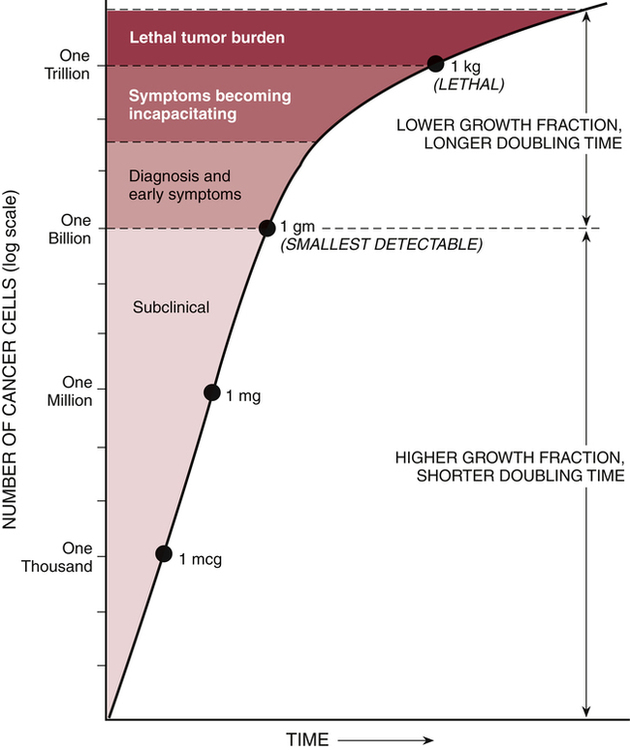
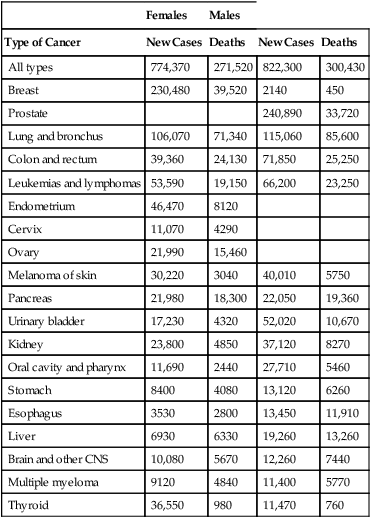
CHAPTER 101 As mortality from infectious diseases has declined, thanks to antimicrobial drugs and public health measures, cancer has emerged as the second leading cause of death. The American Cancer Society estimated that 571,950 Americans died from cancer in the year 2011. Only heart disease kills more people. Among women ages 30 to 74, neoplastic diseases lead all other causes of mortality. Among children ages 1 to 14 years, cancer is the leading nonaccidental cause of death. As shown in Table 101–1, among women, the most common cancers are cancers of the breast, lung, colon, and rectum. Among men, the most common cancers are cancers of the prostate, lung, colon, and rectum. TABLE 101–1 Estimated New Cancer Cases and Deaths, United States, 2011 Data from American Cancer Society. Cancer Facts & Figures 2011. Atlanta: American Cancer Society, 2011. The modern era of cancer chemotherapy dates from 1942, the year in which “nitrogen mustards” were first used for cancer. Since the introduction of nitrogen mustards, chemotherapy has made significant advances. For patients with some forms of cancer (Table 101–2), drugs can often be curative. Cancers with a high cure rate include Hodgkin’s disease, testicular cancer, and acute lymphocytic leukemia. For many patients whose cancer is not yet curable, chemotherapy can still be of value, offering realistic hopes of palliation and prolonged life. However, although progress in chemotherapy has been encouraging, the ability to cure most cancers with drugs alone remains elusive. At this time, the major impediment to successful chemotherapy is toxicity of anticancer drugs to normal tissues. TABLE 101–2 Some Cancers for Which Drugs May Be Curative* *“Cure” is defined as a 5-year disease-free interval following treatment. †These are representative regimens. Other regimens may also be highly effective. ‡Chemotherapy is combined with surgery and/or radiotherapy in these cancers. The cell cycle is the sequence of events that a cell goes through from one mitotic division to the next. As shown in Figure 101–1, the cell cycle consists of four major phases, named G1, S, G2, and M. (The length of the arrows in the figure is proportional to the time spent in each phase.) For our purpose, we can imagine the cycle as beginning with G1, the phase in which the cell prepares to make DNA. Following G1, the cell enters S phase, the phase in which DNA synthesis actually takes place. After synthesis of DNA is complete, the cell enters G2 and prepares for mitosis (cell division). Mitosis occurs next during M phase. Upon completing mitosis, the resulting daughter cells have two options: they can enter G1 and repeat the cycle, or they can enter the phase known as G0. Cells that enter G0 become mitotically dormant; they do not replicate and are not active participants in the cycle. Cells may remain in G0 for days, weeks, or even years. Under appropriate conditions, resting cells may leave G0 and resume active participation in the cycle. Why are cytotoxic anticancer drugs so harmful to normal tissues? Because these drugs lack selective toxicity. That is, they cannot kill target cells without also killing other cells with which the target cells are in intimate contact. We first encountered this concept in Chapter 83 (Basic Principles of Antimicrobial Therapy). As noted there, successful antimicrobial therapy is possible because antimicrobial drugs are highly selective in their toxicity. Penicillin, for example, can readily kill invading bacteria while being virtually harmless to cells of the host. This high degree of selective toxicity stands in sharp contrast to the lack of selectivity displayed by cytotoxic anticancer drugs. We have no way of knowing when 100% cell kill has been achieved. As a result, there is no definitive method for deciding just when chemotherapy should stop. As indicated in Figure 101–2, symptoms disappear long before the last malignant cell has been eliminated. Once a cancer has been reduced to less than 1 billion cells, it becomes undetectable by usual clinical methods; all signs of disease are absent, and the patient is considered in complete remission. It is obvious, however, that a patient harboring a billion malignant cells is by no means cured. It is also obvious that further chemotherapy is indicated. However, what is not so obvious is just how long therapy should last: Because the patient is already asymptomatic, we have no objective means of determining when to stop treatment. The clinical dilemma is this: If therapy continues too long, the patient will be needlessly exposed to serious toxicity; conversely, if drugs are discontinued prematurely, relapse will occur. Early detection of cancer is rare. Cancer of the cervix, which can be diagnosed with a Papanicolaou (Pap) test, is the primary exception. All other forms of cancer are significantly advanced by the time they have grown large enough for discovery. The smallest detectable cancers are about 1 cm in diameter, have a mass of 1 gm, and consist of about 1 billion cells (see Fig. 101–2). Detection at this stage cannot be considered early. Even though truly early detection is largely impossible, every effort at relatively early detection should be made. Why? Because the smaller a cancer is when treatment begins, the better the chances of long-term survival. Hence, even if a cancer has 1 billion cells when it’s detected, that’s still far better than a gazillion. Accordingly, the American Cancer Society recommends routine testing for several cancers, including cancers of the prostate, breast, uterus, rectum, and colon. Table 101–3 indicates who should be tested, how often, and what test or procedure should be performed. With breast cancer, a yearly mammogram can detect disease before it becomes widely invasive, thereby greatly increasing survival—even though more than a billion cells may be present at the time of discovery. Along with routine testing, patients should be counseled about ways to reduce cancer risk, especially avoiding tobacco and excessive exposure to ultraviolet radiation, and receiving a human papillomavirus (HPV) vaccination to protect against cervical cancer (see Chapter 68). TABLE 101–3 Tests that find cancer and precancerous polyps • Flexible sigmoidoscopy (FSIG) every 5 years or • Colonoscopy every 10 years or • Double-contrast barium enema every 5 years or • Computed tomographic colonography (virtual colonoscopy) every 5 years
Basic principles of cancer chemotherapy

Females
Males
Type of Cancer
New Cases
Deaths
New Cases
Deaths
All types
774,370
271,520
822,300
300,430
Breast
230,480
39,520
2140
450
Prostate
240,890
33,720
Lung and bronchus
106,070
71,340
115,060
85,600
Colon and rectum
39,360
24,130
71,850
25,250
Leukemias and lymphomas
53,590
19,150
66,200
23,250
Endometrium
46,470
8120
Cervix
11,070
4290
Ovary
21,990
15,460
Melanoma of skin
30,220
3040
40,010
5750
Pancreas
21,980
18,300
22,050
19,360
Urinary bladder
17,230
4320
52,020
10,670
Kidney
23,800
4850
37,120
8270
Oral cavity and pharynx
11,690
2440
27,710
5460
Stomach
8400
4080
13,120
6260
Esophagus
3530
2800
13,450
11,910
Liver
6930
6330
19,260
13,260
Brain and other CNS
10,080
5670
12,260
7440
Multiple myeloma
9120
4840
11,400
5770
Thyroid
36,550
980
11,470
760

Type of Cancer
Drug Therapy†
Hodgkin’s lymphoma
Doxorubicin + bleomycin + vinblastine + dacarbazine
Burkitt’s lymphoma
Cyclophosphamide + vincristine + methotrexate + doxorubicin + prednisone
Choriocarcinoma
Methotrexate ± leucovorin
Small cell cancer of lung
Etoposide + either cisplatin or carboplatin
Testicular cancer
Cisplatin + etoposide ± bleomycin
Wilms’ tumor‡
Dactinomycin + vincristine ± doxorubicin ± cyclophosphamide
Ewing’s sarcoma‡
Cyclophosphamide + doxorubicin + vincristine alternating with etoposide + ifosfamide (with mesna)
Acute myeloid leukemia
Daunorubicin + cytarabine + etoposide
Breast cancer‡
Fluorouracil + doxorubicin + cyclophosphamide
Colorectal cancer‡
Fluorouracil + leucovorin + oxaliplatin
Acute lymphocytic leukemia
Vincristine + prednisone + asparaginase + daunorubicin or doxorubicin ± cyclophosphamide
The growth fraction and its relationship to chemotherapy
The cell cycle
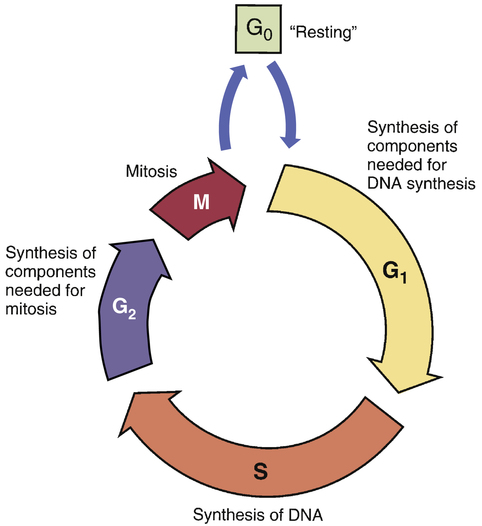
 The cell cycle.
The cell cycle.
Obstacles to successful chemotherapy
Toxicity to normal cells
Cure requires 100% cell kill
When should treatment stop?
Absence of truly early detection

Type of Cancer
Recommendation
Breast
Women age 40 and older should have an annual mammogram and an annual clinical breast examination (CBE) by a healthcare professional. Ideally, the CBE should be conducted before the scheduled mammogram. Women ages 20–39 should have a CBE at least every 3 years. Beginning in their early 20s, women may perform periodic breast self-examinations, in addition to receiving recommended CBEs.
Colon and rectum
Beginning at age 50, men and women should follow one of the 7 examination schedules below:
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

Basic principles of cancer chemotherapy
Only gold members can continue reading. Log In or Register to continue
Get Clinical Tree app for offline access