Linda L. Steele, PhD, APRN, ANP-BC and James R. Steele, MSN, APRN, NP-C On completion of this chapter, the reader will be able to: 1. Describe the physiologic and environmental factors that contribute to the increased risk of cancer in older adults. 2. Identify the malignancies most commonly found in older adults. 3. Discuss the nurse’s role in cancer prevention and early detection. 4. Design therapeutic nursing plans of care by applying principles of cancer treatment to older adults. 5. Develop strategies to manage symptoms experienced by older adults receiving cancer treatment. 6. Discuss unique dimensions of psychosocial problems encountered by older adults with cancer. 7. Analyze ethical concerns related to the care of older adults with cancer. 8. Identify appropriate resources for older adults with cancer. Cancer is a disease of aging. The American Cancer Society’s 2005 statistical report, which has the latest data available for heart disease, showed that for the first time cancer killed more Americans younger than age 85 than did heart disease: in 2005, 476,009 people younger than age 85 died of cancer compared with 450,637 who died of heart disease. Currently, it is only the very oldest Americans who die of heart disease more often than cancer. An estimated 1,372,910 new cancer cases and 562,340 cancer deaths were estimated for 2009. The risk of cancer increases steadily beyond middle age and continues to rise thereafter. Although individuals older than age 65 represented about 12.4% of the population in the year 2000, they accounted for an estimated 60% of all cancer cases and 69% of all cancer deaths; thus a very small percentage of the population bears a disproportionate burden of the disease (American Cancer Society, 2008). Throughout the twentieth century, the older population 65 years or older in the United States grew from 3 million to 37 million people, which accounts for slightly more than 12% of the total population. In the last decade there has been a 12% increase in older adults, while the general population increased only 2.5 times. By the year 2030, persons older than age 65 will represent more than 18% of the population. The oldest-old population (those ages 85 or older) grew from slightly more than 100,000 in 1900 to 5.3 million in 2006, which represents the highest percentage increase per age group. The U.S. Census Bureau projects that the population age 85 or older could grow from 5.3 million in 2006 to nearly 21 million by 2050 (Healthy People, 2010). In 2011, the baby boomers (those born between 1946 and 1964) will start turning 65, which will dramatically increase the number of older people during the 2010 to 2030 period. The older population in 2030 is projected to be twice as large as in the year 2000, growing from 35 million to 71.5 million and representing nearly 20% of the total U.S. population. However, from 2030 onward the growth rate of the older population is projected to slow, when the last baby boomers enter the ranks of the older population and the proportion of those ages 65 or older will be relatively stable, at around 20% (Healthy People, 2010). The impact on the future U.S. cancer burden is estimated based on the growing and aging U.S. population. Cancer incidence refers to the number of new cases in a given time period, usually a year, in the general population. The leading types of cancer in men are prostate, lung, and colorectal. The leading types of cancer in women are breast, lung, and colorectal. Incidence differs from mortality. Mortality is the rate of deaths per number of incidences. Many persons survive cancer, and some cancers have relatively high incidence rates and relatively low death rates. For example, the incidence of breast cancer is 27% in women, up from 18% in 2003, whereas the death rate is 15%. In contrast, the incidence of lung cancer in women is 14%, whereas the death rate is 26% (American Cancer Society, 2008). In other words, although more women die from breast cancer overall, fewer women who get lung cancer survive that disease. The National Cancer Institute (2009d) estimates that approximately 11.1 million Americans alive today have a history of cancer. This has increased from 7.4 million Americans in 2003. Of the survivors, some may be completely cured, whereas others have some evidence of disease. Cancer accounts for 22.9% of all deaths. Between 1950 and 2001 death rates from cancer have remained stable. The 5-year relative survival rate for all cancers diagnosed between 1996 and 2004 is 66%, which is up from 50% in 1975 to 1977. The improvement in survival reflects progress in diagnosing certain cancers at an earlier stage and improvements in treatment. Nonetheless, in 2010, Americans are expected to die of cancer at the rate of more than 1500 people a day. In the United States, cancer accounts for nearly one of every four deaths (American Cancer Society, 2008). Both incidence and death rates from all cancers combined showed a statistically significant (P < .05) decrease in men and women overall and in most racial and ethnic populations in the last 5 years. These decreases reflect declines in both incidence and death rates for the three most common cancers in men (lung, colorectum, and prostate) and for two of the three leading cancers in women (breast and colorectum). There has also been a leveling off of lung cancer death rates in women. Although the national trend in female lung cancer death rates has stabilized since 2003 after increasing for several decades, there are significant prominent state and regional variations. This decrease in overall cancer incidence and death rates is encouraging, but the rates among women increased in 18 states, 16 of them in the South or Midwest. California was the only state with decreasing lung cancer incidence and death rates in women (Horner et al, 2008). Based on 2001 through 2003 data, the likelihood of developing cancer during one’s lifetime is approximately one in two for men and one in three for women. The 5-year relative survival rate for all cancers diagnosed between 1996 and 2004 is 66%, up from 50% in 1975 to 1977 (American Cancer Society Cancer, 2008). The 5-year relative survival rate for all types combined ranges from 16% for patients with lung cancer to 99% for patients with prostate cancer. Cancer survival varies by stage of disease and race; survival rates are lower in blacks compared with whites (National Cancer Institute, 2009c). During the past 15-year period, the cancer death rate among men dropped by 19.2% while the cancer death rate for women fell by 11.4%. Cancer incidence rates also declined from 1.8% a year among men from 2001 to 2005 and 0.6% a year from 1998 to 2005 among women. A decrease of 1% or 2% per year equates to 650,000 cancer deaths avoided over 15 years (American Cancer Society, 2008). Like the rest of the population in the United States, the aging population is becoming more diverse. In addition to whites of European descent, four other main racial and ethnic groups are present in the American population: African Americans, Hispanic Americans, Asian/Pacific Islanders, and Native Americans. Cancer affects Americans of all racial and ethnic groups; however, the incidence of cancer does demonstrate patterns according to racial and ethnic origins. African Americans have higher overall incidence rates than whites, whereas Hispanic Americans and Native Americans have lower incidence rates overall (Tables 19–1 and 19–2). TABLE 19–1 CANCER INCIDENCE RATES (NUMBER OF NEW CASES EACH YEAR)∗ ∗Statistics are for 2000 to 2006, age adjusted to the 2000 U.S. standard population, and represent the number of new cases of invasive cancer per year per 100,000 of both sexes, males and females, respectively. From National Cancer Institute. (2009). Surveillance epidemiology and end results (SEER). Retrieved June 2009, from http://seer.cancer.gov/faststats/selections.php#Output. TABLE 19–2 CANCER DEATH RATES (NUMBER OF DEATHS EACH YEAR)∗ ∗Statistics are for 2000 to 2006, age adjusted to the 2000 U.S. standard population, and represent the number of new cases of invasive cancer per year per 100,000 of both sexes, males, and females, respectively. From National Cancer Institute. (2009). Surveillance epidemiology and end results (SEER). Retrieved June 2009, from http://seer.cancer.gov/faststats/selections.php#Output. Because the incidence of cancer has demonstrated patterns by race and ethnicity, both these factors are important in determining which groups are at risk. When incidence is examined by race, several cautions are in order. First, race and ethnicity are both prone to misclassification. The U.S. Census Bureau has defined race and ethnicity (Box 19–1), but there is no accepted scientific definition for race. Persons with mixed-race parents lack a single classification. Second, as demonstrated by Freeman (1989) in his landmark investigation of genetics and cancer, there is no known genetic basis to explain the major racial differences in cancer incidence. Third, race and ethnicity may be viewed as a rough indicator for certain lifestyle and environmental factors. Race and ethnicity are highly correlated with socioeconomic status. Persons living in poverty tend to lack education, employment, adequate housing, good nutrition, preventive health practices, and access to health care. Within any one race or cultural group, economic status is the major determinant for cancer risk and outcome. Economic status as a risk factor for cancer is demonstrated globally. For most cancers, there are notable geographic variations in incidence rates that reflect socioeconomic differences, particularly differences between developing and developed countries (Hansen, 1998). Freeman (1989) concluded that correcting poverty among groups of people, regardless of their race or ethnic origin, would lead to decreased cancer incidence and increased survival rates. The leading cancers among white men are prostate, lung, colorectal, urinary bladder, melanoma, and non–Hodgkin’s lymphoma. White men have higher urinary bladder cancer incidence rates than men of any other racial or ethnic group: the rates are almost two times higher than those of Hispanic men, who have the second highest rates along with African American men. Breast cancer rates among white women are higher than those for women of any other racial or ethnic group. African American men have a higher overall cancer incidence rate than any other racial or ethnic group in America (666.4 vs. 558.3 for white men per 100,000). In contrast, white women have the highest cancer incidence rate among all ethnic groups (424.6 per 100,000) (Tables 19–3 and 19–4). In the United States the incidence rate for all cancers combined was 16% higher among African American men than white men (a decrease of 4% in the last 5 years), whereas the incidence rate for all cancers combined was 7% higher for white women than African American women (an increase of 2% over the last 5 years) (American Cancer Society Cancer Facts and Figures for African Americans, 2007 to 2008). TABLE 19–3 CANCER INCIDENCE RATES OF AFRICAN AMERICAN AND WHITE MALES TABLE 19–4 CANCER INCIDENCE RATES OF AFRICAN AMERICAN AND WHITE FEMALES Cancer incidence rates vary considerably among the subgroups of Asian/Pacific Islanders. Although Asian/Pacific Islanders experience lower rates overall compared with other groups, they do experience higher death and incidence rates for certain cancers, especially liver and stomach cancer for both sexes. For men, the top three sites among Chinese, Filipinos, Hawaiians, and Japanese are prostate, lung, and colorectal; among Koreans the top sites are lung, stomach, and colorectal; among Vietnamese the top sites are lung, liver, and prostate. Stomach cancer rates among Korean men and liver cancer rates among Vietnamese men are higher than those among men of any other racial or ethnic group. The top three cancer sites among Asian/Pacific Islander women are breast, lung, and colorectal with the following exceptions: The stomach is the leading site among Japanese and Korean women, and the cervix is the leading site among Vietnamese women. Cervical cancer incidence rates among Vietnamese women are more than 2½ times higher than for any other racial or ethnic group. Asian Americans have the highest overall incidence of liver, bile duct, and stomach cancer for both men and women (American Cancer Society, 2008). Information on cancer incidence among Native Americans is based on 54% of the U.S. Indian/Native American population in 624 counties. Alaskan Natives have the highest cancer incidence rates among any racial group for the kidney and pelvis. Alaskan Natives have a relatively high incidence of cancers of the esophagus, stomach, liver, gallbladder, and pancreas. According to the National Cancer Institute: Surveillance epidemiology and end-results program (1975-2006), American Indians who live in New Mexico and Arizona have excessive incidence rates for stomach, cervix, uterine, liver, and gallbladder cancer. American Indians have the highest gallbladder cancer incidence rate of any racial group, including blacks, whites, or Hispanics (National Cancer Institute, 2009c). The leading cancer sites for Hispanic men and women are the same as those for whites—prostate, breast, lung, and colorectal. Other cancers commonly diagnosed among Hispanics include cancers of the urinary bladder and stomach in men. Hispanic women have the highest overall incidence of cervical cancer (American Cancer Society SEER Results, 2008). (See Cultural Awareness Box.) External or environmental factors are believed to contribute to decreased regulation of cell growth by causing cell damage and then promoting the replication of damaged cells (Cohen, 1994). The process of cancer growth is believed to occur in three stages: initiation, promotion, and progression (Fig. 19–1). Initiation results from intense or prolonged exposure to an external agent that causes mutation of genetic material. The mutations are nonlethal, but they are passed on to future cell generations during replication. An initiated cell will continue to produce the mutations with each cell replication; however, the mutations alone are not enough to lead to cancer. Cancerous cell growth begins when an initiated cell encounters a promoting agent; thus the second stage of cancer development is called promotion. Promoting agents are external or environmental agents. A number of substances may be considered promoters of cancer in humans; they may come from a variety of sources, including air, water, or soil, and they may be naturally occurring or chemically produced. Promoting agents share the common property of inducing replication of an initiated mutant cell, thus transforming the initiated cell into a cancer cell. The promoting agent cannot cause cellular transformation unless the cell has been initiated, regardless of the intensity of exposure to the promoting agent. Promotion is dose dependent in its effect, and although promotion can transform a cell immediately after initiation, promotion is thought to be most successful when it involves repeated exposure to an initiated cell. Prevailing thought is that initiation is irreversible, whereas promotion is reversible. This belief has been demonstrated clinically in tobacco smokers: once the exposure to the promoter (tobacco) is withdrawn, the incidence of cancer is reduced. Although some results of cancer genome mapping may not be ready for use in cancer treatment today, discoveries from this research may lead to better tests for diagnosing cancer, as well as more effective treatments (American Society of Clinical Oncology, 2007). Immune function declines with age. Although immunogenic tumors can be more aggressive in older adults, solid tumors influenced by hormones are usually less aggressive. Environmental influences over the course of time are likely to have an effect on older adults in relation to prolonged exposure to carcinogens. Heredity may also contribute to cancer etiology. In addition to exposure to environmental carcinogens, the older adult may be at increased risk of developing cancer because of mutations in DNA repair or metabolic detoxification. Aging may also predispose older adults to increased cancer risks if they have inherited mutated genes. It remains unknown whether cancer in older adults is caused by any one of these factors or a combination of them (Coleman, Hutchins, & Goodwin, 2004). Several mechanisms have been proposed to explain the way in which the aging process directly influences the cancerous transformation of cells (Box 19–2): • Aging increases the duration of exposure to substances that may act as promoting agents. Because the effects of promoters are dose dependent, older adults can accumulate a significant dose over decades. Also, cellular transformations and progression of cancer cells occur over time. Cancer cells grow at various rates, and in some cases significant time is needed for the small cluster of cancer cells to grow large enough to cause signs and symptoms. • Aging cells demonstrate a tendency toward abnormal growth. Aged cells are more vulnerable to damage; thus aging likely increases the susceptibility of cells to substances that cause genetic mutations. • Once an aged cell is damaged by an initiating substance, it is more difficult to repair. • Oncogene activation might be increased in older persons, resulting in increased loss of regulation of cell growth and the development of cancer cells. • Decreased immune surveillance, or immunosenescence, may contribute to increased development of cancers and their progression, although the evidence on the role of the immune system in the development of cancer is inconclusive (Crawford & Cohen, 1987; Pfeifer, 1997a). Breast cancer is the leading cause of death in women ages 55 to 74. Late-stage diagnosis is a serious concern for older adults. The primary presenting symptom is a lump in the breast; however, approximately 10% of women show symptoms of metastasis as the first indication of disease. The lung, liver, bones, and adrenal glands are predominant metastatic sites for breast cancer. Specific symptoms are related to the metastatic site and extent of disease (American Geriatric Society [AGS], 2000). Although all women are at risk for developing breast cancer, the older a women is, the greater her chances of developing breast cancer. Breast cancer is more common in white women than in other racial or ethnic groups, but according to the most recent data, death rates are continuing to decline in white women. According to the SEER program of the National Cancer Institute (2009a), white Hawaiian and African American women have the highest incidence of invasive breast cancer in the United States. Korean, American Indian, and Vietnamese have the lowest incidence of invasive breast cancer in the United States. African American women have the highest death rate from breast cancer and are more likely to be diagnosed with a later stage of breast cancer in the age group 55 to 69. However, in the age group that is 70 years or older the death rate is higher for white women than for African American women (American Cancer Society, 2004a) (see Cultural Awareness Box). menopause (after age 55), lengthy exposure to postmenopausal estrogen, recent use of oral contraceptives, and never having given birth or having first given live birth at a late age (after age 35) (Reigle, 2000). The highest incidence of breast cancer is in women ages 50 to 59. A first peak of incidence occurs between ages 45 and 49, when women are either premenopausal or menopausal. The high incidence in this age group is thought to be related to ovarian estrogen. A second peak of incidence occurs in women between ages 65 and 69, most of whom are postmenopausal. The second peak appears to be related to an imbalance of adrenal estrogen. Given these findings, breast cancer appears to be two separate diseases differentiated by menopausal status. Although hormones are not inherently mutagenic, they may act as initiators or promoters by altering cell reproduction and growth (Pfeifer, 1997a). A major advance in understanding breast cancer is that the disease has a genetic basis. Approximately 5% to 10% of breast cancers are hereditary. The genes involved in most inherited breast cancers are BRCA1 and BRCA2. These are tumor-suppressor genes that also serve to protect and preserve DNA. Mutation of these genes has been linked to hereditary breast and ovarian cancer. A woman’s risk of developing breast and/or ovarian cancer is greatly increased if she inherits a deleterious BRCA1 or BRCA2 mutation. Men with these mutations also have an increased risk of breast cancer. Up to 40% of inherited breast cancers are due to mutations in BRCA1, and mutations in BRCA2 are responsible for up to 30% of inherited cancer (Cummings & Olopade, 1998; American Cancer Society, 2008). Genetic tests are available to check for BRCA1 and BRCA2 mutations. Federal and state laws help ensure the privacy of a person’s genetic information and provide protection against discrimination in health insurance and employment practices.Currently many research studies are being conducted to discover newer and better ways of detecting, treating, and preventing cancer in carriers of BRCA1 and BRCA2 mutations (American Cancer Society, 2008). High-fat diets and obesity have been suggested as risk factors, although the evidence is inconclusive. In animal studies the proliferation of breast tissue may be altered by changes in estrogen levels and pituitary and thyroid function, which are all sensitive to dietary intake (London & Willett, 1989). The risk associated with obesity may differ according to menopausal status and is thought to be associated with the metabolism of estrogen. Additional risk factors associated with breast cancer include pesticide and other chemical exposure, alcohol consumption, and physical inactivity (Knobf, 1996). Mammography is able to detect breast tumors before they present physical findings. A tumor must be about 10 mm to be palpable. A 10-mm tumor contains about 109, or 1 billion cells. Mammography screening can detect 107 cells. Mammography screening is more accurate for older women because breast tissue is less dense than that of younger women, making tumors easier to visualize. The American Cancer Society recommends mammography screening every 2 years for women between ages 40 and 49 and annually for women ages 50 and older (American Cancer Society, 2004b). Although Medicare pays for mammography screening every year, the use of mammography by women older than age 70 remains low, particularly among minority populations. Radiotherapy is indicated postlumpectomy. Postoperative breast irradiation is well tolerated by older women; therefore age is not a contraindication to breast preservation treatment (Wyckoff et al, 1994). In general, older women treated for breast cancer do not experience greater complications or treatment toxicities when compared with younger women (Masetti et al, 1996). Although mastectomy is not the treatment of choice, if mastectomy is done, breast reconstruction is an option, depending on personal preference and the extent of the disease. As with all women, older women should be given information and support to help make treatment decisions. Breast cancer should be treated promptly but is not an emergency. Nurses should provide a supportive atmosphere and encourage family members to participate in treatment decisions. There is a sharp increase in lung cancer incidence starting between ages 45 and 50, and the peak incidence occurs between ages 70 and 74 for women and 75 and 79 for men. A close relationship exists between incidence rates and death rates, indicating that most individuals diagnosed with lung cancer die of the disease (American Cancer Society, 2008). Cigarette smoking is by far the most important risk factor in the development of lung cancer, both for active smokers and nonsmokers exposed to environmental, or passive, tobacco smoke (also known as secondhand smoke). Ninety percent of all lung cancer deaths can be attributed to tobacco. Tobacco smoke is considered a cancer promoter demonstrating a dose-response relationship; that is, the risk of lung cancer increases with the quantity of cigarettes smoked. The greatest lifetime cumulative exposure to cigarette smoking occurs between ages 70 and 80. It has been known for some time that the risk of lung cancer decreases over time for exsmokers, the risk of lung cancer is increased for both current and former smokers compared with never smokers, and the risk declines for former smokers with increasing duration of abstinence. (Ebbert et al, 2003). Other risk factors include exposure to certain industrial substances that act as initiators and promoters, such as arsenic, ether, chromates, coke-oven fumes, nickel, and petroleum products and oils. Organic chemicals linked to a risk of lung cancer include radon and asbestos, and exposed persons who also smoke have an increased risk. Radiation exposure from occupational, medical, and environmental sources is also a risk factor. Air pollution contains several substances that, with repeated exposure, may increase the risk of lung cancer; however, air pollution is only hypothesized, not proven, to increase risk. Close to 342,000 Americans die of lung disease annually. Most lung diseases are chronic and diminish the quality of life for those persons living with the disease (American Lung Association, 2004). The presenting signs and symptoms of lung cancer are vague and may be attributed to other problems, especially in older adults who have underlying lung or other chronic illnesses. The classic clinical presentation of lung cancer is a persistent cough, sputum streaked with blood, chest pain, and recurring pneumonia or bronchitis. This constellation of symptoms is also associated with cigarette smoking, and their significance as indicators of cancer may be overlooked. Other symptoms include more systemic complaints such as anorexia, weight loss, and fatigue. Symptoms of local metastasis may include hoarseness in the presence of laryngeal nerve involvement, shoulder pain when the brachial plexus is involved, dyspnea representing esophageal compression or invasion, and head and neck swelling signaling superior vena cava involvement. Older persons more often experience dyspnea and weight loss, while pain is less frequent (Shell, Bulson, & Vanderlugt, 1997). Lung cancer can grow for years before exhibiting clinical symptoms. Because symptoms often do not appear until the disease is advanced, early detection is difficult. When used in combination, chest x-ray studies and cytologic examination of sputum cells can detect small tumors. Both tests are expensive, requiring special facilities and personnel. The Mayo Lung Project, conducted between 1972 and 1982, examined the benefits of screening for lung cancer and determined that, although screening programs achieved earlier diagnoses and longer survival times, no significant reduction in mortality was demonstrated (Woolner, Fontana, & Cortese, 1984). Early detection appears to lengthen the interval between diagnosis and death without increasing total life span. Currently, the American Cancer Society does not recommend routine screening for lung cancer in asymptomatic persons. In a retrospective review of lung cancer among male veterans, older men were found to have more local disease at diagnosis than younger men. This finding was attributed to earlier diagnosis in the older group, who likely had lung cancer detected serendipitously by chest radiology used to monitor other chronic conditions such as cardiopulmonary disorders, which were prevalent among the group. Small cell types represent about 20% to 25% of all lung cancers and are strongly associated by dose response with cigarette smoking. Because of small cell cancers’ high growth rate and tendency to metastasize early and widely, clients with small cell cancers have a poor prognosis. Small cell lung cancers are very sensitive to both chemotherapy and radiotherapy; in some cases chemotherapy (alone, or in combination with radiation) is used instead of traditional surgery as the treatment of choice. With surgery alone, small cell lung cancers tend to relapse, but with combination chemotherapy and radiotherapy, more persons with small cell lung cancer experience longer periods of remission (American Lung Association, 2004). Of the non–small cell cancers, the squamous cell type, like the small cell type, is strongly linked by dose response to cigarette smoking. Squamous cell cancers tend to arise in central airways and invade the bronchi; therefore surgery to remove the obstructing tumor is usually the treatment of choice. Adenocarcinomas are the most common lung cancer, first seen as slow-growing masses in peripheral lung tissue. Surgical resection of the lung is the treatment of choice. Because lung cancer has usually metastasized by the time it is discovered, radiotherapy and chemotherapy are also necessary. Evidence has suggested that older adults with non–small cell lung cancers, particularly those individuals older than age 75, do not receive the same level of treatment as younger persons: surgery is omitted more often in older persons. Omission of surgery may reflect age bias, or it may indicate that an older adult is a poor surgical candidate. Guadagnoli et al (1990) noted that older adults who received less aggressive treatment had more comorbid disease. The presence of comorbid disease can increase the demands of illness, which in turn influence not only physical but also psychologic adjustment to lung cancer. From 2002 to 2006, the median age at diagnosis for cancer of the prostate was 68 years of age. Approximately 0.0% were diagnosed younger than age 20; 0.0% between 20 and 34; 0.6% between 35 and 44; 8.7% between 45 and 54; 29.0% between 55 and 64; 35.6% between 65 and 74; 21.4% between 75 and 84; and 4.7% at 85 years of age or older. The age-adjusted incidence rate was 159.3 per 100,000 men per year. These rates are based on cases diagnosed in 2002 to 2006 from 17 SEER geographic areas. Prostate cancer accounts for approximately 23% of all cancer in men in the United States and approximately 12% of all cancer deaths. Prostate cancer is the second most common cancer in American men, and the highest rate of prostate cancer in the world is among African Americans. Prostate cancer incidence rates are 66% higher for African American men than for white men, and prostate cancer death rates are more than two times higher for African American men than for white men (American Cancer Society, 2004a). It is unclear what the exact prostate cancer risk is for men who have a first-generation relative with prostate cancer. Some studies have shown a twofold to elevenfold increase in men with a positive family history (American Cancer Society, 2004a). In the United States men have a 15% lifetime risk of a diagnosis of prostate cancer, but only a 3% lifetime risk of dying from it (American Cancer Society, 2004a). Annual digital rectal examination (DRE) and prostate-specific antigen (PSA) testing are the two primary screening tests for prostate cancer in the United States. However, only a portion of the prostate can be palpated using the digital technique. Studies suggest that DRE alone detects less than 60% of prostate cancers. Adding PSA testing detects 26% more cancers than DRE testing alone. The American Cancer Society (2004a) recommends that every man older than age 40 have a DRE as part of a regular annual physical examination. Ultrasound of the prostate is expensive and to date has not been demonstrated to be more predictive of prostate cancer than DRE. PSA is an immunologically distinct antigen made exclusively by prostate tissue. The American Cancer Society (2004a) recommends that men ages 50 or older have an annual PSA blood test. If either test result—DRE or PSA—is questionable, a complete evaluation is necessary. While the highest rate of prostate cancer is among African Americans, Collins (1997) found that, when surveyed, only 21% of African American men correctly identified the screening recommendations and early symptoms of prostate cancer. Like surgery, radiotherapy, in the form of either external beam radiation or internal implantation of seeds, can be a primary treatment for prostate cancer. External beam radiation is administered daily in small doses for approximately 40 treatments. Often the radiation treatments are coupled with hormonal therapy with agents such as luteinizing hormone–releasing hormone agonists. The benefits of the use of accompanying hormone therapy are a decrease in the serum testosterone level and shrinkage of the tumor in preparation for radiation (Millikan & Logothelis, 1997). Both surgery and radiotherapy are effective. Convincing proof of the superiority of either approach is not available. The choice of treatment often depends on the presence of comorbidity, availability of treatment facilities, requisite medical expertise, and individual preference. Erectile dysfunction can be a consequence of any of these procedures. Dr. Anthony D’Amico (2008) states, “In order to get the highest cure rate for men with high-risk prostate cancer, it appears that five weeks of external beam radiation and at least four months of hormonal therapy should be added to brachytherapy,” (D’Amico et al, 2008). Because treatments can have long-term consequences for sexual performance, the selection of a treatment should include a discussion with the older man and his sexual partner about preservation of sexual function. Age alone does not dictate sexual interest or performance. Some degree of sexual activity is preserved in approximately 75% of persons older than age 70. Loss of sexual function may have a serious impact on the quality of life of older adults. Careful consideration of sexual needs should be part of a treatment decision for clients of any age (see Chapter 13).
Cancer
Incidence
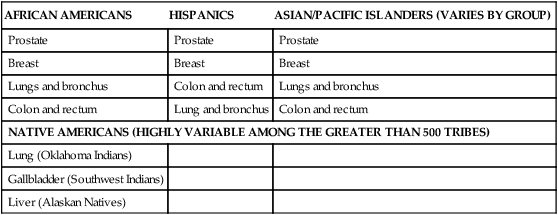
Racial and Ethnic Patterns
GROUP
BOTH SEXES
MALES
FEMALES
African American
467.30
582.95
388.10
White
458.13
524.68
412.53
Asian/Pacific Islander
297.80
228.35
280.07
Hispanic/Latino
331.00
379.86
300.14
American Indian/Alaskan Native
307.37
303.31
312.78
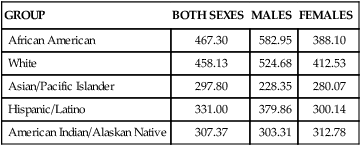
GROUP
BOTH SEXES
MALES
FEMALES
African American
218.79
287.74
176.91
White
180.09
218.74
153.43
Asian/Pacific Islander
107.77
128.23
93.31
Hispanic/Latino
119.49
145.57
101.49
American Indian/Alaskan Native
150.40
171.27
136.40
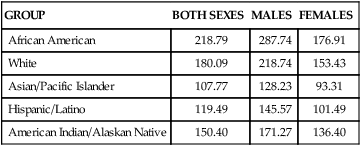
TYPE
AFRICAN AMERICAN
WHITE
ABSOLUTE DIFFERENCE
RATE RATIO
All Types
666.4
558.3
108.1
1.2
Prostate
258.3
163.4
94.9
1.6
Lung/Bronchus
112.2
81.7
30.5
1.4
Urinary/Bladder
19.8
30.2
-20.4
0.5
Skin Melanoma
1.1
-26.5
-25.5
<0.1
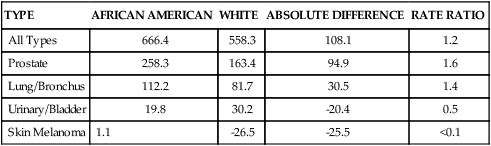
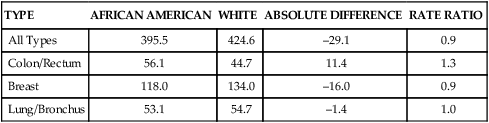
TYPE
AFRICAN AMERICAN
WHITE
ABSOLUTE DIFFERENCE
RATE RATIO
All Types
395.5
424.6
–29.1
0.9
Colon/Rectum
56.1
44.7
11.4
1.3
Breast
118.0
134.0
–16.0
0.9
Lung/Bronchus
53.1
54.7
–1.4
1.0
Aging and Its Relationship to Cancer

Common Malignancies in Older Adults
Breast Cancer
Risk Factors
Early Detection
Treatment
Lung Cancer
Risk Factors
Signs and Symptoms
Early Detection
Treatment
Prostate Cancer
Risk Factors
Early Detection
Treatment
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Cancer
Get Clinical Tree app for offline access