8 Cardiovascular Disorders
Etiologies of chd: noninherited and genetic factors
Congenital heart disease (CHD) encompasses many structural defects or disorders of the heart that are present at birth; they may be diagnosed in utero, at birth, or later in life. Although the general population risk for any birth defect is 4%,567 the risk of a congenital heart defect is 0.4% to 1%,264 making the heart the most common organ to be affected by a birth anomaly. Approximately 25% of the children with congenital heart disease, as well as 70% of spontaneous abortions and stillborn fetuses with CHD also have at least one extracardiac anomaly.506
In the past quarter century, two population studies, the New England Regional Infant Cardiac Program290 and the Baltimore-Washington Infant Study,263,264 increased understanding of the incidence and etiology of CHD. Recent years have brought the human genome project, advances in molecular biology, and expanded capabilities for chromosomal analysis. Small chromosome deletions and duplications can be identified through fluorescence in situ hybridization (FISH) and, more recently, array comparative genomic hybridization (A-CGH), which opens the door to identification of a greater number of genetic diagnoses. All of these factors combined with the epidemiologic results of the population studies mentioned above are contributing to the identification of genes that cause syndromes as well as isolated congenital heart defects, classification of the pathologic processes of embryology that are triggered by genetic abnormalities, and correlation between genotypes and various phenotypes (the characteristic features).265,657,699 These methods are enabling identification of chromosome anomalies and gene abnormalities that are a result of new or inherited mutations. These and other new developments are leading to better genetic testing, improved diagnostic and prognostic capabilities, future research opportunities, and enhanced care planning and counseling.932
Whether the triggering event is a teratogen, a genetic abnormality, or unknown, the pathogenesis of congenital heart defects follow six mechanisms as proposed by Clark181,182,506,747:
I. Ectomesenchymal tissue migration or neural crest abnormalities, resulting in conotruncal (ventricular outflow) and aortic arch anomalies;
II. Abnormalities of intracardiac blood flow, resulting in hypoplasia of structures through which flow was diminished or absent;
III. Cell death abnormalities, resulting in Ebstein malformation and muscular ventricular septal defects;
IV. Extracellular matrix abnormalities, resulting in anomalous formation of the endocardial cushions (atrioventricular septal and canal defects);
V. Abnormal targeted growth, resulting in anomalies of pulmonary venous and left atrium formation; and
VI. Abnormal situs and looping, resulting in malposition of organs, structures, and vascular connections.
Exploration of anomalies in terms of a common pathogenetic mechanism allows researchers to learn more about familial patterns. As more information about the interaction of genes, environment, and proteins involved in developmental pathways is available, our understanding of the biologic basis of normal and abnormal cardiovascular embryologic development (morphogenesis) will be refined.506 Further information on cardiovascular morphogenesis is provided in the section, Fetal Development of the Heart and Great Vessels.
Although most congenital heart disease was previously believed to be of multifactorial etiology (a combination of a number of genetic factors from both parents, environmental influences, and random events), new information has led to the belief that most human CHDs result from single gene defects506 and some from exposure to teratogens. Nevertheless, at this point, for most patients with CHD there is not a precise identifiable cause.506 Congenital heart defects are thought to be related to teratogens in 2% to 4%, genetics in 10% (although this is probably underestimated), and unknown in 85% to 90%.190,277 As discoveries continue, the percentage with unknown cause should decrease.
Noninherited Risk Factors for CHD (Many Potentially Modifiable)
Maternal Disorders or Biologic Teratogens
Maternal disorders or biologic teratogens associated with increased incidence of CHD include phenylketonuria (PKU), diabetes, infections, obesity, systemic lupus erythematosus, and epilepsy.277 When a mother has untreated PKU during pregnancy, the fetus may have growth and mental retardation and has a 20% to 25% incidence of CHD. Maternal control of blood phenylalanine concentration and adequate maternal nutrition before and throughout the pregnancy must occur to reduce the risk. All babies conceived when the mother was receiving inadequate nutrition should have an echocardiogram.558 The most frequently associated defects are tetralogy of Fallot, ventricular septal defect (VSDs), patent ductus arteriosus (PDA), and single ventricle.414
Maternal pregestational insulin dependent diabetes increases fetal risk of CHD. The most common defects are malformations with laterality or embryologic heart tube looping defects, transposition of the great arteries, conotruncal defects (e.g., tetralogy of Fallot, interrupted aortic arch, truncus arteriosus), VSDs, atrioventricular septal (AV canal) defects, hypoplastic left heart syndrome, outflow tract defects, PDA, and hypertrophic cardiomyopathy (which may resolve).414 Less frequently, gestational diabetes also has been associated with CHDs, and these cases may represent women who have undetected type 2 diabetes. Adequate blood sugar control before and during pregnancy does reduce the risk.414,795
Maternal infections have long been associated with CHD. Maternal rubella has been associated with PDA, pulmonary valve abnormalities, peripheral pulmonary stenosis, and VSDs. Immunization of women against rubella can eliminate this risk. More recently other maternal febrile illnesses (such as influenza) in the first trimester have been associated with a variety of heart defects, but it is unknown whether the fever, infectious agent, or medication to treat the fever and infection cause the effect.190,414 Although HIV infection in utero increases the risk of dilated cardiomyopathy and left ventricular hypertrophy, it has not been associated with structural cardiac defects.414
Maternal obesity before pregnancy has been associated with CHD, but findings are inconsistent. The many complex variables with obesity and nutrition make this a difficult area for drawing conclusions.414 Connective tissue disorders such as systemic lupus erythematosus in women are associated with congenital heart block in infant offspring. Maternal connective tissue disorders have not been associated with structural cardiac malformations.414
Maternal epilepsy is associated with increased incidence of CHD. The therapy of anticonvulsant drugs and their potential impact on folate metabolism—rather than the seizures themselves—may increase the risk.414
Maternal Drug Exposure (Chemical Teratogens)
Of note, use of maternal multivitamins and folic acid in the periconceptual period may reduce the risk of CHDs, but the evidence is not yet conclusive. These supplements may reduce the risk of CHD when used with some other agents that are associated with an increased risk of CHD, for example maternal febrile illness. Further studies are needed.414
Exposure to chemical agents can alter cellular development, and the timing of such exposure can influence the effect on risk. Identification of timing related to fetal vulnerability could help ensure counseling about treatment options to avoid critical exposures for a susceptible embryo.190,277,414 Many therapeutic drugs used preconceptually or during pregnancy have been linked with possible associated risk of congenital heart disease. (The interested reader can find a list on the Evolve Website. Please see Evolve Box 8-1 in the Chapter 8 Supplement on the Evolve Website.) Because much of the evidence is inconclusive and there are always reports from new studies, research and caution is urged before use of any therapeutic drugs during the periconceptual period and pregnancy.
Nontherapeutic drugs used preconceptually and/or during pregnancy that have been associated with increased risk of CHD include: alcohol, cocaine, marijuana, cigarette smoking, and vitamin A in high doses. Again, the evidence is often inconclusive, so actual risks can be difficult to determine. Many other agents have been studied but the data are insufficient to determine risks for cardiac defects.414,754
Environmental Exposures (Chemical, Biologic, and Physical Teratogens) and Influences
Increased risk of a variety of CHDs has been associated with maternal occupational exposure to organic solvents (compositions of solvents can include degreasers, dyes, lacquers, paints, glycol ethers, and mineral oil products); heavy metals; herbicides, pesticides, and rodenticides associated with maternal employment in the agriculture industry; air quality (increased levels of ambient carbon monoxide, ozone, and dioxide); and parental exposure to groundwater contamination with trichloroethylene. There are other environmental exposures, such as hazardous waste sites and occupational exposure to ionized radiation, but no consistent association with CHD has been found.414,866
Evaluation of maternal sociodemographic characteristics has shown that maternal age is not associated with nongenetic CHDs as a group. Some specific defects are more likely with advanced maternal age, and young maternal age is associated with tricuspid atresia.414 Some studies have shown disparity of incidence between white and black infants, with many defects being more prevalent in white infants, whereas pulmonary stenosis is more prevalent in black infants. Other studies have not shown variations in prevalence of birth defects in general among white, black, and Hispanic infants.414 Reproductive problems (miscarriage, stillbirth, or preterm birth) have been associated with increased incidence of tetralogy of Fallot, nonchromosomal atrioventricular canal defects, ASDs, and Ebstein’s anomaly, where the association could represent exposure to teratogens or an inherent susceptibility.414 Maternal stress associated with job loss, divorce, separation, or death of a close relative or friend, especially in mothers who were not high school graduates, was found to be associated with a greater prevalence of conotruncal heart defects.414
Paternal exposures and factors may also play a role in noninherited cardiac defects. Older fathers have been associated with Marfan syndrome. Studies have suggested that increasing paternal age is associated with ASDs, VSDs, PDA, and tetralogy of Fallot, whereas children of men less than 20 years of age were also at higher risk for septal defects.414 Other paternal exposures have been investigated in a limited number of studies, with the suggestion or trend toward increased risk of CHD associated with paternal exposure to marijuana, cocaine, cigarette smoking, and alcohol.414
A unique environmental influence may be present for some monochorionic twins. The smaller twin is more often affected with CHD, which may result from abnormal cord insertion.373
Prevention of some CHDs may be accomplished by following these recommendations for mothers who wish to become pregnant: Take a multivitamin with folic acid daily, obtain prenatal and preconceptual care for management of maternal disorders associated with increased risk of CHD, discuss use of any drugs with the healthcare provider, avoid contact with people who have the flu or other febrile illnesses, avoid exposure to organic solvents,414 and follow any employer guidelines established to avoid exposures that may increase risks.
Genetic Factors Associated with CHD
New findings with molecular genetic studies indicate that the genetic contribution to the etiology of CHD has been underestimated. With the rapid changes in this field it is certain that our understanding will be evolving and the identifiable genetic etiologies of CHD will continue to expand.700 Review of current literature will always be necessary to have an accurate understanding of these factors.
Humans normally have 46 chromosomes (23 pairs). The first 22 pairs are autosomal (non-sex) chromosomes and the 23rd pair determines gender (XX = female, XY = male). Each chromosome has two arms held together by a centromere—a short arm (p) and a long arm (q). There are more than 35,000 pairs of genes on our chromosomes,190 and each gene is composed of hundreds or thousands of base pairs. An abnormality in a single base pair can cause a malfunction. Changes in the deoxyribonucleic acid (DNA) sequence (a mutation) in a single gene changes the path of a protein, which is like changing one part of a recipe. (The results can range from asymptomatic to disastrous malformations.) Mutations may occur de novo (a new mutation that is not inherited from a parent) or may result from autosomal-dominant or -negative inheritance. Many gene mutations associated with cardiovascular malformations are now being identified.190,277,700
Before advanced cytogenetic tests were available, chromosome abnormalities were found in approximately 8% to 13% of neonates with CHD,263 but with new testing the prevalence of chromosome aberrations is now estimated to be much higher.700 These abnormalities can be aneuploidies (abnormal number of chromosomes) such as trisomies (an extra chromosome), or tetrasomies. Other chromosome abnormalities are caused by deletions (a missing piece), duplications (extra genetic material on chromosome), or translocations (genetic material transferred from one chromosome to another).190,277 Single gene defects have been thought to account for about 3% to 5% of those CHDs with a genetic etiology, but new findings indicate that this range substantially underestimated the problem. Any genetic defect can start an embryonic chain reaction, which can create mild to severe phenotypic expression of the abnormality.803
Cardiovascular malformations can occur as a part of a group or pattern of anomalies. A syndrome, a combination of multiple anomalies occurring together resulting from a single cause (the cause is often a genetic error but the cause may be unknown), is thought to cause about 5% of the CHD. More than 400 genetic syndromes list CHD as a possible manifestation.651,803 Associations, a group of anomalies that occur in a recurrent pattern, may also involve CHDs. There may be no known genetic basis for an association. Inherited metabolic diseases have a variety of genetic etiologies and may include cardiovascular problems.
For more information on characteristics and etiologies of specific defects and conditions see Tables 8-1 and 8-2. There are hundreds of syndromes and conditions with multiple manifestations that involve cardiovascular malformations. Table 8-2 includes an abbreviated table, and a more comprehensive table with genetic associations is provided in Evolve Table 8-1 in the Chapter 8 Supplement on the Evolve Website.
Table 8-1 Cardiac Defects and Associated Genetics, Teratogens, and Exposures328,414,700,871,932
| Cardiac Defect | Associated Genetics, Teratogens, and Exposures |
| Any congenital heart defect | Maternal PKU, pregestational diabetes, febrile illness, influenza, rubella, epilepsy, anticonvulsants, NSAIDS/Ibuprofen, sulfasalazine (antiinflammatory), thalidomide, trimethoprim-sulfonamide, and vitamin A congeners/retinoids. Many illnesses, medical and substance exposures, as well as sociodemographic factors have been studied, but there have been insufficient data to determine risks for CHD with these. Somatic mutations (occur after fertilization and therefore only affect some cells or tissues) are hypothesized to be an important cause of isolated CHDs. |
| Aortic atresia | See HLHS |
| Coarctation of the aorta | Turner syndrome. Familial left-sided obstructive heart defects. Deletion of chromosome locus 18p. Duplications in chromosome 4p, 4q, 6q, or 10p. Trisomy 8 or 9. Maternal exposure to organic solvents. |
| Supravalvular aortic stenosis | Williams-Beuren syndrome. Deletion or translocation (rare) in chromosome locus 7q11. Elastin gene mutations. |
| Aortic valve or LV outflow tract obstruction | Deletion of chromosome locus 11q or 10q. Trisomy 13 or 18. Duplications of chromosome locus 1q, 2p, 2q, 6q, or 11q. NOTCH 1 gene mutations. Noonan, Turner, or Jacobsen syndromes. Pregestational diabetes. Maternal vitamin A exposure. |
| Atrial septal abnormalities | Holt Oram, Ellis-van Creveld, Noonan, Rubinstein-Taybi, Kabuki, Williams, Goldenhar, thrombocytopenia-absent radius, Klinefelter, or (rare) Marfan syndrome. Mutations of TBX5 gene on chromosome 12q24.1, NKX2.5 gene on chromosome 5, EVC gene on chromosome 4p16.1, MYH6 gene, or GATA 4 gene. Deletions on chromosome 1, 4, 4p, 5p, 6, 10p, 11,13,17,18, or 22. Trisomy 18 or 21. Pregestational diabetes. Familial ASDs with AV conduction disturbances without extracardiac manifestations (may also have VSD, TOF, and others) has been associated with mutations of NKX2.5 on chromosome 5. Familial ASD without AV conduction disturbances without extracardiac manifestations (may also have VSD and/or PS) has been associated with mutations of GATA 4 gene with variable expression and autosomal dominant inheritance. This condition has also been associated with mutations of NKX2.5 on chromosome 5. |
| Atrioventricular septal abnormalities/atrioventricular canal/endocardial cushion defects | Trisomy 21, 13 or 18. Deletions of chromosome 3p25, 8p2, or 22q. Duplications of chromosome 10q, 11q, 22q. Holt-Oram, Noonan, Smith-Lemli-Opitz, or Ellis-van Creveld syndrome. Mutation of gene on chromosome 1p21-p31. CRELD1 gene mutations. Chondrodysplasias. Pregestational diabetes. Maternal exposure to organic solvents. Familial AVSD (partial or complete) without extracardiac manifestations has been associated with gene locus on 1p21-p31 mutation with autosomal dominant inheritance. |
| Bicuspid aortic valve | Turner syndrome. Familial left-sided obstructive heart defects. Deletion of chromosome locus 10p. Duplications in chromosome 6q. Trisomy 13 or 18. BAV without extracardiac manifestations may be associated with other CHDs (especially CoA) and ascending aortic aneurysm is associated with Notch1 gene mutations with autosomal dominant inheritance. |
| Conotruncal defects (tetralogy of Fallot, truncus arteriosus, interrupted aortic arch and others) | Deletion of chromosome 22q11.2. Mutations of NKX2.5 and 2.6. Pregestational diabetes. Maternal exposure to organic solvents. |
| Double-outlet right ventricle | Trisomy 9, 13, or 18. Duplications on chromosome 2p or 12p. Deletion of 22q11 (rare). |
| Ebstein anomaly | Most cases are sporadic. Chromosome abnormalities are rare. Has been reported with Trisomy 21, abnormalities of 11q with renal malformation, and Pierre Robin sequence. Familial occurrences are rare but are associated with family members with mitral valve abnormalities or with familial atrial conduction problems. Animal studies are suggestive of a genetic connection with genes on chromosome 17q. Maternal marijuana. Maternal exposure to organic solvents. |
| Heterotaxy syndromes with complex CHD—laterality and looping abnormalities | Chromosome locus 2 (CFC1 gene encoding CRYPTIC protein), 6q (HTX3 gene), LEFTY A gene, or X-linked q26.2 or Xq24-47 (ZIC3 gene). Pregestational diabetes. |
| Hypoplastic left heart syndrome | Deletion of chromosome locus 11q (Jacobsen syndrome). Turner or Wolf-Hirschhorn (deletion of 4p) syndromes. Trisomy 13 or 18. Familial left-sided obstructive heart defects. Pregestational diabetes. Maternal exposure to organic solvents. |
| Interrupted aortic arch | Deletion 22q11. |
| Left-sided obstructive heart disease—familial—CoA, aortic atresia/HLHS, BAV | Increased occurrence of these lesions in first-degree relatives. Inheritance patterns may be multifactorial, autosomal dominant with reduced penetrance, or autosomal recessive. |
| Left superior vena cava persistence | 60% have other anomalies, 87% have other CHD, and 42% syndromes or other conditions (VACTERL, Down’s syndrome, CHARGE). |
| Patent ductus arteriosus | Char syndrome. Mutations of TFAP2B. Pregestational diabetes. Indomethacin tocolysis. |
| PA branch stenosis | Alagille, congenital rubella, Ehlers-Danlos, Noonan, Costello, Cardiofaciocutaneous, LEOPARD, or Williams-Beuren syndromes. Deletions in chromosome locus 20p12. JAG1 gene mutation. Pregestational diabetes. Maternal vitamin A exposure. Maternal exposure to organic solvents. |
| Pulmonary Valve Obstruction | Noonan, Alagille, Costello, or LEOPARD syndromes. Mutations of PTPN11, KRAS, SOS1, and HRAS genes. Chromosome deletions of 1p, 8p, 10p, or 22q. Chromosome duplications of 6q, 15q, or 19q. Trisomy 8. Maternal vitamin A exposure. Maternal exposure to organic solvents. Maternal rubella. |
| Tetralogy of Fallot | Deletion of 22q11, 5p or many other chromosomes. Duplication on chromosome 22 and many other chromosomes. Alagille (JAG1 gene), Noonan (PTNP11), Cat-eye, and nearly 50 other syndromes. Trisomy 18 or 21. Partial trisomy 8q. Translocation 1p36. Maternal exposure to organic solvents. Isolated TOF is associated with NKX2.5 mutations. |
| Total anomalous pulmonary venous return | Most cases are sporadic. Trisomy 8. Familial cases have been reported with familial scimitar syndrome and a chromosome 4p13-q12 abnormality with autosomal dominant inheritance and variable expression (a large Utah-Idaho family). Maternal exposure to organic solvents. |
| Transposition of great arteries | Rarely associated with chromosome abnormalities or syndromes. Pregestational diabetes. Maternal exposure to organic solvents. |
| Tricuspid atresia | Most cases are sporadic. Chromosome abnormalities are rare with tricuspid atresia, but deletions of 22q11 and 4p and duplications of chromosome 22 have been reported. Familial occurrences are rare but have been reported. A gene mutation has been associated in mice, which suggests a genetic basis for this disease. |
| Truncus arteriosus | Deletion on chromosome 22q11 or 10p. Trisomy 8. An autosomal recessive form has been mapped to chromosome 8p21. |
| Ventricular septal abnormalities | Holt Oram, Rubinstein-Taybi, Goldenhar, Costello, Williams, Kabuki, Cornelia de Lange, Apert, or Carpenter syndrome. VACTERL association. Familial ASD with or without AV conduction disturbances. TBX5 or GATA 4 mutation. Deletions or duplications of many chromosomes. Trisomy 13, 18, or 21. Pregestational diabetes. Maternal marijuana. Maternal exposure to organic solvents. Septal defects without extracardiac manifestations are associated with mutations in MYH6 and CITED2 genes. |
ADD, Attention deficit disorder; AS, aortic stenosis; ASD, atrial septal defect; AV, atrioventricular; AVC, atrioventricular canal; AVSD, atrioventricular septal defect; BAV, bicuspid aortic valve; CA, coronary artery; CHD, congenital heart defect; CNS, central nervous system; CoA, coarctation of aorta; CV, cardiovascular; DCM, dilated cardiomyopathy; DORV, double-outlet right ventricle; GI, gastrointestinal; GU, genitourinary; HCM, hypertrophic cardiomyopathy; HLHS, hypoplastic left heart syndrome; IAA, interrupted aortic arch; IVC, inferior vena cava; LSVC, persistent left superior vena cava; LV, left ventricular; LVOTO, left ventricular outflow tract obstruction; MV, mitral valve; NSAIDs, nonsteroidal antiinflammatory drugs; PA, pulmonary artery; PAPVR, partial anomalous pulmonary venous return; PAtresia, pulmonary atresia; PDA, patent ductus arteriosus; PKU, phenylketonuria; PPS, peripheral pulmonary stenosis; PS, pulmonary stenosis; RVOTO, right ventricular outflow tract obstruction; SVC, superior vena cava; SVT, supraventricular tachycardia; TAPVR, total anomalous pulmonary venous return; TEF, tracheoesophageal fistula; TGA, transposition of great arteries; TOF, tetralogy of Fallot; VSD, ventricular septal defect.
See Table 8-2 for more information on specific genes, syndromes, and conditions.
Genetic Testing, Counseling, and Nursing Implications
Genetic testing can reveal important genetic patterns that are critical for identifying other important organ system involvement; gaining prognostic information; learning important reproductive risks for the family; and considering the appropriate testing of other family members.700 Genetic testing can be performed on blood lymphocytes, cord blood, skin, amniotic fluid, chorionic villi, and bone marrow. Current genetic tests available to be used in assessing CHD include:
• Standard chromosome analysis—reveals standard karyotype and is used to identify many chromosomal disorders, especially those in which there is an abnormal number of chromosomes.700
• High resolution banding—better defines chromosomal structure abnormalities such as duplications, deletions, and translocations of genetic material.700
• Fluorescence in situ hybridization (FISH)—specific DNA probes can be used to diagnose more subtle structural abnormalities such as microdeletions, tiny duplications, and/or subtle translocations.700
• Array comparative genomic hybridization (A-CGH)—even more sensitive than FISH for testing for tiny structural abnormalities of chromosomes.868
• Gene discovery—cloning techniques are used to identify genes that produce a protein associated with cardiogenic gene mutations.700
• DNA mutation analysis—identifies changes in the coding sequence of a gene to find small deletions, duplications, or substitutions that alter the resulting protein structure. Once a change is found, it must be determined if it constitutes a disease-causing mutation.700
As more etiologic genes are identified, progress can be made in determining mechanisms and interactions that cause various phenotypes. That may lead to the development of targeted therapies for patients and fetuses.871
Genetic counseling is warranted when there is one major or two minor birth defects and may be employed to attempt to determine exact causes of anomalies in individual situations. Testing must be accurately ordered to direct the proper studies to obtain complete results. Simply ordering a karyotype will not achieve the targeted exploration that can be achieved with FISH or A-CGH studies. Information gathered enables practitioners to counsel the family on problems associated with a condition, allowing them to be proactive in their child’s care. Discussions with the family should be informative but not directive so that parents may make their own decisions.190,651
Recurrence risks for chromosomal trisomies are low if there are no other birth defects in the family and maternal age is not advanced. For a parent with an autosomal dominant genetic anomaly, there is a 50% risk of recurrence. In parents who have an autosomal negative genetic anomaly (without the evidence of the problem), both parents must have the anomaly to cause the resulting disorder. In these cases the recurrence risk for the couple is 25%. In X-linked recessive anomalies, the risk is 50% for a male to be affected and 50% for a female to be a carrier. In cases of unknown etiology (possibly multifactorial inheritance), if no other child in the family is affected the recurrence risk is presumed to be 3% to 5%, but this changes if a subsequent sibling is affected.190,651
Information about genetic conditions is widely available via websites and targeted support groups and associations (e.g., Online Mendelian Inheritance in Man, www.ncbi.nlm.nih.gov/Omim/). Web-based databases and support groups that provide valuable information are provided in the Chapter 8 Supplement on the Evolve Website.
The nursing implications of genetics involvement in congenital heart defects are significant. Nurses who are familiar with the patterns of anomalies can have a heightened index of suspicion when a single malformation is noted and findings can be unmasked. Nursing knowledge can be enhanced by familiarity with the most current scientific statements from the American Heart Association Congenital Heart Defects Committee, Council on Cardiovascular Disease in the Young on both the genetic basis and the noninherited risk factors for congenital heart disease.414,700
This quote from Joey’s Journey: Our Life with Lissencephaly, at http://lfurlotte.tripod.com expresses how parents may feel when they begin a journey with a child affected by anomalies and the support others can provide: “Life will never be the same again. However, with a little different perspective, life does go on and happiness does return.”415
Fetal development of the heart and great vessels
Formation of the Heart Tube: Day 22
The cardiovascular system is the first system to function in the embryo. The critical period of cardiovascular growth begins at 15 to 18 days of gestation and is initiated by a period of rapid cell proliferation. During this period, the developing heart is most susceptible to teratogens (factors that can be harmful). Early development of the cardiovascular system is necessary because of the increasing need for nutritional and oxygen requirements by the rapidly growing embryo and for elimination of carbon dioxide and waste products. Moore et al.,627 Ransom and Srivastava,723 and Sander et al.,764 describe heart development as involving five primary steps:
1. Migration of precardiac cells from the primitive streak and assembly of paired cardiac crescents at the myocardial plate
2. Coalescence of the cardiac crescents to form the primitive heart tube, an event that establishes the definitive heart
3. Cardiac looping, a complex process that assures proper alignment of the future cardiac chambers
4. Septation and heart chamber formation
5. Development of the cardiac conduction system and coronary vasculature
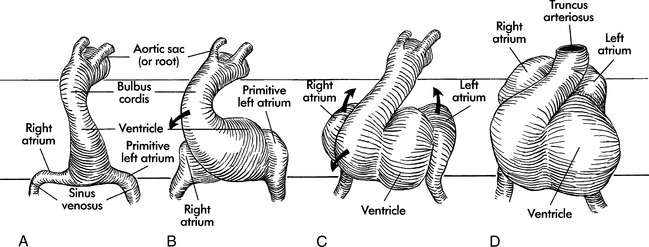
The primordial heart and vascular system appear in the middle of the third week. The cardiovascular system is primarily derived from embryonic mesoderm and neural crest cells. The precardiac stem cells (mesoderm) migrate to form the cardiac crescents that fuse to form the single heart tube at 22 days (Fig. 8-1).627,723,764,902
At this time (22 days), circulation begins with ebb-and-flow blood flow from the venous to arterial poles. Premyocardial cells and neural crest cells continue to migrate into the region of the heart tube. The regulation of the mesoderm is partially controlled by retinoids, isoforms of vitamin A, that bind to specific nuclear receptors and regulate gene transcription and by extracellular matrix proteins, such as fibronectin, that direct cellular migration. The teratogenic effects caused by interaction of retinoid-like drugs on cell receptors are seen clinically (see Noninherited Risk Factors).79
Formation of the Heart Loop: Day 22 to 28
Before and during the looping process, sections of the single heart tube begin to develop specialized cells that will ultimately become the chambers of the mature heart. A cascade of genes is expressed in the anterior (ventricular) and the posterior (atrial) portions of the tube. These genes regulate the cellular processes that transform the heart tube into a four-chambered heart. This transformation occurs through a balance of cell growth, cell differentiation, and cell death (apoptosis). Disruptions in these genetic mechanisms and specific cellular signaling processes result in cardiac malformations seen in congenital heart disease. Problems include defects in cardiac looping, septation, and chamber formation.764
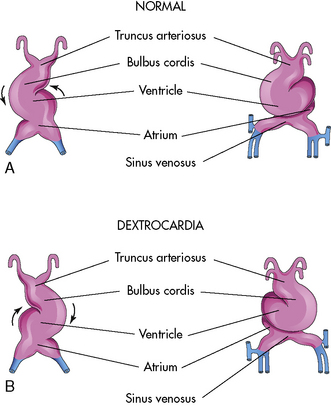
The endocardial heart tube begins to expand and elongate and develops areas of dilation: the bulbus cordis (including the truncus arteriosus, conus arteriosus, and conus cordis), ventricle, atrium, and sinus venosus. This expansion and elongation results in coiling of the heart tube anteriorly and to the right (this is referred to as “dextral,” or “D-looping”), with creation of a bulboventricular loop (see Fig. 8-1, B and C).
Because the venous and arterial poles of the heart tube are fixed during this time of coiling, torsion occurs within the anterior portion of the loop, the truncus arteriosus. This torsion will later contribute to the formation of a spiral septum within the truncus. By the 26th day of gestation, a truncus arteriosus is visible in the center of the anterior portion of the heart, and a common atrium and ventricle are recognizable (see Fig. 8-1, D). By day 28 the looping process is complete.
The embryonic heart begins to contract by day 26 to 28, with cycles that are similar to those in mature hearts.72 In this “in-series circulation,” blood flows from the morphologically right atrium to the morphologically left atrium, left ventricle, right ventricle and then the truncus arteriosus.902
Malrotation during the formation of the ventricular loop may cause various cardiac malpositions (such as dextrocardia) and malformations. As noted, the normal direction for the ventricular loop is to the right, or D-looping. If the ventricles loop to the left instead, L-looping has occurred, so the morphologic left ventricle is located to the right, and the morphologic right ventricle is on the left (Fig. 8-2).
Formation of Cardiovascular Septation: Day 26 to 49
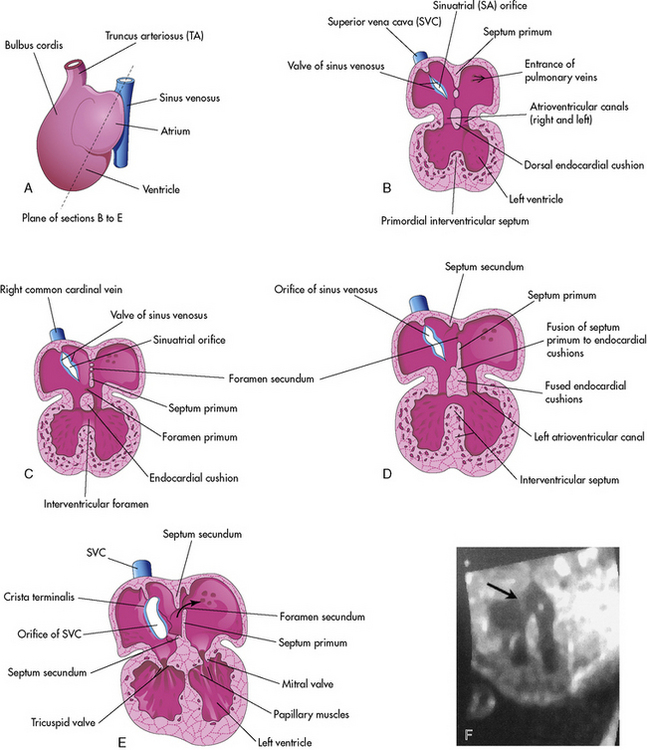
Cardiac septation occurs after the looping process is complete. During the septation process, septae are formed that ultimately close the ostium primum, the central atrioventricular canal, and the interventricular foramen. At the end of this period, the in-series circulation becomes two parallel circulations.902
Endocardial Cushion Development: Day 26 to 40
Septation begins on day 26 with the ingrowth of large tissue masses, the endocardial cushions. These cushions form on the dorsal and ventral walls of the atrioventricular (AV) canal separating the primordial atrium from the primordial ventricle.627 These cushions contain mesenchymal cells, derived from endocardium, and cardiac jelly, derived from endothelium. The endothelial cells line the atrioventricular canal and conotruncal segments. Neural crest cells migrate into the pharyngeal arches and then to the endocardial cushions, where they play a role in the septation of the cardiac chambers, outflow tracts, and heart valves. From the endocardial cushions, the neural crest cells then invade the myocardium.
Genetic disruptions in neural crest cell development are not fully understood, but patients with DiGeorge, Alagille, and Noonan syndrome have congenital heart defects that are influenced by cardiac neural crest cells.764
Atrial Septation: Day 30 to 35
Septation of the atria begins around day 30. Two septae develop and are modified to form a flapped orifice, the foramen ovale as illustrated in Fig. 8-3. The first septum to form is the septum primum, which grows from the anterior, superior portion of the atrium and extends toward the center of the heart. The development of the septum primum leaves a gap, the ostium primum, in the inferior portion of the atrial wall; this gap normally is closed by the fusion of the endocardial cushions. As the ostium primum is closing, perforations produced by apoptosis begin to form in the central portion of the septum primum. These perforations coalesce to create the ostium secundum (see Fig. 8-3, C and D).627
The septum secundum is the second septum to form within the atria. It is a thick crescent-shaped muscular fold that grows immediately adjacent to the septum primum. As it grows, it overlaps the foramen secundum in the septum primum. The atrial partition is incomplete, forming an oval foramen (in Latin, the foramen ovale).627
The portion of the septum primum that is attached to the cranial portion of the atrium gradually disappears. The remaining portion of the primum septum that is attached to the fused endocardial cushions forms the flap of tissue called the valve of the oval foramen.627
Before birth the foramen ovale allows the oxygenated blood from the placenta entering the right atrium via the inferior vena cava to flow to the left atrium and to the fetal brain. (See section, Fetal Circulation that follows.) The flap of the oval foramen normally prevents flow in the opposite direction.627
Atrial septal defects occur when the primum septum or the secundum septum or both do not completely form. Atrial septal defects include an ostium primum, secundum atrial septal defect, or common atrium. An ostium primum atrial septal defect is located near the atrioventricular valves. This defect will not close spontaneously. An ostium secundum atrial septal defect is thought to result from excessive apoptosis of the septum primum, so that a large defect is present in the area of the foramen ovale. Some secundum defects decrease in size and some undergo spontaneous closure.723
Ventricular Septation: Day 25 to 49
Septation of the ventricles begins around day 25 with protrusions from the inlet (primitive ventricle) and outlet (bulbus cordis) segments of the primordial heart. The muscular intraventricular septum initially grows from the apex of the ventricle and increases with dilation of both ventricles. With rapid proliferation of the myoblasts in the septum, the muscular septum extends toward the center of the heart.79,627 At first there is a crescent-shaped intraventricular foramen between the septum and endocardial cushions. The right and left bulbar ridges and the fused endocardial cushions join with the intraventricular muscular septum to form the membranous septum, obliterating the intraventricular foramen (refer again to Fig. 8-3, B-E).
As the ventricular cavities develop, the walls form a sponge work of muscular bundles. Some of the bundles become the papillary muscles and the tendinous cords (Latin, chordae tendineae) of the atrioventricular valves.627 Ventricular septal defects can occur in any location in the developing ventricular septum. Some muscular defects will close spontaneously.723
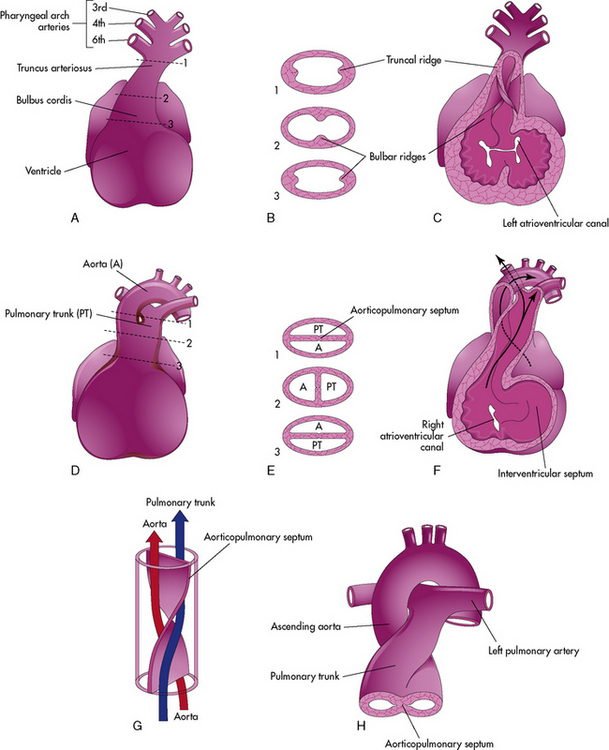
Septation of the Truncus Arteriosus: Day 26 to 42
Active proliferation of the mesenchymal cells in the walls of the bulbus cordis form the conotruncal or bulbar ridges. Similar ridges form in the truncus arteriosus (Fig. 8-4).
The mesenchymal cells in these ridges are primarily from neural crest cells. As cell proliferation occurs, the truncal and bulbar ridges create a 180-degree spiraling. This spiraling may be enhanced by the streaming of blood from the ventricles, as illustrated, or may result from passive untwisting as the pulmonary artery passes from the anterior pulmonary valve to the posterior pulmonary bifurcation.902
The spiraling of the conotruncal septum aligns the future pulmonary artery to the anterior and rightward right ventricle, where it joins with the outflow tract (infundibulum) created by incorporation of the bulbus cordis into the right ventricle. The future aorta communicates with the posterior and leftward left ventricle at the aortic vestibule created by incorporation of the bulbus cordis below the aortic valve.627 Differences in cell growth create a separation between the tricuspid and pulmonary valve. Disappearance of this segment below the aortic valve provides fibrous continuity between the mitral and aortic valve.79
Defects in the conotruncal and aortic arch development do not close spontaneously. Congenital heart defects that result from defective conotruncal development include: truncus arteriosus, tetralogy of Fallot, pulmonary atresia, double-outlet right ventricle, interrupted aortic arch, and aortopulmonary window. These defects are often associated with genetic deletions of chromosome 22q11 (see section, Genetic Factors Associated with CHD).627,723 Failure of appropriate conal reabsorption of the subaortic conus is thought to produce improper truncal rotation and result in d-transposition of the great vessels.
Formation of the Cardiac Valves: Day 34 to 42
The semilunar valves form when truncal septation is nearly complete. They develop from three swellings of subendocardial tissue around the openings of both the aortic and pulmonary trunks. The signaled transformation of endocardial cells to mesenchymal cells creates the differentiation of fibrous valve tissue.30 This fibrous tissue is hollowed out and reshaped to form three thin-walled cusps for each valve.627 Congenital heart defects that result during valve formation include pulmonary valve stenosis or atresia, pulmonary infundibular stenosis, a bicuspid aortic valve, and aortic stenosis.
The atrioventricular valves develop from localized proliferation of tissue around the atrioventricular canals.627 Most of the atrioventricular valve tissue comes from the ventricular myocardium in a process that involves undermining the ventricular walls. This process is asymmetric and positions the tricuspid valve annulus closer to the apex of the heart than the mitral valve annulus. Physical separation of these two valves creates the atrioventricular septum. Absence of this septum is the common defect in children with atrioventricular septal defects. Ebstein anomaly is thought to result from incomplete undermining of the ventricular wall.79
Classification of Complex Cardiac Malpositions and Malformations
Complex cardiac malformations and malpositions can be described according to a labeling system proposed by Van Praagh.902 This classification included 10 cardiac segments, but three were used most often. The positions of the viscera, the ventricular loop, and the great arteries are all labeled separately, using three letters within curved brackets, such as {A, B, C}.
The cardiac chambers and organ segments are identified by morphology (i.e., structure and appearance); the segments then can be referred to as concordant (consistent in position), or discordant (inconsistent in position). Although this segmental description of congenital heart defects is not used for the most common forms of defects, it is used in the classification of cardiac malpositions (e.g., dextrocardia or dextroversion) and complex transpositions. A variation of the Van Praagh segmental classification has been published recently343 using six segments (systemic and pulmonary veins, atrial situs, atrioventricular connection, ventricles and infundibulum, ventricle to artery connection, and great arteries and the ductus arteriosus). The following section identifies the three most commonly used segments for identification.
Position of the Abdominal Viscera and Atria (S, I, or A)
The atria can be labeled definitively during cardiac surgery, when characteristic atrial morphology can be identified. When the atrial and visceral positions are normal (S, for situs solitus), the morphologic right atrium is on the right side; it is identifiable because it is joined to the suprahepatic portion of the inferior vena cava.903 In addition, the abdominal viscera are in typical position; the liver is on the right and the stomach and spleen are on the left.
Description of the Ventricular Loop (D or L)
Position of the Great Vessels (d– or l-Normal, or d– or l-Transposition)
As noted, the position of the great vessels is determined by the relationship of the semilunar valves. Normal (dextral) position of the great vessels is present if the aortic valve is posterior and to the right of the pulmonic valve; this position also is occasionally referred to as the situs position of the great vessels. Abnormal position of the great vessels may be indicated by the letters d or l. Abnormal dextral or d-position of the great vessels is present if the vessels are located abnormally and the aortic valve is located to the right but anterior to the pulmonic valve. The great vessels are labeled as position l (levo or leftward) if the aortic valve is to the left of the pulmonic valve. Since dextral position may be either normal or associated with great vessel malposition, the letters d or l (capital letters are not used here) are usually modified by the terms “normal” or “transposition”29 to indicate the relationship between the great vessels and the ventricles.
Corrected transposition (l-transposition) with normally related abdominal viscera would be labeled TGA (S, L, l-transposition): situs solitus is present, a left ventricular loop has occurred, and the aortic valve lies to the left of the pulmonic valve.903 The aorta arises from the morphologic right ventricle.
Development of the Aortic Arch: Day 28 to 49
Two large arteries form at the distal end of the truncus arteriosus during the fourth and fifth weeks of fetal development. Although these original arteries ultimately disappear, they give rise to six pairs of arteries, the six aortic arches. By the end of the fourth week the first two pairs and the fifth pair of aortic arches have disappeared, and the sixth pair of arches now is joined to the pulmonary trunk and contributes to the development of the ductus arteriosus. Ultimately the third aortic arch will form the common carotid artery, the external carotid artery, and part of the internal carotid artery. The fourth aortic arch forms part of the final aortic arch and the proximal portion of the right subclavian artery. The sixth aortic arch provides the proximal segment of both pulmonary arteries and the ductus arteriosus, and a branch develops with the lung buds to provide pulmonary blood flow (see Evolve Fig. 8-1 in the Chapter 8 Supplement on the Evolve Website). Abnormalities in formation of the aortic arches can result in an interrupted aortic arch, aortic atresia, patent ductus arteriosus, vascular rings (including double aortic arches), and aberrant origin of the right subclavian artery.
Development of the Ventricular Myocardium, Conduction System, and Coronary Circulation
After the neural crest cells enter the endocardial cushions, they migrate to the myocardium. The myocardium then develops into a working myocardium and the cardiac conduction system. Originally the atrium acts as the pacemaker of the heart. The sinoatrial node develops during the fifth week. Additional cells from the right atrial wall join with cells from the atrioventricular region to form the atrioventricular node and His bundle located just above the endocardial cushions. Bundle branches are found throughout the ventricular myocardium.627
Additional cells develop from a small, transient organ, the proepicardium, on the dorsal thoracic wall. The proepicardial cells contain mesothelial cells that migrate to the heart and form the epicardium that lines the heart. These cells migrate further and differentiate to form the coronary vasculature and cardiac fibroblasts.764
In prenatal life myocardial cells undergo significant changes to increase in number (hyperplasia) and size (hypertrophy). The myocardial cells also change shape from round to cylindrical, become more regular in the orientation of the myofibrils (the contractile element), and have increasing proportions of myofibrils. Developmental changes are also seen in the sarcolemma (plasma membrane) and sarcoplasmic reticulum. Both control the ion channels and transmembrane receptors that regulate cardiac function, depolarization, and repolarization. Maturation of these cells and functions continues into the neonatal period.79
Fetal Circulation
Fetal circulation differs anatomically and physiologically from postnatal circulation in several important ways. In the fetus, oxygenation of the blood occurs in the placenta, which is a relatively inefficient oxygenator. The fetus is hypoxemic, with an aortic arterial oxygen tension of approximately 20 to 30 mm Hg; the saturation of fetal hemoglobin is higher at this oxygen tension than is normal hemoglobin, so oxygen saturation is approximately 60% to 70%. The fact that the oxygen tension is normally much lower in the fetus than postnatally may account for the ability of the neonate to tolerate cyanotic heart disease.278 Despite this arterial hypoxemia, the fetus does not have tissue hypoxia because fetal cardiac output is higher than at any other time in life, averaging approximately 400 to 500 mL/kg per minute. Approximately 20% of the normal fetal oxygen consumption of 8 mL/kg per minute is required to develop new tissue.278 Fetal cardiac output is constant (at approximately 400 to 500 mL/kg per minute) at a heart rate greater than 120 to 180/min. Changes in preload result in very little change in cardiac output, and changes in afterload are not well tolerated.278
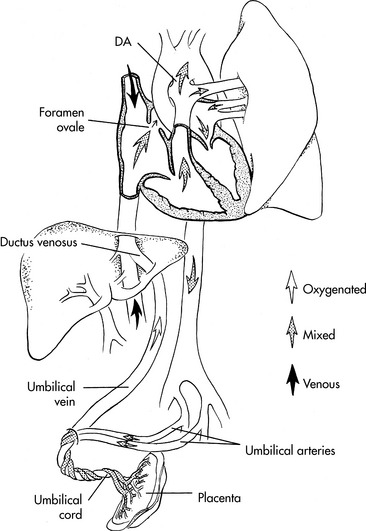
Fetal circulation is designed to deliver the best-oxygenated and nutrient rich blood from the placenta to the fetal brain and heart (Fig. 8-5). This blood enters the fetus through the umbilical vein. The blood flow splits in the liver with almost half going through the hepatic veins and portal system of the liver and the rest through the ductus venosus, where it joins the inferior vena cava near its junction with the right atrium. In the right atrium the blood from the inferior vena cava (with a PO2 of 32 to 35 mm Hg and an oxygen saturation of 70%—the highest fetal PO2) is divided into two streams. About 40% of this blood passes through the foramen ovale into the left atrium, where it joins the small amount of blood from pulmonary venous return. It then passes through the mitral valve to the left ventricle and then to the ascending aorta where it supplies the coronary, carotid, and subclavian arteries. The preferential streaming of this blood results in an ascending aortic arterial oxygen tension (PaO2) of 26 to 28 mm Hg and an oxygen saturation of 65%.278,686 Ten percent of the blood flow from the left ventricle passes through the aortic arch into the descending aorta, where it joins 90% of the blood leaving the right ventricle. The fetal right ventricle pumps more than two thirds of the combined ventricular output, so the right ventricle is relatively muscular.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Be sure to check out the supplementary content available at
Be sure to check out the supplementary content available at