. Patency of the conduit can be confirmed by auscultation when a shunt murmur should be heard, or by cardiac ECHO examination.
Total Caval Pulmonary Connection (TCPC) (Fontan Procedure)
The original Fontan procedure involved connecting the right atrium to the pulmonary artery and using it as the source of pulmonary blood flow. Complications with right atrial distension, atrial dysrhythmias and compromised Fontan flow have led to modifications to this procedure. Although not technically the same as the original Fontan, the terms TCPC and (modified) Fontan are often used interchangeably. The aim of the surgical procedure is to separate systemic and pulmonary circulation, resulting in a child who is no longer cyanosed but has arterial saturations of at least 90%.
Usually the TCPC or Fontan will be preceded by a Glenn shunt where the SVC is attached to the right pulmonary artery in order to increase pulmonary blood flow. In a TCPC/Fontan the Glenn shunt is left in place and the inferior vena cava (IVC) is also attached to the right pulmonary artery so that all systemic venous return is now directed directly to the pulmonary circuit and the remaining single ventricle is only supporting systemic circulation.
The procedure can be further categorised as an internal or external TCPC/Fontan. An internal TCPC/Fontan involves diverting the IVC flow through a tunnel within the right atrium and then attaching the superior portion of the tunnel to the underside of the right pulmonary artery. An external TCPC/Fontan involves the construction of a conduit from the transected IVC around the outside of the right atrium to the underside of the right pulmonary artery.
TCPCs/Fontans may also be fenestrated. This is when a window or opening is left between the tunnel or conduit and the right atrium. If pulmonary blood flow becomes congested then the TCPC/Fontan circuit will offload via the fenestration to the right atrium. This will mean that the child’s arterial saturations will drop as desaturated blood is again mixing with the systemic flow, but it also means that when pulmonary vascular resistance is high and pulmonary blood flow is reduced systemic output is maintained as the blood that shunts through the fenestration maintains preload of the systemic ventricle. In addition, the avoidance of pulmonary venous congestion reduces the incidence and duration of pleural effusions. If necessary, the fenestration can be closed at cardiac catheter, although in some children it occludes naturally.
Early postoperative extubation is aimed for, as the self-ventilating child has lower pulmonary pressures than when receiving positive pressure ventilation and this will facilitate flow through the TCPC/Fontan circuit. Volume boluses may be required to maintain a high preload, a high central venous pressure and good systemic venous return as it is the systemic venous return that is generating the pressure for pulmonary blood flow. Postoperatively the child will be nursed in a head-raised or sitting position, with their legs elevated to optimise systemic venous return and maximise flow through the TCPC/Fontan. A heparin infusion should be commenced in the early postoperative period until enteral feeds are established when oral aspirin or warfarin will be commenced to reduce the risk of the shunt clotting.
Cardiopulmonary Bypass
Cardiopulmonary bypass is an extracorporeal circuit which is used to maintain circulation and gas exchange during open heart surgery. Open heart surgery refers to any operation where one of the chambers of the heart needs to be surgically opened for the surgery to be performed. In order to achieve this, the blood flow must be diverted away from the operative field to ensure the surgeon has a relatively blood-free and static view. A combination of cardiopulmonary bypass and circulatory arrest may be required.
Closed heart surgery refers to an operation which focuses on the vessels arising from the heart and to complete this surgery the heart does not need to be opened. Examples of closed heart surgical procedures include coarctation repair, ligation of a patent ductus arteriosus, or completion of a Blalock–Taussig shunt.
Cardiopulmonary bypass is established by inserting venous drainage cannula in either the right atrial appendage or the SVC and the IVC. The venous blood is then pumped through an oxygenator where CO2 is removed and oxygenation occurs. The blood will then be pumped through a heat exchanger which is used to maintain the child’s core temperature before being filtered and returned via a cannula in the ascending aorta. The blood that is drained into the bypass circuit does not flow through the child’s heart and lungs. The circuit is responsible for maintaining gas exchange and cardiac output and to achieve this pump flows will normally be 100–120ml/kg/min, but can be up to 200ml/kg/min. It should be acknowledged that the venous drainage cannula will never be 100% efficient at catching all the venous return so there will always be a small residual volume of blood within the child’s heart and lungs.
To maintain patency of the bypass circuit the child’s circulating volume must be anti-coagulated. To achieve this, heparin boluses will be given and the activated clotting time (ACT) will be maintained at around 400 seconds. At the end of the bypass period the heparin will need to be reversed by administering protamine.
In order to provide the surgeon with a motionless operative area the heart needs to be arrested. This is achieved by instilling cold cardioplegia solution into the aortic root so that it perfuses the coronary arteries. Typically, cardioplegia contains large doses of both potassium and magnesium which, combined with hypothermia, induce asystole. Top-up doses are needed approximately every 20 minutes to maintain arrest and the aorta will have been cross-clamped to prevent systemic infiltration of the cardioplegia solution.
The required hypothermia is achieved through a combination of topical cooling of the child with the use of ice packs and core cooling by using the heat exchanger within the bypass circuit to reduce the temperature of the child’s blood. Hypothermia is used to reduce the metabolic rate and decrease the tissue’s oxygen requirement. Deep hypothermia is a core temperature of 18–22°C and is required for long, complex, open heart procedures, however most surgery will be performed under moderate hypothermia (a core temperature of 30–32°C).
As blood cools it naturally becomes more viscous and more likely to clot. To counteract these undesirable effects the child is given additional volume to dilute the circulation and reduce the viscosity. This is referred to as haemodilution and decreases the formation of micro-emboli which may impair renal and/or cerebral blood flow. Haemodilution is not without complications. The priming solution used in the bypass circuit plays an important role in haemodilution given the relatively low circulating blood volume of newborns and infants compared to that of adults. The prime volume may be as much as three times the actual blood volume of the neonate. As a consequence, the effects of haemodilution are markedly enhanced in neonates compared with adults, as evidenced by decreased levels of plasma protein, coagulation factors and haemoglobin. This increases organ oedema, coagulopathy and transfusion requirements. The use of modified ultrafiltration (MUF) as part of the bypass process helps remove inflammatory mediator-rich fluid from the patient and bypass circuit and aims to reduce the impact of some of the effects detailed (Allen et al. 2009).
Postoperative Management of the Child After Open Cardiac Surgery
A child undergoing open cardiac surgery will be exposed to two separate but interdependent processes which can cause significant harm (Table 5.5):
- Cardiopulmonary bypass (CPB).
- Cardioplegia, which is the use of extreme cold and induced hyperkalaemia to preserve the myocardium, which paralyses the muscle fibres and reduces the oxygen/metabolic demands of individual myocytes.
Table 5.5 Potential systemic effects of cardiopulmonary bypass
| Body system | Effects and care considerations |
| Pulmonary |
|
| Cardiovascular |
|
| Renal |
|
| CNS |
The body is cooled by the CPB pump to enable it to withstand no blood flow. The heart is stilled with cardioplegia and the pump is turned off (pump catheters are removed), or the child receives very low flow bypass support during procedure (pump catheters remain in situ). The length of TCA and child’s core temperature determine the possible neurological consequences (Barry et al. 2010). Between 18 and 20 °C Between 40 and 60 minutes of TCA is possible with minimal risk of ongoing deficits, but studies have cited 11–20% of patients suffering postoperative seizures with variable neurological sequelae demonstrated during long-term follow-up (Goldberg et al. 2007; Markowitz et al. 2007; Wypij et al. 2003). |
| Endocrine |
|
| Inflammatory response | In response to the instigation of bypass the body will mount a generalised inflammatory response to the perceived threat. The response is mediated by the release of bradykinin and complement, the effects of which include:
|
These two processes have significant effects on all the major body systems and anticipation of the consequences of the procedure are part of the essential postoperative management of the infant or child.
General Postoperative Management Principles
Timely assessment of the infant or child, identification of problems and prompt intervention improve outcomes. On receiving the infant or child back from theatre, it is essential that the following information is elicited:
- Type of lesion.
- Any previous cardiac surgery.
- Aim of the current procedure – correction, palliation or shunt.
- Post-repair anatomy.
- Bypass statistics (e.g. times/use of MUF).
- Intra-operative problems.
Most postoperative complications will result from primary cardiovascular instability, which will in turn impact on all other systems, but given the possible effects of bypass, consideration should also be given to the other major systems: respiratory, gastrointestinal, haematological, renal and CNS.
Cardiovascular System
This may be related to the heart itself or be a consequence of poor cardiac performance (Table 5.6) and can be attributed to any or all of the following:
- Myocardial dysfunction.
- Dysrhythmias.
- Inadequate tissue perfusion.
Table 5.6 Cardiovascular complications post-cardiac surgery
| Complication | Notes and considerations |
| Myocardial dysfunction | The myocardium is very sensitive and responds adversely to being handled. There is usually a period of relative stability (between 4 and 8 hours postoperatively) after which the effects become apparent. An awareness of pre-operative cardiac function is important. Myocardial dysfunction may be indicated by ECG abnormalities or increasing pressures such as the LAP or RAP rising above 12mmHg or the CVP above 15–18mmHg (unless high CVP is necessary). Interventions to stabilise or improve myocardial function include:
|
| Dysrhythmias | Any number of rhythm disturbances may manifest in the postoperative period depending on which part of the conduction pathway has been disturbed. Management strategies include:
|
| Inadequate tissue perfusion | May be a consequence of inadequate preload caused by fluid volume deficit, or excessive afterload caused by increased SVR, increased PVR, or both. Management strategies include:
|
Pulmonary Hypertension
Pulmonary hypertension is defined as a mean pulmonary artery pressure of >25 mmHg or a systolic pulmonary artery pressure of >35 mmHg. In infants and children with high pre-operative pulmonary blood flow there is a significant risk of postoperative pulmonary hypertension as a consequence of the changes in the muscular (middle layer) of the pulmonary artery in the face of increased and or turbulent pulmonary blood flow or blood flow under relatively high pressure into the pulmonary circuit. Infants and children at particular risk for postoperative pulmonary hypertension can be identified and divided into four broad categories based on their cardiac disease and the mechanisms responsible for pulmonary hypertension:
The mechanisms involved in the triggering of pulmonary hypertension post-cardiac surgery are multifactorial and are not solely dependent on the child’s diagnosis. The effects of cardiopulmonary bypass (Table 5.5),which produces endothelial injury within the pulmonary vasculature and may generate a transient elevation in PVR, are thought to be significant in pulmonary hypertension alongside contributing factors such as impaired nitric oxide production, increased release of endothelin and the inflammatory response to cardiopulmonary bypass, which all increase the possibility of hypoxia-induced increased pulmonary vascular resistance (Taylor and Laussen 2010).
Pathophysiology of Pulmonary Hypertensive Crisis
A pulmonary hypertensive crisis can occur if the PA pressure rises to or above systemic pressures. The pathophysiology of pulmonary hypertension in the postoperative period includes not only the increased PVR and elevated PA pressures but also the physiological consequences of RV pressure overload and ventricular dysfunction (Figure 5.9).
Postoperative Assessment
This may include the provision of a pulmonary artery (PA) line placed at time of surgery, although this will be based on an individual surgeon’s preferences and their use has become less frequent in the last few years. In the absence of a PA line, assessment of pulmonary artery pressures can be undertaken through the use of cardiac ECHO. Clinical signs of pulmonary hypertension in the absence of direct PA pressure monitoring are systemic hypotension, acute desaturations with a corresponding decrease in PaO2 and decreased lung compliance (reduced tidal volumes on the ventilator or difficulty in eliciting chest wall movement with manual hyperinflation in the presence of a patent ETT). The presence of acidosis, hypercapnia and hypoxia may all precipitate an episode of pulmonary hypertension, therefore caution should be exercised when undertaking routine cares such as ETT suctioning.
Management of Pulmonary Hypertension
The primary aim of management strategies is twofold: first, to reduce the PA pressures; and second, to support right ventricular function, preventing a pulmonary hypertensive crisis.
- Maintain effective analgesia and sedation to avoid the infant/child becoming distressed.
- Use of muscle relaxants if clinically unstable.
- Use of inhaled nitric oxide therapy 5–20 ppm to reduce pulmonary vascular resistance (see Chapter 4). The use of inhaled nitric oxide inhibits the production of endothelium-derived relaxing factor (EDRF) and it can be difficult to wean the child from low-dose nitric therapy after a period of time. In these cases, caution should be taken to avoid a rebound increase in pulmonary pressure after discontinuation of therapy. Consideration should be given to use of other pulmonary vasodilators such as sildenafil (or more rarely phenoxybenzamine) to assist with the transition.
- Use of dopamine and milrinone to support RV function.
Pulmonary Hypertensive Crisis
In the event of an acute deterioration first-line actions are:
- Hand-ventilating the infant/child with a FiO2 of 1.0 (plus nitric oxide if already receiving therapy), aiming for a normal to slightly low PaCO2 (vasodilatory effect).
- Bolus of analgesia and sedative agents.
- Bolus dose of muscle relaxants.
- Increase in level of inotropic support.
Respiratory Complications
Common postoperative respiratory complications include:
- Atelectasis.
- Depressed cough reflexes; secretion clearance impaired.
- Pulmonary oedema and impaired gas exchange.
- Pleural effusion.
- Haemothorax, chylothorax, pneumothorax.
The management of these complications is detailed in Chapter 4. Cardiovascular surgery is the most common cause of acquired diaphragmatic palsy secondary to phrenic nerve damage which occurred in between 0.28 and 5.6% of children included in four studies published since 2005 (Talwar et al. 2010). The clinical signs of diaphragmatic palsy include an inability to wean from ventilatory support or dependency on non-invasive support such as nasal CPAP, as well as a CXR finding of a right or left hemi-diaphragm. The management of these infants/children remains a discussion point in the published literature, however surgical plication of the diaphragm is often the suitable treatment, particularly in the infant population, given the importance of the diaphragm in their breathing patterns.
Gastrointestinal Complications
Major complications are unusual post-cardiac surgery in the older and young child, however there is a significant risk of necrotising enterocolitis (NEC) in the neonatal population, both pre- and postoperatively.
Neonates and NEC
NEC is one of the commonest gastrointestinal emergencies in the newborn and although the highest mortality is associated with the most premature infants, there is still a significant associated mortality in the term infant, particularly in infants with cyanotic heart disease or duct-dependent lesions with reduced lower body perfusion (e.g. coarctation of the aorta). The pathogenesis of NEC is uncertain, however there are a number of contributory factors (see Chapter 8 for the management priorities).
Infants and older children care considerations:
- Auscultate for bowel sounds – palpate abdomen.
- Nasogastric tube placed to relieve dilation which is common.
- Monitor feed absorption and tolerance.
Neurological Complications
Potential postoperative complications are often difficult to assess in the first few hours post-surgery due to the use of analgesic/sedative agents and muscle relaxants and some complications such as vocal cord palsy will not manifest until the infant is extubated. A careful, detailed neurological assessment is important to rule out events such as:
- Paraplegia secondary to spinal cord ischaemia (after coarctation of the aorta repair).
- Vocal cord paralysis secondary to ligation of a patent ductus arteriosus or shunt insertions.
- Thromboembolic events secondary to bypass.
- Haemorrhage secondary to systemic anticoagulation.
Haematological Complications
These usually occur as a consequence of the effects of systemic anticoagulation and the extracorporeal bypass circuit. They include:
- Haemolysis resulting from pump trauma.
- Irreversible platelet deactivation – platelets are usually transfused at the end of surgery prior to the child coming off bypass.
- Heparin used for anticoagulation prevents the formation of anti-thrombin III and increases the risk of haemorrhage. Heparin is reversible by the administration of protamine.
Any child with a low cardiac output state (e.g. seen 4–6 hours postoperatively secondary to myocardial dysfunction) is at increased risk of developing disseminated intravascular coagulopathy (DIC).
Infectious Complications
These are relatively rare, but there is always a risk of surgical wound infection secondary to microorganism entry as well as the potential for systemic infection secondary to the presence of multiple invasive monitoring lines and/or a urinary catheter, as well as localised infections (e.g. lower respiratory tract infections).
Acyanotic Heart Lesions
Atrial Septal Defect (ASD)
Anatomy
An ASD results from an error during atrial septation at weeks 4–6 of gestation. The most common type of ASD is a secundum ASD and this occurs if there is inadequate growth of the septum secundum or excessive reabsorption of the septum primum. It results in an opening in the centre of the atrial septum. Failure of the septum primum to meet the endocardial cushion results in an ASD low in the atrial septum, referred to as a primum ASD, and it may be associated with mitral valve anomalies. A defect high in the atria close to the insertion of the SVC is referred to as a sinus venosus ASD and may be associated with anomalous pulmonary venous drainage.
Altered Haemodynamics
Blood will shunt from left to right through the ASD as pressure is higher in the left atrium than the right atrium. The volume of the shunt will be low as the pressure within the atria is low and the pressure gradient between the left and right atria is minimal.
Clinical Presentation
A child with an ASD will typically be asymptomatic in early childhood due to the low volume of the left-to-right shunt. If the defect is undiagnosed, the child may develop signs of tachypnoea, dyspnoea and crepitus on auscultation, all associated with increased pulmonary blood flow. Rarely, signs of right ventricular dysfunction, such as hepatomegaly, may be evident.
On examination a murmur will be heard and chest X-ray may reveal increased pulmonary vascular markings.
Management
ASDs can be closed with a septal occlusion device during a cardiac catheter procedure or, if the defect is large, they may require stitch or patch closure during open heart surgery.
Transient atrial dysrhythmias are potential postoperative complications specific to an ASD.
Ventricular Septal Defect (VSD)
Anatomy
A VSD results from an error during ventricular septation at weeks 4–8 of gestation. The majority of VSDs are membranous (or peri-membranous), which means they are located in the upper portion of the septum. Muscular VSDs are located lower in the ventricular septum.
Altered Haemodynamics
Blood will shunt from left to right through the VSD as pressure is higher in the left ventricle than the right ventricle. The volume of the shunt will depend on the size of the defect and the pressure gradient between the left and right ventricles. However, even a small defect will generate turbulent flow, which will be heard on auscultation. The left-to-right shunt will increase the volume load within the right ventricle and will result in increased pulmonary blood flow. This will be more pronounced with a larger VSD.
Clinical Presentation
If the VSD is small, the child may be relatively asymptomatic. Symptoms will be more prevalent with larger defects or with increased time before diagnosis and will include tachypnoea, dyspnoea and rhonchi or crepitus on auscultation, all associated with increased pulmonary blood flow. These symptoms may result in feeding difficulties and faltering growth.
On examination a murmur will be heard and CXR may reveal increased pulmonary vascular markings.
Management
Small defects may close spontaneously. Larger defects, or those where the child is symptomatic, require stitch or patch closure via a right trans-atrial approach during open heart surgery. Potential postoperative complications include:
- Heart block or bundle branch block which may occur if the VSD was located close to the conduction pathways.
- Pulmonary hypertension may occur if the VSD was large and/or closed late.
Persistent Ductus Arteriosus (PDA)
Anatomy
The ductus arteriosus connects the pulmonary artery and the aorta. It is a normal part of foetal circulation, but should start to close within hours of birth, with full closure achieved within the first 7–10 days of life. The main stimulus for the ductus arteriosus to remain patent in postnatal circulation is hypoxia and as such a persistent ductus arteriosus may be seen in premature babies who have a degree of lung disease. PDA may also occur in conjunction with congenital rubella syndrome, Down’s syndrome or a cyanotic heart lesion.
Altered Haemodynamics
Blood will shunt left to right from the high pressure aorta to the low pressure pulmonary artery. The volume of the shunt and the subsequent increase in pulmonary blood flow will depend on both the diameter and length of the ductus arteriosus and the pressure gradient between the aorta and pulmonary artery. As pulmonary pressures continue to reduce in the initial postnatal period the volume and the significance of the ductal shunt may increase.
Clinical Presentation
If the PDA is small, the child may be relatively asymptomatic. Symptoms will be more prevalent with larger defects and are more significant in premature babies with chronic lung disease. The increased pulmonary blood flow from the left-to-right shunt will result in tachypnoea, dyspnoea and rhonchi or crepitus on auscultation. A widened pulse pressure may also be noted as pressure within the aorta is poorly maintained during diastole as blood ‘runs off’ from the aorta to the low-pressure pulmonary artery.
On examination a murmur will be heard and CXR may reveal increased pulmonary vascular markings.
Management
The first-line approach for closure of a PDA in a premature baby is with the intravenous administration of indomethacin, which inhibits prostaglandin synthesis and therefore promotes ductal constriction and closure.
If medical management is unsuccessful or the child presents beyond the effective time frame, closure may be achieved through use of an occlusion device at cardiac catheter. Large PDAs may be unsuitable for insertion of an occlusion device and need to be ligated during closed heart surgery through a thoracotomy incision. Potential postoperative complications following thoracotomy and ligation of the ductus arteriosus include:
- Bleeding.
- Ligation of the wrong vessel (left pulmonary artery or aorta).
- Laryngeal or phrenic nerve damage.
Atrio-Ventricular Septal Defect (AVSD)
Anatomy
The endocardial cushion is involved in the closure of the atrial septum, the ventricular septum and in the formation of the tricuspid and mitral valves, all between 4–8 weeks of gestation. Failure of correct development of the endocardial cushion can result in abnormalities to the valves and/or the atrial septum and/or the ventricular septum.
An AVSD (also known as an endocardial cushion defect or an atrio-ventricular canal) can be categorised as complete, partial or transitional depending on the components involved. A complete AVSD includes a common AV valve which straddles both the right and left ventricles, an ostium primum ASD and a VSD. A partial AVSD usually involves an ostium primum ASD and a cleft mitral valve and a transitional AVSD has some, but not all, of the components of a complete AVSD.
Altered Haemodynamics
For a child with a complete AVSD the presence of a common, incompetent AV valve will mean that there will always be significant regurgitation from the left ventricle to the left atrium with the associated increase in pressure causing pulmonary venous congestion and a left-to-right atrial shunt. There will be further left-to-right shunting through the ventricular component of the septal defect resulting in volume loading of the right side of the heart and a significant increase in pulmonary blood flow. The pulmonary hypertension that results from both the increased pulmonary blood flow and the pulmonary congestion caused by the high left atrial pressures can cause irreversible damage to the pulmonary vasculature. Early, effective management is therefore crucial.
For a child with a partial AVSD the altered haemodynamics will be determined by the severity of the mitral incompetence. If the mitral regurgitation is only mild, then the increase in pressure within the left atrium will be minimal and the child will present similarly to a child with an isolated ostium primum ASD. If the mitral incompetence is moderate to severe, then the regurgitation and subsequent volume loading of the left atrium will increase the volume of the left-to-right atrial shunt. Additionally, the increased left atrial pressure will impede flow from the pulmonary circulation to the left atrium, resulting in pulmonary venous congestion.
The altered haemodynamics, and hence the symptoms exhibited by a child with a transitional AVSD, will be determined by the size of the left-to-right shunt at ventricular level and the severity of regurgitation through the AV valve.
Clinical Presentation
A child with a complete AVSD will present early in infancy with symptoms of congestive heart failure. These will be related to the pulmonary congestion caused by the increased left atrial pressures and to the increased pulmonary blood flow, which results from the large left-to-right ventricular and atrial shunts.
A child with a partial AVSD may be relatively asymptomatic if the mitral valve is only mildly incompetent. Symptoms will be more obvious if the AV regurgitation is more severe and will reflect pulmonary congestion and congestive heart failure.
On examination a murmur will be heard and CXR may reveal increased pulmonary vascular markings.
Management
Pre-operatively, any congestive heart failure will be managed with diuretics. Captopril may also reduce left ventricular afterload and improve left ventricular function. Complete, partial and transitional AVSDs will require open heart surgery for their repair. The septal defects will be patched and the AV valve will be repaired to make it as efficient as possible. Valve replacements in the young tend to be avoided because of the need for frequent up-sizing as the child grows and the increased operative risks associated with frequent surgeries. Potential postoperative complications include:
- Pulmonary hypertension and pulmonary hypertensive crisis. The management strategies discussed in the generic postoperative cardiac care section should be employed.
- Increased risk of heart block following repair of an AVSD related to any oedema or trauma close to the location of the atrio-ventricular node. This is usually transient and the child may require temporary epicardial pacing until the oedema resolves and normal conduction returns. Permanent trauma to the conduction tissue may necessitate insertion of a permanent pacing system.
Repair of the AV valve will never be fully effective and any residual regurgitation can cause ongoing problems with poor forward flow from the left ventricle and left ventricular failure. The use of drugs to reduce afterload, such as intravenous milrinone and subsequently oral captopril, are useful in optimising left ventricular function and cardiac output.
Coarctation of the Aorta
Anatomy
Coarctation of the aorta refers to a narrowing of the lumen of the aorta and results from abnormalities in aortic arch development at 5–8 weeks of gestation. Most commonly, the narrowing occurs between the origin of the left subclavian artery and the insertion of the ductus arteriosus; this is referred to as a pre-ductal coarctation. Alternatively, the narrowed segment may be at the site of insertion of the ductus arteriosus (a juxta-ductal coarctation), or after the insertion of the ductus arteriosus (a post-ductal coarctation).
There are two main theories relating to the aetiology of coarctation of the aorta: the haemodynamic theory and the ductal tissue theory (reviewed by Raeside 2009).
The haemodynamic theory suggests that the coarctation results from abnormal flow in the aortic arch with blood in the ascending aorta streaming to the head and upper body and blood from the ductus arteriosus flowing into the descending aorta with little flow across the aortic isthmus between the subclavian artery and the ductus arteriosus. Coarctations are associated with lesions such as ventricular septal defects or bicuspid aortic valves and the decreased ascending aortic flow associated with these defects lends weight to the theory that reduced flow across the aortic isthmus contributes to the formation of a coarctation.
The ductal tissue theory suggests that ectopic ductal tissue within the aorta creates a thickened tunica media and intima, which reduces isthmic flow. Flow is then further compromised as additional constriction occurs with ductal closure.
The haemodynamics, signs and symptoms, management and postoperative complications are different for a pre-ductal coarctation compared to a post-ductal or juxta-ductal coarctation. They will therefore be discussed separately.
Pre-Ductal Coarctation of the Aorta
Altered haemodynamics: Oxygenated, high-pressure blood flow from the left ventricle will flow as normal into the ascending aorta and perfuse the head and upper body via the brachiocephalic artery, the left common carotid artery and the left subclavian artery. However, little of this oxygenated, high-pressure flow will cross the coarctation to perfuse the descending aorta and the lower body. Desaturated, low-pressure blood flow from the right ventricle will flow as normal into the pulmonary artery. However, some of this desaturated blood will shunt right to left across the patent ductus arteriosus to the low-pressure, relatively empty descending aorta. This means that the head and upper body are perfused by a high-pressure oxygenated blood flow while the lower body is perfused by a low-pressure desaturated blood flow. When the ductus arteriosus closes, typically at 7–10 days of life, perfusion of the lower body is further compromised.
Clinical Presentation
Typically, the infant with a pre-ductal coarctation of the aorta will present collapsed with a metabolic acidosis at 7–10 days of age as the ductus arteriosus closes and the already present signs and symptoms intensify.
Assessment will reveal a differential cyanosis: normal saturations in both arms but saturations of 65–75% in the lower limbs. Similarly, blood pressure will be higher in the arms than the lower limbs and palpation of pulses will identify that femoral pulses will be weak and delayed compared to brachial or axillary pulses. This reflects that the left ventricle is delivering oxygenated high-pressure blood to the head and upper body but the weaker right ventricle is delivering desaturated blood, via the ductus arteriosus, to the lower body. The difference in blood pressure is referred to as relative hypertension and there needs to be a systolic variance of over 20 mmHg for it to be considered significant.
The low-pressure, desaturated perfusion of the lower body will fail to meet the oxygen requirements of the tissues and the subsequent anaerobic metabolism and production of lactic acid produces a metabolic acidosis. This will become more evident as the duct closes and the infant will become tachypnoeic and tachycardic with poor peripheral perfusion, reduced urine output and a decreased consciousness level as a consequence.
Management
If the infant presents collapsed, then appropriate resuscitation must be provided, although ultimately suspicion of a duct-dependent cardiac lesion and commencement of a prostaglandin infusion are needed to dilate the ductus arteriosus and improve systemic perfusion.
Once the infant has stabilised, systemic perfusion has been optimised and any metabolic acidosis has resolved or reduced as far as is possible, surgery will be required.
Surgery is via a thoracotomy incision and is closed heart procedure. The aorta is clamped on either side of the coarctation, the narrowed segment of aorta is resected and an end-to-end anastomosis performed. Alternatively, a subclavian flap repair may be used, in which the left subclavian artery is isolated and divided distally. The vessel is then opened longitudinally with extension of the incision on to the aorta. The subclavian artery flap is then folded down over the area of aortic narrowing and sutured into place.
Postoperative complications are related to decreased perfusion of the lower body both preoperatively and intra-operatively when the aorta is cross-clamped.
- Spinal cord ischaemia can lead to paralysis, so it is important to assess the infant’s legs for movement and strength as soon as possible in the postoperative period. For this reason, the use of muscle relaxants is usually avoided.
- An ischaemic bowel can lead to poor tolerance of feeds, a distended abdomen and potentially necrotising enterocolitis. Enteral feeds should nevertheless be commenced early in the postoperative period once bowel sounds are heard but regular assessment of their tolerance must occur.
Hypertension can also be a problem postoperatively and this is thought to be related to imbalances in sympathetic activity, increased baroreceptor sensitivity and/or an increase in circulating renin and angiotensin (Gargiulo et al. 2008). The hypertension is usually transient and can be managed with intravenous infusions of a vasodilator, such as sodium nitroprusside.
Post-Ductal and Juxta-Ductal Coarctation of the Aorta
Altered Haemodynamics
When the area of coarctation is at the site of, or distal to, the insertion of the ductus arteriosus, systemic blood ejected from the left ventricle is obstructed from perfusing the lower body by the coarctation, and blood from the right ventricle is unable to perfuse the lower body as the coarctation is distal to the insertion of the ductus arteriosus. As this situation exists from 5–8 weeks gestation, collateral circulation will develop from above the coarctation to below it and systemic perfusion will be via these collateral vessels. Blood flow to the lower body will be at a lower pressure and pulses slightly delayed when compared to the upper body due to the restricted flow through the collateral vessels.
When the infant is born the ductus arteriosus will close as normal at 7–10 days of age, however systemic perfusion is not compromised as in pre-ductal coarctation because of the collateral flow.
Clinical Presentation
Children with a post-ductal or juxta-ductal coarctation will usually present later than those with a pre-ductal coarctation, even into their teenage or adult years. They may have relatively subtle signs such as frequent headaches or epistaxis related to upper body hypertension. Investigation into hypertension may be ongoing and anti-hypertensive therapy may have been commenced prior to diagnosis of a post- or juxta-ductal coarctation. If upper and lower limb blood pressures are taken, a difference in the systolic pressure will be seen and this is indicative of aortic coarctation. Further examination will reveal weak, delayed lower body pulses and the child may have a history of complaining of cold feet. A CXR in an older child may demonstrate rib notching, which is a defect caused by the collateral vessels affecting rib growth. Auscultation reveals a systolic or continuous murmur, usually heard in the left infraclavicular area and under the left scapula. An ejection click may signify an associated bicuspid aortic valve which is present in about 85% of cases. A thrill or hum due to flow in aberrant collateral vessels may be present over the chest or abdominal wall. Occasionally post- or juxta-ductal coarctations will present within the first few weeks or months of life, with signs of left ventricular failure, pulmonary congestion and dyspnoea.
Management
As with the pre-ductal diagnosis, surgery is required to resect the area of coarctation and the method of repair is essentially the same. Potential postoperative complications are usually related to spinal cord ischaemia, as with the preductal repair, or to hypertension. Hypertension in this group may be challenging as it will be long established and therefore prolonged anti-hypertensive therapy may be necessary.
Long-Term Effects of Coarctation of the Aorta
While the majority of infants and older children will have a successful primary repair, long-term follow-up is necessary as there are a number of complications which may manifest in later life. The principal problems are a re-coarctation, aneurysm formation around the site of surgical repair, persistent hypertension and/or the development of premature coronary and cerebrovascular disease.
Aortic Stenosis
Anatomy
Aortic stenosis is a lesion which obstructs outflow of the left ventricle. It can be valvular, supravalvular (above the valve) or subvalvular (below the valve).
The normal aortic valve consists of three cusps and develops between 6 and 9 weeks of gestation. Valvular aortic stenosis is caused by an abnormality of this process which results in a unicuspid or bicuspid valve, or a valve in which the commissures are fused. This results in anything from mild to severe valvular stenosis and an associated left ventricular hypertrophy which is proportional to the degree of the stenosis.
Supravalvular aortic stenosis can be caused by the presence of a fibrous membrane, by an hourglass deformity with a narrow segment of restricted lumen size or by a more extensive section of narrowing. The stenosed area is usually distal to the coronary artery ostia, but occasionally there may be some coronary artery malformation, particularly on the left.
Subvalvular aortic stenosis can be caused by the presence of a fibrous membrane, a narrow fibromuscular ring or a more extensive fibromuscular narrowing of the left ventricular outflow tract (LVOT). Subvalvular aortic stenosis usually develops over a period of time and presentation during infancy is rare. Abnormalities of the aortic and mitral valve can occur as their function becomes restricted by the evolving fibromuscular tissue. Aortic regurgitation increases as the pressure gradient over the LVOT increases and the subsequent aortic regurgitation exacerbates the volume loading of the left ventricle.
Altered Haemodynamics
In severe neonatal aortic stenosis cardiac output is limited and deoxygenated blood will shunt right to left across the ductus arteriosus into the relatively empty, low-pressure aorta. The left ventricle will become volume-loaded and distended as it fails to empty effectively and signs of left ventricular failure and pulmonary congestion will be evident.
Mild aortic stenosis can produce essentially no haemodynamic changes, although increasing aortic incompetence as the degree of stenosis evolves can result in signs of left-sided failure.
Clinical Presentation
Severe aortic stenosis results in reduced cardiac output, poor coronary artery perfusion and myocardial ischaemia. As the duct closes at 7–10 days of age systemic perfusion is compromised and the child will present collapsed with a metabolic acidosis, tachycardia, hypotension, tachypnoea and signs of increased respiratory effort, such as intercostal recession. Peripheral pulses will be poor in all limbs in contrast to a child with a pre-ductal coarctation who will have weaker pulses in their legs compared to their arms.
Mild aortic stenosis may be reasonably well tolerated and is often asymptomatic, although a systolic murmur can be heard on auscultation. Children may have a history of syncope and chest pain on exertion, or report a history of avoiding exercise.
Management
If the infant presents collapsed, then appropriate resuscitation must be provided, although as with any suspicion of a duct-dependent cardiac lesion, commencement of a prostaglandin infusion is needed to dilate the ductus arteriosus and improve systemic perfusion.
The infant/child may require inotropic support to optimise their systemic perfusion and reduce or resolve any metabolic acidosis. A balloon aortic valvuloplasty at cardiac catheter will be performed to dilate the obstructed area, hopefully without creating a significantly regurgitant valve. Occasionally, this will be ineffective or unsuitable and a surgical valvotomy will be required. Ultimately, these children usually require an aortic valve replacement.
The child with mild aortic stenosis diagnosed by the presence of a murmur usually requires no initial management but close follow-up to monitor the progress of the evolving stenosis. Surgery is considered as left ventricular function deteriorates. Complications post-balloon aortic valvuloplasty or a surgical valvotomy include aortic valve regurgitation. Other complications of a balloon valvuloplasty include myocardial perforation or dysrhythmias, but overall the potential risks of balloon valvuloplasty are similar to those of conventional surgery.
Cyanotic Heart Lesions
Tetralogy of Fallot
Anatomy
This defect consists of four coexisting lesions: pulmonary stenosis (usually infundibular), right ventricular hypertrophy, a ventricular septal defect (VSD) and an overriding aorta. These are thought to result from malformation of the pulmonary conus at 4–8 weeks of gestation. The pulmonary conus contributes to the formation of the pulmonary infundibulum and ventricular septation and subsequently affects the positioning of the aorta. The right ventricular hypertrophy is a consequence of the pulmonary stenosis.
Altered Haemodynamics
The altered haemodynamics are essentially determined by the degree of pulmonary stenosis and the size of the VSD. The extent of the pulmonary stenosis can vary from mild and relatively insignificant to pulmonary atresia.
The child with severe pulmonary stenosis or pulmonary atresia will have greatly reduced pulmonary blood flow. Pressure within the right ventricle will be high because of the obstructed right ventricular outflow tract and will result in a right-to-left shunt across the VSD. The aorta is displaced to the right and receives deoxygenated blood from the right ventricle as well as blood from the left ventricle, which will also have lower than normal oxygenation due to the right-to-left shunt across the VSD. Consequently, deoxygenated blood perfuses the systemic circulation resulting in cyanosis.
Blood will also shunt left to right across the ductus arteriosus from the high pressure aorta to the lower pressure pulmonary artery. If the pulmonary stenosis is severe or there is pulmonary atresia, this will constitute the main source of pulmonary blood flow. Children with pulmonary atresia may also develop multiple aorto-pulmonary collateral arteries (MAPCAs) for pulmonary blood flow.
The child with only mild or relatively insignificant pulmonary stenosis will not have the same increase in right ventricular pressure as is seen with severe pulmonary stenosis or pulmonary atresia. Consequently, there may be minimal right-to-left shunting through the VSD and the child may only become cyanosed when crying or on exertion.
The degree of pulmonary stenosis will worsen with time as the pulmonary infundibulum becomes more hypertrophied. A child who was relatively asymptomatic at birth may become progressively more cyanosed as the pulmonary blood flow decreases, the right ventricular pressure increases and the right ventricular hypertrophy develops.
Children with Fallot’s may also experience hypercyanotic spells (commonly referred to as ‘spelling episodes’ or ‘tet spells’) in which there is intermittent worsening of right ventricular outflow tract obstruction (RVOTO) through infundibular spasm. This results in an acute and potentially fatal decrease in pulmonary blood flow. Clinical signs of a hypercyanotic spell are agitation and or increasing distress, tachypnoea, worsening cyanosis and potential syncope.
Clinical Presentation
The infant with severe pulmonary stenosis or pulmonary atresia will present with cyanosis, hypoxia, tachypnoea and metabolic acidosis, all of which will become more evident as the ductus arteriosus constricts. These children will need early definitive surgery or a modified Blalock–Taussig shunt followed by definitive repair.
If the pulmonary stenosis is mild, then the child may initially be relatively asymptomatic. As the infundibular pulmonary stenosis worsens the symptoms will become more evident and the child will become more cyanotic and dyspnoeic, and will demonstrate poor feeding and faltering growth. Clubbing of the fingers and toes, a symptom associated with chronic hypoxia, may also be observed. A murmur will be heard on auscultation.
The right ventricular hypertrophy will be identifiable on CXR as a boot-shaped heart and the lungs will appear oligaemic, reflecting the decreased pulmonary blood flow due to the pulmonary stenosis.
Management of Hypercyanotic Spells
- Oxygen administration to improve cyanosis and as a pulmonary vasodilator.
- Sedation (fentanyl) to reduce the child’s distress and therefore reduce tachypnoea.
- Volume to improve cardiac output.
- Positioning – either the child will squat or can be placed in a knee–chest position. It is thought that this position increases peripheral vascular resistance and so increases pressure in the left side of the heart, reduces the right-to-left shunt at ventricular level and subsequently increases pulmonary blood flow.
- Medications:
- Propranolol – relaxes the infundibular muscle, thus reducing the RVOTO and improving pulmonary blood flow.
- Phenylephrine – increases systemic vascular resistance therefore reduces the right-to-left shunt through increased left ventricular pressure.
- Propranolol – relaxes the infundibular muscle, thus reducing the RVOTO and improving pulmonary blood flow.
If the infant or child is experiencing an increased number of hypercyanotic episodes, urgent referral for surgical repair is usually indicated.
Management
Infants with severe pulmonary stenosis or pulmonary atresia who present during the neonatal period will need early surgical intervention. A decision will be made whether to perform a definitive surgical repair or to insert a modified Blalock–Taussig (BT) shunt.
The modified BT shunt is largely considered to be a lower-risk surgical procedure than the open heart surgery required for a definitive repair. It can be a useful palliative procedure to alleviate symptoms and improve pulmonary blood flow, particularly in children of low birth weight for whom the complications of cardiopulmonary bypass can be significant. If a modified BT shunt is performed, then the child will return for elective removal of the shunt and definitive repair at a later stage.
For children with mild pulmonary stenosis definitive surgical repair is usually planned for 4–6 months of age. This involves open heart surgery, resection of the hypertrophied right ventricular muscular infundibulum, patch enlargement of the right ventricular outflow tract and closure of the VSD.
Potential Postoperative Complications
Following a modified BT shunt the child’s arterial saturations will usually be in the region of 70–80%, for, while the shunt has improved the volume of pulmonary blood flow, the right-to-left shunt at ventricular level, the pulmonary stenosis and the overriding aorta remain. Close monitoring of the child’s oxygenation is important as poor flow through the modified BT shunt will be indicated by decreasing saturations and an increasing metabolic acidosis. Patency of the conduit can be confirmed by auscultation, when a shunt murmur should be heard, or by cardiac ECHO examination.
Following definitive repair of tetralogy of Fallot there is invariably some degree of right ventricular failure. Function can be optimised by using volume boluses to maintain an adequate preload. Much of the surgical repair focuses on the area close to the site of atrio-ventricular node and rhythm disturbances such as junctional ectopic tachycardia (JET) or heart block may occur. Active cooling measures may be required to maintain normothermia or mild hypothermia, thus reducing the risk of tachydysrhythmias. Administration of IV amiodarone may also be useful in the management of episodes of JET. The child will have temporary epicardial pacing wires in situ postoperatively and atrio-ventricular sequential pacing may be required to facilitate conduction and optimise cardiac output.
The poor right ventricular function results in elevated right-sided heart pressures. As a consequence of this pleural effusions are a common postoperative complication. Pleural drains may need to be inserted and may remain in situ for an extended period of time. In addition, the infant/child may develop ascites secondary to venous engorgement due to increased right atrial pressure.
Tricuspid Atresia
Anatomy
The tricuspid valve should form from the endocardial cushion tissue and the myocardium at 4–8 weeks of gestation. Failure can result in an absent or imperforate tricuspid valve and tricuspid atresia. The right ventricle is poorly developed (hypoplastic) as a result of the lack of flow from the right atrium and the pulmonary artery is also relatively hypoplastic for the same reasons. Most children with tricuspid atresia will also have a patent foramen ovale or an ASD and a small VSD.
Altered Haemodynamics
Venous return entering the right atrium is unable to flow through to the right ventricle and the subsequent increase in right atrial pressure will force the foramen ovale to remain open if there is no ASD. Blood will shunt right to left at the atrial level through the foramen ovale or the ASD and then mix with the oxygenated blood in the left atrium before flowing through to the left ventricle. Some of the blood from the left ventricle will flow as normal into the aorta and a small volume will shunt left to right through the VSD and perfuse the pulmonary artery and the lungs. The volume of the ventricular shunt is limited as the small right ventricle and the narrow pulmonary artery produce a high resistance to flow and elevated right ventricular pressures. Pulmonary blood flow will be enhanced by a left-to-right shunt across the ductus arteriosus from the high pressure aorta to the lower pressure pulmonary artery. If the child does not have a VSD, then this left-to-right shunt across the ductus arteriosus will be the sole source of pulmonary blood flow. The child will be cyanosed as the right-to-left atrial shunt will result in some deoxygenated blood from the right side of the heart perfusing the aorta.
Clinical Presentation
Infants with tricuspid atresia usually present within the first few days of life with significant cyanosis, tachypnoea, a metabolic acidosis and a murmur. The cyanosis will be more evident as the ductus arteriosus constricts and pulmonary blood flow is limited. Signs of left ventricular failure, such as poor cardiac output and pulmonary congestion, will be evident because of the volume overloading of the left ventricle. If the foramen ovale or ASD is small and/or restrictive, then the ensuing right atrial engorgement will produce signs of systemic venous congestion. A CXR will have decreased pulmonary vascular markings and show an enlarged heart.
Management
If the infant presents in a collapsed state, appropriate resuscitation must be provided according to current Resuscitation Council guidelines, although ultimately, as with any suspicion of a duct-dependent cardiac lesion, commencement of a prostaglandin infusion is needed to dilate the ductus arteriosus and improve the pulmonary blood flow.
The infant will usually need intubating, ventilating and the support of inotropes to stabilise their condition, optimise pulmonary blood flow and resolve or reduce the metabolic acidosis. A three-stage univentricular heart repair will then be planned. The first of these stages is a modified BT shunt and this will be performed within the first few days of presentation. The modified BT shunt will establish improved and reliable pulmonary blood flow and enable the infant to be discharged home until the second stage of surgery, which is a takedown of the modified BT shunt and insertion of a Glenn shunt. This is usually performed between 6 and 9 months of age. The final stage of the repair, a total caval pulmonary connection (TCPC) or Fontan, will be performed when the child is 2–3 years of age or when clinically indicated.
Potential Postoperative Complications
Specific complications relating to the modified BT shunt, the Glenn or the TCPC/Fontan are discussed earlier in the chapter and are applicable to the postoperative care of children with tricuspid atresia.
Transposition of the Great Arteries (D-TGA)
Anatomy
Between weeks 3 and 4 of gestation the truncus arteriosus should divide into the pulmonary artery and the aorta. Failure of the trunconal ridges to spiral and septate the truncus arteriosus normally can result in displacement of the pulmonary artery so that it arises from the left ventricle and posterior to the aorta which arises from the right ventricle. This is D-TGA and the child will often have a patent foramen ovale, a patent ductus arteriosus and sometimes a VSD.
Altered Haemodynamics
Systemic venous return enters the right atrium and the right ventricle as normal, but is then ejected into the aorta and recirculates around the body, returning to the right atrium. Oxygenated blood in the left atrium enters the left ventricle as normal, but is then ejected into the pulmonary artery and recirculates around the lungs, returning to the left atrium. Initially, right atrial pressures are higher than normal as the right side of the heart is supporting the systemic circulation. This tends to keep the foramen ovale open and there is usually a bi-directional shunt through it. The initial high pulmonary vascular resistance also forces a shunt from the pulmonary artery to the aorta and some oxygenated blood will perfuse the systemic circulation. As the pulmonary vascular resistance falls this shunt changes and becomes predominantly from the aorta to the pulmonary artery. The now increased pulmonary blood flow volume loads the left atrium and the atrial shunt changes from bi-directional to predominantly left to right. It is this left-to-right atrial shunt which is now providing some oxygenated blood for the systemic circulation. If a VSD is present, blood will shunt from right to left at the ventricular level, as systemic pressure is higher than pulmonary pressure. This further increases pulmonary blood flow, increases left atrial volume and pressure, and increases the left-to-right atrial shunt.
Clinical Presentation
The infant with D-TGA and an intact ventricular septum usually presents within the first few hours of birth. As the ductus arteriosus starts to constrict, the volume of the aorta to pulmonary artery shunt is reduced and this subsequently reduces the left atrial volume load and the left-to-right atrial shunt. As this is the only source of systemic oxygenation the reduced left-to-right atrial shunt will result in severe cyanosis, hypoxia, tachypnoea and a metabolic acidosis. Signs of biventricular failure may also be evident as the right ventricle struggles to provide the strength of flow necessary for the systemic circulation and the left ventricle and left atrium are over-distended due to the aorta to pulmonary artery ductal shunt. A murmur may be heard, reflecting the shunt through the patent ductus arteriosus.
If the infant has a D-TGA with a VSD, then symptoms will be similar to those described above although the infant may present slightly later and with less cyanosis as the right-to-left ventricular shunt will maintain volume loading of the left atria and the left-to-right atrial shunt even as the ductus arteriosus starts to constrict.
Management
The primary aim of initial management is to create a communication between the two circulations, which may be achieved initially through the maintenance of a patent ductus arteriosus with an intravenous prostaglandin infusion. Once the infant has been resuscitated and stabilised, an atrial balloon septostomy needs to be performed under cardiac ECHO guidance to establish adequate atrial mixing and sufficient oxygenated systemic flow to reduce or resolve the metabolic acidosis. Intubation, ventilation and inotropic support may also be required to achieve this. The infant will then need an arterial switch procedure which is electively performed at 7–14 days of age, although the infant’s clinical condition will also influence the timing of surgery.
An arterial switch is an open heart procedure in which the aorta and the pulmonary artery are transected above the semilunar valves and repositioned in the anatomically correct position. The coronary arteries must also be detached from the base of the aorta as it arises from the right ventricle and then reinserted into the base of the aorta when it is re-implanted on the left ventricle. This is the technically challenging and delicate part of the procedure. The surgery is electively performed during the first couple of weeks of life as there is concern that beyond this time frame the pulmonary pressures drop significantly and the left ventricle adjusts its pressures accordingly. The left ventricle may then struggle to maintain systemic output when the aorta is attached to it. Similarly, there will be increased problems with pulmonary hypertension postoperatively if the right ventricle has to support the systemic circulation for a prolonged period as it will be generating too high a pressure for the newly attached pulmonary artery.
Potential Postoperative Complications
Infants are at risk of pulmonary hypertension post-arterial switch repair because of the increased pulmonary blood flow in the preoperative period. Other complications may be related to kinking or spasm of the re-implanted coronary arteries and this can compromise flow of oxygenated blood to the myocardium and lead to myocardial ischaemia. This will be evident on the infant’s ECG as ST elevation and is usually associated with signs of poor cardiac output. Use of vasodilators, (e.g. glyceryl trinitrate – GTN), can help to relax the coronaries and improve myocardial blood flow.
Cardiac output may also be compromised postoperatively as the left ventricle, which has adjusted to pumping blood around the low-pressure pulmonary system, now has to increase its effort and generate the pressures required to perfuse the systemic circulation. Vasoactive drugs may be required to support the left ventricle during this period.
Truncus Arteriosus
Anatomy
Between weeks 3 and 4 of gestation the truncus arteriosus should divide into the pulmonary artery and the aorta. Failure of the trunconal ridges to spiral and septate the truncus arteriosus normally can result in persistence of the truncus arteriosus into postnatal life. This common vessel arises from both ventricles, and coronary, pulmonary and systemic vessels arise from it. A VSD is always present and there is a single truncal valve. Truncus arteriosus can be further categorised (I–IV) according to the configuration in which the pulmonary arteries arise from the truncal vessel.
Altered Haemodynamics
Blood from both ventricles is ejected via the truncal valve into the common truncal artery. The volume of blood perfusing either the pulmonary or systemic circulations is determined by the pulmonary and systemic pressures. In the initial post-delivery phase pulmonary pressures are relatively high and systemic pressures relatively low. The systemic circuit will be well perfused at the expense of pulmonary flow and while having an adequate blood pressure the infant’s saturations will be extremely low due to the minimal pulmonary blood flow. Over the next 48 hours pulmonary pressures will gradually fall and pulmonary blood flow will improve, resulting in the child having increased saturations but a lower blood pressure. The challenge for medical care is to balance the infant’s circulation by manipulating pulmonary pressures in order to maintain adequate pulmonary blood flow and reasonable saturations while also having an adequate systemic flow and a reasonable blood pressure. Poor systemic flow and excessive pulmonary flow will result in ‘wet’ lungs and a combined respiratory and metabolic acidosis due to compromised gas exchange and poor tissue perfusion; too much systemic flow and poor pulmonary flow will result in a metabolic acidosis due to hypoxia.
Clinical Presentation
Infants will usually present within the first few days of life with cyanosis. Signs of congestive heart failure will become more pronounced after the first 48 hours as pulmonary pressures start to fall.
Management
The aim of pre-operative management is to balance the flow between the pulmonary and systemic circulation to reduce or minimise any acidosis and mitigate any congestive heart failure.
If a metabolic acidosis, which is the result of excessive pulmonary blood flow and diminished systemic flow, develops, the infant needs to be intubated and ventilated on settings which maintain the PaCO2 at values which are the high side of normal (40–45 mmHg/5.3–6.0 kPa) The effect of this will be to produce pulmonary vasoconstriction, restrict pulmonary blood flow and so increase systemic blood flow. If this is insufficient to balance the circulation, the infant may need to be ventilated using a hypoxic gas mix. This is achieved by infiltrating nitrogen into the inspiratory gases to give a FiO2 < 0.21. Again, the net effect is to cause pulmonary vasoconstriction, restrict pulmonary blood flow and increase systemic blood flow. Vasoactive drugs and/or diuretics may also be needed to manage any congestive heart failure which is present.
Surgical management involves open heart surgery and resection of the pulmonary arteries from the truncal vessel. A valved conduit is then inserted from the right ventricle to the pulmonary arteries. The VSD is also closed.
Potential Postoperative Complications
Postoperative management strategies should be aimed at preventing or minimising pulmonary hypertensive episodes and optimising cardiac output.
Total Anomalous Pulmonary Venous Drainage (TAPVD)
Anatomy
TAPVD results from an abnormality during foetal cardiac development in which the pulmonary veins errantly do not connect to the left atrium. It can be categorised into one of three types:
- Supracardiac – the pulmonary veins connect to the SVC, which then drains into the right atrium.
- Cardiac (intracardiac) – the pulmonary veins connect to the right atrium via the coronary sinus.
- Infracardiac (infradiaphragmatic) – the pulmonary veins connect to the ductus venosus and drain through the liver to the IVC, which in turn connects to the right atrium. There is an increased incidence of obstruction to pulmonary venous drainage in the infracardiac TAPVD as flow through the aberrant pulmonary veins is often restricted as it passes through the muscular diaphragm.
Infants with a TAPVD will always also have a patent foramen ovale or an ASD.
Altered Haemodynamics
Oxygenated blood in the pulmonary veins, which should flow to the left side of the heart, is redirected via the anomalous pulmonary connection to the right side of the heart. It will mix with venous blood either in the SVC or IVC (depending on the anomalous connection) before draining to the right atrium. The resulting volume loading of the right atrium will increase right atrial pressures and force a right-to-left shunt through the ASD or foramen ovale. There will also be an increased volume of flow from the right atrium through to the right ventricle and the pulmonary artery and this will lead to pulmonary hypertensive changes if surgery is not timely.
The infant’s cardiac output is determined by the volume of the right-to-left atrial shunt as this is the only source of filling for the left ventricle.
If the pulmonary venous drainage is obstructed, which most commonly occurs in infracardiac TAPVD but can occur in any category, the infant will present with profound and life-threatening cyanosis. The pulmonary venous obstruction causes pulmonary congestion and increased right-sided pressures. The right-to-left atrial shunt will be predominantly poorly saturated venous blood as there is minimal forward flow of oxygenated blood through the obstructed pulmonary veins. Consequently, systemic oxygenation is massively compromised producing life-threatening hypoxia.
Clinical Presentation
The infant presentation is largely determined by the degree of obstruction to the pulmonary veins (Table 5.7).
Table 5.7 Clinical presentation of TAPVD
| Unobstructed | Obstructed |
| Presentation normally between 3 months and 2 years of age. Clinical signs:
| Presentation normally in first few days of life and most common with infra-cardiac type. Clinical signs:
|
Management
For infants with an obstructed TAPVD who present collapsed and shocked, management aims to improve pulmonary blood flow and drainage from the pulmonary veins to the right side of the heart in order that the right-to-left atrial shunt can then fill the left side of the heart and improve the cardiac output. Strategies such as high rate and tidal volume ventilation are used to keep the PaCO2 on the low side of normal (35 mmHg/4.6 kPa) and the PaO2 as high as is possible to counteract the pulmonary vasoconstriction associated with acidosis. A prostaglandin infusion may help to keep the ductus arteriosus patent and allow some shunting of venous blood from the pulmonary artery to the aorta. While this may help to improve systemic blood flow, the systemic saturations will remain low and the metabolic acidosis associated with poor cardiac output and poor oxygen delivery to the tissues may persist. Vasoactive drugs may be used to manipulate the child’s cardiac output, but ultimately the obstruction to pulmonary drainage cannot be managed medically and early surgical intervention is vital.
If the TAPVD is unobstructed, surgery will be planned as a semi-elective procedure. This involves open heart surgery and resection and re-implantation of the anomalous pulmonary arteries to the left atrium.
Potential Postoperative Complications
Pulmonary hypertension is likely in the postoperative period and preventative measures should be taken to avoid any acute episodes.
Kinking or stenosis of the re-implanted pulmonary veins can cause obstruction to pulmonary drainage and haemodynamic instability related to poor left-sided filling and poor cardiac output, combined with pulmonary congestion. A cardiac ECHO will demonstrate pulmonary venous obstruction and further surgical management may be required.
Hypoplastic Left Heart Syndrome (HLHS)
Anatomy
HLHS refers to a collection of anomalies associated with the development of the left side of the heart. The mitral valve, which should form from the endocardial cushion tissue and the myocardium at 4–8 weeks of gestation, is either absent or atretic. As a consequence of the restricted flow through the valve in utero the left ventricle is poorly developed and unable to support systemic circulation. The aortic valve is narrowed, absent or atretic and the aorta itself is hypoplastic, all due to the restrictive mitral valve. Infants with HLHS will also have an ASD.
Altered Haemodynamics
At birth, oxygenated blood from the lungs returns to the left atrium as normal, however, the abnormalities of the mitral valve, the left ventricle, the aortic valve and the aorta mean that little of this blood flows forward through the left side of the heart and into the aorta. Consequently, left atrial pressures are higher than normal and oxygenated blood shunts left to right through the ASD. This oxygenated blood will then mix with venous blood in the right side of the heart, enter the pulmonary artery and some of it will shunt right to left from the pulmonary artery across the ductus arteriosus to the aorta. The blood that shunts across the ductus arteriosus, combined with the minimal flow from the left ventricle to the aorta, constitutes the infant’s systemic blood flow.
Within the first 48 hours of birth the infant’s pulmonary vascular resistance continues to fall and the ductus arteriosus starts to constrict which affects the haemodynamics of the infant with HLHS. As the pulmonary pressures fall less blood shunts right to left across the ductus arteriosus to the aorta and more blood from the pulmonary artery will flow preferentially into the low-pressure pulmonary circuit. This increase in pulmonary blood flow results in a decrease in systemic blood flow and although the infant’s saturations will be improved they will be poorly perfused, have a poor cardiac output and develop a metabolic acidosis. The challenge of medical management is to balance the infant’s circulation so they have adequate pulmonary blood flow and saturations and an adequate systemic blood flow and blood pressure. To achieve this balanced circulation medical management will aim to manipulate the pulmonary vascular resistance.
Additionally, as the ductus arteriosus constricts, the reduction in systemic blood flow is significant and the compromised cardiac output will lead to collapse, shock and a profound metabolic acidosis.
Clinical Presentation
HLHS is often diagnosed antenatally at a routine scan as the heart does not have four clearly identifiable chambers. In this situation the infant’s management will be structured and planned from the moment of delivery.
If there is no antenatal diagnosis, the infant will usually present soon after birth as the pulmonary pressures fall and the infant’s circulation becomes unbalanced and/or as the ductus arteriosus starts to constrict and the systemic perfusion is significantly compromised. The infant will often present obtunded, cyanosed, pale and poorly perfused with feeble pulses and a poor cardiac output. They may be tachypnoeic with sternal recession or be gasping and at the point of cardio-respiratory arrest.
Management
If there is an antenatal diagnosis, then the infant’s parents will receive support and counselling regarding the treatment options. As the long-term surgical survival rates are relatively low with actuarial survival rate after staged reconstruction being 70% at 5 years, termination of pregnancy or palliative care on delivery will be offered as treatment options alongside surgical repair (Syamasundar Rao 2011). If the parents opt for surgical repair, the infant’s care will be closely managed from the point of delivery. A prostaglandin infusion will be commenced to maintain ductal patency and the infant will preferably be nursed self-ventilating in air. A cardiac ECHO will be performed to confirm diagnosis and also to clarify the size of the ASD and the ductus arteriosus. Close monitoring of the infant’s respiratory status and cardiac output is essential in order that any signs of increased pulmonary flow and poor cardiac output (tachypnoea, increased respiratory effort, increased crepitus on auscultation, cyanosis, poor peripheral perfusion and decreased blood pressure) can be responded to immediately.
If the infant’s circulation becomes unbalanced as the pulmonary pressures fall, then it will be necessary to intubate and ventilate the infant on settings which maintain the PaCO2 at values which are the high side of normal (40–45 mmHg/5.3–6.0 kPa). The effect of this will be to produce pulmonary vasoconstriction, restrict pulmonary blood flow and so increase systemic blood flow. If this is insufficient to balance the circulation, the infant may need to be ventilated using a hypoxic mix (see management of Truncus Arteriosus).
The prostaglandin infusion may need to be increased if the ductus arteriosus is not widely patent and vasoactive drugs may also be needed to improve ventricular function and cardiac output.
If the infant presents obtunded or arrested, prompt resuscitation is vital and survival depends on early suspicion of a duct-dependent cardiac lesion, cardiac ECHO to confirm the diagnosis and manipulation of the child’s circulation. Any profound or prolonged acidosis may result in multi-organ failure and compromise the infant’s survival.
Once stabilised a three-stage surgical repair (the Norwood procedure) is recommended. This aims to establish the right ventricle as the systemic pumping chamber and re-route pulmonary blood flow so that it arises from the SVC and IVC.
The first stage involves open heart surgery and is ideally performed within the first week of life. The ductus arteriosus is ligated and an atrial septectomy is performed to allow unrestricted mixing at atrial level. The distal end of the main pulmonary artery is detached from the pulmonary artery branches and the main pulmonary artery trunk is then used to enlarge the hypoplastic aortic trunk. This means that a mixture of oxygenated and de-oxygenated blood from both ventricles is now ejected into the new ‘aorta’ and perfuses the body. A modified Blalock–Taussig shunt is then inserted to provide pulmonary blood flow.
Alternatively, a hybrid procedure for interim management of HLHS can be performed. The first two parts are usually performed through interventional radiology and consist of:
- Enlargement of the septal defect.
- Stenting of the ductus arteriosus to maintain patency.
The purpose is to enable the right heart to pump blood more easily around the body reducing the stress on the RV. The third part of the hybrid procedure is a PA band, which is usually carried out at the same time in hybrid catheter suites.
The second stage of surgery involves removal of the modified BT shunt and insertion of a Glenn shunt; this is usually performed at 6–9 months of age. The final stage of the repair, a total caval pulmonary connection (TCPC) or Fontan, will be performed when the child is 2– 3 years of age, or when clinically indicated.
Potential Postoperative Complications
Bleeding can be a significant problem in the immediate postoperative period and this is related to the extensive suture lines required to anastomose the pulmonary trunk and the hypoplastic aorta which are then exposed to systemic pressures.
Specific complications relating to the modified BT shunt, the Glenn or the TCPC/Fontan are discussed earlier and are applicable to the postoperative care of children with HLHS. Long-term planning for the child and family should include the potential need for cardiac transplantation as well as the effects on the quality of life for the child and their family.
Extracorporeal Membrane Oxygenation (ECMO)/Extracorporeal Life Support (ECLS)
In the UK, ECMO/ECLS services are provided by a small number of supra-regional centres for all age groups, and as such are a highly specialised service provision. There is an expectation, however, that all paediatric cardiac surgical centres should be able to offer ‘rescue support’ to children who are unable to come off cardiopulmonary bypass.
In addition to providing cardiac support, ECMO may be used in the management of primary respiratory failure (e.g. meconium aspiration in the neonate), while ECLS may be used in the management of septic shock in children who are refractory to conventional therapies.
The standard criteria for ECMO/ECLS are:
- Acute severe, reversible respiratory failure.
- Inability to wean from CPB after correcting anomaly or providing surgical interventions.
- Underlying disease process treatable or reversible.
- High predicted mortality with conventional therapy.
- Child otherwise has good prognosis, neurological and other organ function is intact or recoverable.
Exclusion Criteria for ECMO/ECLS Support
All children:
- Chronic lung disease.
- Severe neurological deficits.
- Malignancy.
- Underlying irreversible disease.
- Mechanical ventilation >10 days.
Neonates:
- Birth weight < 2kg and gestational age < 35 weeks.
ECLS for Cardiac Support
ECLS is not a cure for underlying disease processes but is a mechanical support system which allows time for the damaged heart (and/or lung tissue) to heal while resting them from the damaging effects of mechanical ventilation or vasoactive drugs.
The role of ECLS post-cardiac surgery is to:
- Maintain tissue perfusion.
- Maintain tissue oxygen delivery.
- Prevent cardiac distension.
- Minimise myocardial work.
As well as being used to support children who are unable to come off CPB, ECLS is used to support children who have had witnessed cardiac arrests or those with cardiomyopathy awaiting cardiac transplant (Extra Corporeal Life Support Organisation 2009). These children are supported on veno-arterial (VA) ECLS.
Advantages of VA ECLS
- Supports both cardiac and pulmonary function.
- Provides rapid patient stabilisation.
- Safer during haemodynamic deterioration.
Disadvantages of VA ECLS
- Potential for arterial emboli.
- Low or non-pulsatile flow.
- Lower myocardial oxygen delivery.
- Possible carotid ligation.
Cardiovascular Considerations
Cardiac performance on ECLS can be optimised by maintaining a higher preload and a lower afterload status. There will always be some native cardiac output from the patient and to achieve full gas exchange, approximately 80% of the cardiac output must be diverted to the oxygenator. Blood flow through the ECLS circuit is calculated in terms of normal cardiac output and ‘full flow’ is achieved at flows of 100 ml/kg/min (normal cardiac output 120 ml/kg/min).
Children on VA support will have a flattened systolic/diastolic waveform due to the non-pulsatile ECLS pump flow. This becomes more flattened as the level of ECLS support is increased and more blood is drained from the right side of the heart, therefore it is important to observe MAP on VA ECLS. There may on occasion be a more pulsatile waveform present on the arterial line trace, because blood from the bronchial blood network returns to the left ventricle filling the heart.
There should always be an ECG trace on the monitor even on full flows and no ECG means that either the leads are not attached or the child is in asystole.
If the heart is not ejecting or is ejecting poorly because of myocardial stun, the BP is determined only by pump flow and vascular tone. If the heart has the ability to eject, adding volume will increase BP but at the cost of myocardial strain.
The pump is load-sensitive, therefore anything that increases resistance in the circuit will decrease the circuit flow. This includes the patient’s vascular system and a sudden change in flow at the same driving pressure (pump speed in RPMs) should alert practitioners to a change in the system resistance.
Weaning from ECLS
Children should remain on ECLS for the minimum length of time necessary and removal of ECLS should occur when there is evidence of improvement, recovery or a complication that will cause irreversible loss of function. In cardiac surgical centres offering ‘rescue’ ECLS post-cardiotomy there should be a clearly documented plan (discussed with the parents) detailing the number of days of support the child will be offered. Rescue ECLS post-cardiotomy is a short-term support, designed to rest the stunned myocardium only and myocardial function should have recovered within 3–5 days post-surgery.
Weaning generally involves decreasing pump flows over a period of time until ‘idling’ flow has been achieved and ventilatory support and inotropic support are increased as ECLS support is decreased.
Weaning failure is indicated by:
- LAP > 10 mmHg – indicative of poor left ventricular function.
- Adrenaline infusion at > 0.2 mcg/kg/min.
- Ventilation – PIP/PEEP > 30/10.
- Lactate > 4.
If the child fails to wean from support, then ideally a period of 48 hours of rest on full support should be given before another attempt is made. In some children, however, where the fail was borderline and myocardial function has improved significantly, further weaning attempts are often made within 24 hours.
Rhythm Disturbances and Temporary Pacing
Rhythm disturbances can be the primary cause of illness (e.g. SVT, LQT syndrome) or secondary to the illness itself (e.g. cardiac surgery, electrolyte imbalance). There are three main types of significant dysrhythmia/arrhythmia, classified as:
- Bradyarrhythmias.
- Tachyarrhythmias.
- Collapse rhythms.
ECG (Figure 5.10)
ECG review – questions to ask
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree