Assisting in Microbiology and Immunology
Learning Objectives
1. Define, spell, and pronounce the terms listed in the vocabulary.
2. Apply critical thinking skills in performing the patient assessment and patient care.
3. Cite the protocols for the collection, transport, and processing of specimens.
4. Identify the elements needed for microbial growth.
5. Compare bacteria with viruses.
6. Describe the characteristics of common viral diseases.
7. Describe the bacterial structures used in identification.
8. Describe various bacterial morphologies.
9. Explain the characteristics of common diseases caused by bacteria.
10. Compare bacteria with fungi, parasites, and protozoa.
11. Describe the unusual characteristics of Chlamydia, Rickettsia, and Mycoplasma organisms.
12. Identify the characteristics of common diseases caused by fungi, protozoa, and parasites.
13. Perform patient education on the collection of a stool specimen for ova and parasite testing.
14. Describe the equipment needed in a microbiology laboratory.
15. List the different growth media used for culturing.
16. Perform the procedure for inoculating a blood agar plate.
18. Perform a screening urine culture test.
19. Prepare a direct smear or culture smear for staining.
20. Compare and contrast the throat culture for Streptococcus pyogenes with the rapid strep test.
21. Perform a rapid strep test.
22. Describe three microbiologic tests that use a rapid identification technique.
23. Describe the method used for antimicrobial susceptibility testing.
24. Explain how pinworm testing is done and when it must be performed.
25. Perform a cellulose tape collection for pinworms.
26. Discuss the purpose of immunologic testing.
27. Describe three rapid immunologic tests that could be done in the physician office laboratory.
28. Perform the Mono-test for mononucleosis.
29. Discuss legal and ethical issues involved in laboratory testing.
Vocabulary
antimicrobial agents Drugs used to treat infection.
arthropods (ahr′-throh-pods) Members of a class of invertebrate animals that includes insects, crustaceans, and arachnids.
broad-spectrum antimicrobial agents Drugs used to treat a wide range of infectious microorganisms.
cysts Small, capsulelike sacs that enclose certain organisms in their dormant or larval stage.
eukaryotes (yoo-kar′-e-ohts) Single-celled or multicellular organisms with cells that contain a distinct membrane-bound nucleus.
fastidious Requiring specialized media or growth factors to grow.
genus A breakdown of a family of microorganisms.
interleukin (in-tehr-loo′-kin) A protein produced by certain white blood cells that regulates immune responses by activating lymphocytes and initiating fever.
in vitro Referring to conditions outside of a living body.
macromolecules The molecules needed for metabolism: carbohydrates, lipids, proteins, and nucleic acids.
microorganisms Organisms of microscopic or submicroscopic size.
molecules Groups of like or different atoms held together by chemical forces.
nanometers Units measuring 1 billionth (10−9) of a meter.
organelles (or-guh-nels′) Differentiated structures within a cell (e.g., mitochondria, vacuoles, and chloroplasts) that perform a specific function.
pathogens Disease-causing microorganisms.
prokaryote (pro-kar′-e-oht) A unicellular organism with cells that lack a membrane-bound nucleus.
prostaglandins (prahs-tih-glan′-dins) Chemicals released from cells that cause smooth muscle contraction and pain.
pure culture A bacterial or fungal culture that contains a single organism.
species A category of microorganisms that is below genus in rank; a genetically distinct group.
tissue culture The technique or process of keeping tissue alive and growing in a culture medium.
transport medium A medium used to keep an organism alive during transport to the laboratory.
viable Capable of living, developing, or germinating under favorable conditions.
Scenario
Infectious diseases are a continuing threat for everyone. Anna McIntyre, CMA (AAMA), knows that some diseases have been effectively controlled with the help of modern technology, but new diseases are constantly appearing, such as the avian flu and West Nile virus infection. In addition, other familiar infectious diseases, such as malaria, tuberculosis, and bacterial pneumonias, now are appearing in forms that are resistant to drug treatment. Anna knows that it is important to identify pathogens quickly so that the proper treatment can begin. The identification of pathogens, she has discovered, can involve many different types of tests, many of which can be performed in the physician office laboratory (POL) where she works.
While studying this chapter, think about the following questions:
• How can Anna protect herself and other patients in the practice from infectious microorganisms?
• How can body fluids or other samples be tested for the presence of pathogenic organisms?
• How are pathogenic organisms differentiated from normal, nonpathogenic species?
Microorganisms get a lot of publicity. Bioterrorism became a reality in the United States in the fall of 2001, when Bacillus anthracis spores sent through the mail caused anthrax, killing three people. Products line our grocery store shelves declaring their ability to keep us germ free. The evening news reports on the latest outbreaks of “flesh-eating bacteria,” contaminated water supplies, and antibiotic-resistant microbes. No wonder most people have the impression that all microorganisms are harmful. In reality, less than 1% of known microorganisms are pathogens. In fact, without microorganisms, we could not survive.
Microorganisms are responsible for decomposition of waste and natural recycling. The organisms that are normally present on and in our bodies ensure that our food is digested, that our blood clots properly as a result of vitamin K production by the organisms inhabiting our intestines, and that pathogens are prohibited from invading our skin, mucous membranes, and gastrointestinal and genitourinary tracts. When the normal flora are disrupted, such as by antibiotic use or hormonal changes, certain organisms that are present normally in low numbers overgrow, causing a superinfection. For example, vulvovaginal candidiasis, a yeast infection of the vaginal tract, is common in women taking broad-spectrum antimicrobial agents.
The study of immunology, or the immune system, is closely tied to microbiology. Microorganisms induce an immune response, leading to the production of a variety of molecules that come to our defense. These molecules include antibodies and mediators of inflammation such as interleukin, prostaglandins, and interferon. Often a bacterial or viral infection must be diagnosed indirectly by testing for antibodies to the infectious agent rather than by isolating the pathogen itself.
As a medical assistant, you need to understand the role of microorganisms in both health and disease. The main objective of microbiology procedures is to identify the organisms responsible for illness so that the physician can properly treat the patient. In addition, your responsibilities will include preventing nosocomial infections and assisting with infection control in the physician office laboratory (POL) and in the patients the POL serves. Microbiology procedures may be performed in the POL or in the microbiology department of a medical referral laboratory.
Chapter 27 discussed the chain of infection and how it can be broken using infection control procedures such as proper hand sanitization, antiseptics and disinfectants, and sterilization methods. This chapter covers the cultivation of microorganisms; detection of infecting organisms by microscopy; detection of specific products of infecting organisms using chemical, immunologic, or molecular techniques; and detection of antibodies produced by the patient in response to an infecting organism (immunodiagnosis).
Specimen Collection and Transport
Specimen collection and handling are among the most critical considerations in patient care, because any results the laboratory generates are directly dependent on the quality of the specimen and its condition on arrival in the laboratory. Specimens for microbiology testing must be collected in such a way as to prevent the introduction of any contaminating microorganisms. This means not only using special sterile collection and transport devices, but also taking steps to prevent environmental and patient contamination. Such steps include using antiseptics on the skin before drawing blood, instructing a patient in the collection of a urine sample using the clean catch midstream (CCMS) technique (see Chapter 52), and avoiding the teeth and tongue when collecting a throat culture specimen on a swab.
When collecting specimens for microbiologic analysis, medical assistants should ask themselves two questions: “In what ways can I prevent contamination of this sample?” and “What can I do to protect myself from becoming infected while I collect this sample?” Answers to the first question include cleansing the area to be sampled with an antiseptic, opening sterile containers only when necessary, and never touching a sterile swab or collection device to a nonsterile surface. Answers to the second question include wearing gloves and a disposable surgical mask or face shield while collecting a throat or sputum culture and wearing gloves when receiving a urine specimen from a patient who has just voided.
Ideally, specimens should be collected during the acute phase of an illness and before antibiotics are prescribed. Many types of samples can be collected. Sterile swabs can be used to collect samples from wounds and the upper respiratory tract. Serum or whole blood can be used to test for infectious organisms. Urine and feces can be collected in containers by patients at home. If patients are expected to collect specimens, it is crucial that they receive clear instructions on how to perform the procedure without contaminating the sample. The referral laboratory is responsible for providing a manual of written instructions to the POL, and the POL is responsible for providing clear instructions (preferably written) to the patient, especially if the patient will be collecting the sample in private or at home.
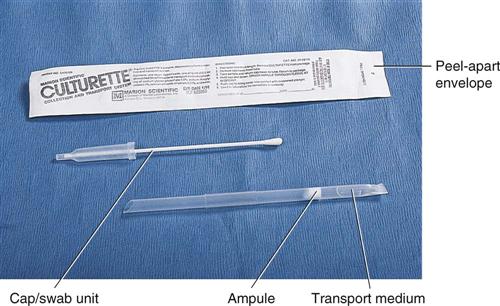
The transport of specimens is also crucial. Many different types of transport devices are available, and close attention must be given to their proper use. Microorganisms are living organisms and must be provided with conditions that permit their survival but do not permit their multiplication. If microorganisms are allowed to multiply after specimen collection, the culture results will not reflect the true disease state. Specialized transport media, such as modified Stuart’s medium or Amie’s medium, often are used in the swabbing devices used for specimen collection (Figure 55-1). These collection devices typically are made of a plastic tube that encases a sterile Dacron swab and a sealed vial of transport medium. After the specimen is obtained on the swab, it is placed in the plastic tube and the transport medium is released, usually by crushing the internal vial. It is essential to follow the manufacturer’s directions to prevent drying of the swab and specimen.
Ideally a specimen should be transported to the laboratory and cultured immediately after collection. In most situations, however, this is not possible, and the transport device must be handled by a courier en route to a referral laboratory or held in the POL until it can be cultured. For specimens that will be transported by a courier, make sure the specimens are safely packaged in leakproof containers marked with warning labels (Figure 55-2). Proper temperature and time of storage are crucial. Most pathogenic organisms prefer temperatures around 37° C (98.6° F) and will remain viable for up to 72 hours if held at room temperature or refrigerator temperature (4° C [39.2° F]). However, some organisms die if exposed to cold temperatures. Always check the referral laboratory’s procedure manual for directions on duration and temperature of storage before plating. Likewise, microorganisms have oxygen requirements; some, called aerobes, require oxygen to stay alive; others, called anaerobes, die if exposed to oxygen. Devices are available for both aerobic and anaerobic collection. Table 55-1 lists the collection, transport, and storage of specimens commonly collected or handled by medical assistants. Figure 55-3 shows some commonly used collection devices.
TABLE 55-1
| SPECIMEN | CONTAINER | PATIENT PREPARATION | SPECIAL INSTRUCTIONS | STORAGE BEFORE PROCESSING |
| Blood | Blood culture media set or Vacutainer brand blood culture tube with SPS | Disinfect venipuncture site with alcohol swab and Betadine | Draw blood during febrile episodes; draw two sets from right and left arms | Deliver to laboratory within 2 hr; incubate at 37° C (98.6° F) on receipt in the laboratory |
| Body fluids (e.g., peritoneal, synovial, pleural) | Sterile, screw-cap container or anaerobic transporter | Disinfect aspiration site with alcohol swab and Betadine | Needle aspirations are preferable to swab collections | Transport immediately and plate specimen immediately on receipt in the laboratory |
| Eye | Aerobic transport swab | Moisten swab with Amie’s or Stuart’s medium before collection | May be stored up to 24 hr at room temperature | |
| Stool | Clean, leakproof container | Transport to laboratory within 24 hr if storing at 4° C (39.2° F) | Plate within 72 hr if storing at 4° C (39.2° F) | |
| Rectal swab | Swab placed directly in enteric transport medium | Insert swab approximately 1 inch past anal sphincter | Store at 4° C (39.2° F), transport within 24 hr to laboratory and plate within 72 hr | |
| Gonorrhea culture | Jembec transport system or transport device with Stuart’s or Amie’s medium | Wipe away exudate before obtaining culture specimen, obtain culture specimen with swab | Do not refrigerate | Transport to laboratory within 2 hr |
| Chlamydia culture | Specialized Chlamydia transport medium containing antibiotics | Urogenital swabs preferred; necessary to obtain epithelial cells, not exudate | Transport immediately on ice to laboratory | Store up to 24 hr at 4° C (39.2° F); inoculate cultures within 15 min of collection if swab is not on ice |
| Skin scraping (fungal culture) | Clean, screw-top tube | Wipe skin with alcohol prep pad | Scrape skin at leading edge of lesion | Can be held indefinitely at room temperature but best to process within 72 hr of collection |
| Sputum | Sterile, screw-cap container | Patient should rinse or gargle with mouthwash before collection | Have patient collect from deep cough; do not collect saliva | Store at 4° C (39.2° F), and plate within 24 hr |
| Throat | Transport swab | Moisten swab with Stuart’s or Amie’s transport medium | Swab pharynx and tonsils, not mouth, tongue, or teeth | Transport and plate within 24 hr; room temperature storage |
| Ova and parasite (O&P) | O&P transport device (with formalin and PVA) | Three specimens collected every other day at a minimum for outpatients | Wait 7-10 days if patient has been taking Pepto-Bismol, Kaopectate, or Milk of Magnesia | Store at room temperature and deliver to laboratory within 24 hr |
| Urine | Sterile, screw-cap container | Instruct patient in clean catch midstream collection | Hold at 4° C (39.2° F) and deliver to laboratory within 24 hr | Hold at 4° C (39.2° F) and plate within 24 hr |
| Superficial wound | Aerobic transport swab | Wipe area with sterile saline or alcohol prep pad before collection | Moisten swab with Amie’s or Stuart’s medium before collection | Transport and plate within 24 hr; room temperature storage |
| Deep wound or abscess | Anaerobic transport device | Wipe area with sterile saline or alcohol prep pad before collection | Aspirate material, excise tissue, or insert swab deep into wound | Transport and plate within 24 hr; room temperature storage |
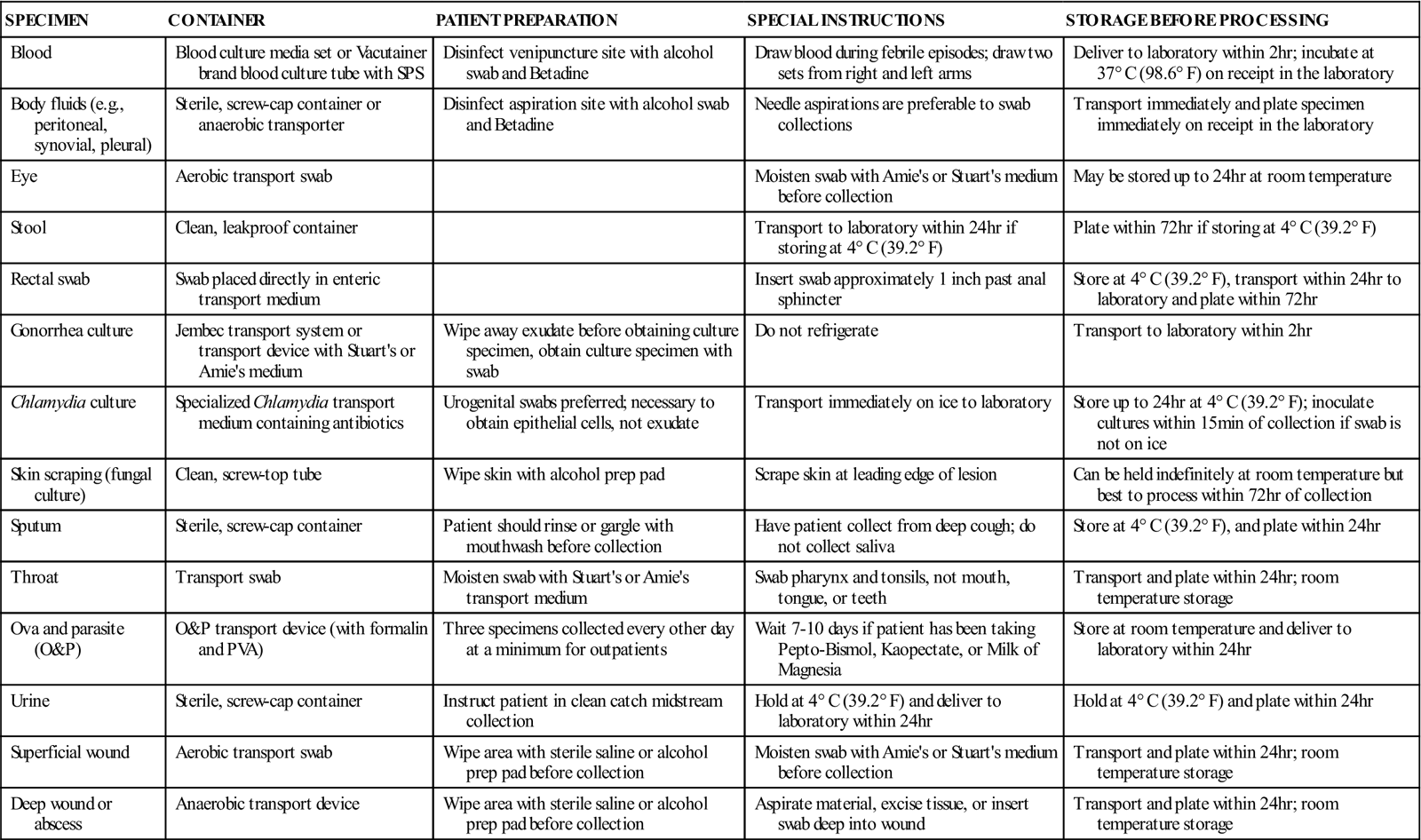
SPS, Sodium polyanetholsulfonate; PVA, polyvinyl alcohol.
Modified from Forbes BA, Sahm DF, Weissfeld AS: Bailey and Scott’s diagnostic microbiology, ed 11, St Louis, 2002, Mosby.
Classification of Microorganisms
Once the specimen reaches the microbiology laboratory, it is analyzed for the presence of infectious microorganisms or their components. Although the medical assistant is not responsible for identifying microorganisms, a working knowledge of the terminology used in the classification of microorganisms is essential.
Most cultures handled by the medical assistant have been ordered to diagnose bacterial infections. Bacteria are one type of microorganism; other microorganisms include fungi and protozoa. Parasitic worms are studied in the microbiology laboratory but are not considered microorganisms.
Many microbiologists do not consider viruses to be microorganisms simply because they are not, by definition, alive. Viruses consist of a core of either ribonucleic acid (RNA) or deoxyribonucleic acid (DNA) covered by a protein shell. Alone, they neither metabolize nor reproduce; however, once inside a host cell, viruses use the host cell’s organelles and macromolecules to multiply. Because of this absolute need for a host cell for replication, viruses are called obligate intracellular parasites, and they cannot be cultured on artificial media such as those used to culture bacteria.
Viruses must be cultured in fertilized eggs or in tissue culture, which is done by referral or hospital laboratories. Often, instead of culturing a specimen for a virus, antigenic products of the virus or antibodies made by a patient to a virus are detected. For example, in the diagnosis of hepatitis B virus infection, the serum is tested for hepatitis B surface antigen (HBsAg), a protein found in the shell of the virus. Table 55-2 lists common diseases caused by viruses.
TABLE 55-2
Common Diseases Caused by Viruses
| DISEASE | VIRUS | TRANSMISSION | SYMPTOMS | TESTS | PREVENTION |
| Smallpox | Variola major | Direct contact; fomites | Vesicles on entire body, including soles and palms | Eradicated (vaccine is still available) | |
| Infectious mononucleosis | Epstein-Barr virus | Direct and airborne | Sore throat, fever, malaise, lymph gland involvement; hepatitis, enlarged spleen | Serology testing for heterophile antibodies; CBC | Avoid direct contact with known cases |
| Influenza | Myxovirus—influenza A and B | Droplet and fomites | Fever, body aches, cough | Nasopharyngeal swab, nasal wash | Immunization for the old, young, and debilitated |
| Warts (verruca) | Human papilloma virus | Direct and indirect contact | Circumscribed outgrowths on skin; most common on hands and feet | ||
| Rabies | Rhabdovirus | Contact with saliva of infected animal (dog, cat, skunk, fox, bat are usual) | Fever, uncontrollable excitement, spasms of the throat, profuse salivation | DFA from brain or hair follicle tissue | Vaccine available; vaccinate pets |
| Mumps | Paramyxovirus—mumps virus | Direct contact | Pain, swelling of salivary glands; fever | Acute and convalescent titers | MMR vaccine |
| Measles | Paramyxovirus—measles virus | Direct contact; droplets | Fever, nasal discharge, red eyes; Koplik’s spots, rash | Serologic titer | MMR vaccine |
| Rubella (German measles) | Direct contact; droplets; congenital | Rash, swollen lymph glands; causes severe birth defects | Serologic titer | MMR vaccine | |
| Common cold | Rhinovirus and many others | Direct; droplets; fomites | Headache, fever, runny nose, congestion | Good hygiene (hand washing) | |
| Polio | Poliovirus | Direct contact; carriers enter via mouth | Fever, headache, stiff neck and back, paralysis of muscles | IPV; SC or IM; four doses | |
| Molluscum contagiosum warts | Molluscipox virus | Direct contact with infected individual | Small pink or white domes found in clusters | Microscopic evaluation | Avoid contact with infected individual; have existing warts removed |
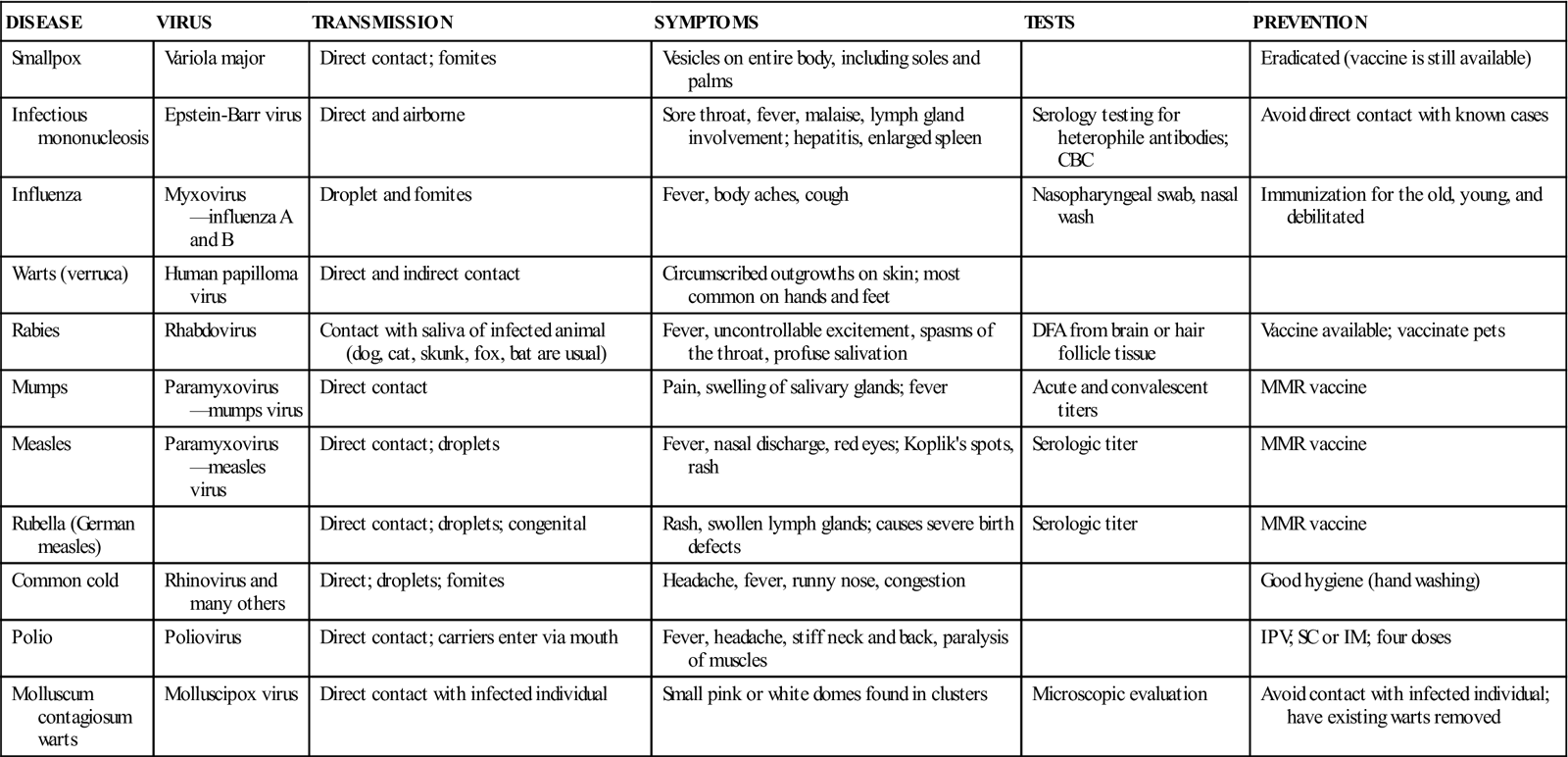
Courtesy Kathleen Moody.
Naming of Microorganisms
Scientists have used the binomial system of nomenclature developed by Swedish botanist Carl von Linné to name all living organisms: animals, plants, fungi, protozoa, and bacteria. This binomial system assigns two names; the first name is the genus, and the second is the species. Both names are either italicized or underlined when written. The genus begins with a capital letter, the species with a lowercase letter. Often the name reveals some characteristic about the organism. For example, Neisseria gonorrhoeae is a bacterium that was studied extensively by Albert Neisser, and it causes the sexually transmitted disease gonorrhea. When culture results are reported, it is essential that both the genus and species names be recorded. Different species may cause different symptoms or require different antibiotic treatment. For example, N. gonorrhoeae causes disease, whereas N. sicca is found in the mouth and does not cause disease under normal conditions.
The genus name of the organism may be represented by a single letter after the organism’s full genus and species name has been written once in a report. For example, Escherichia coli is commonly referred to as E. coli.
Typical Pathogenic Bacteria
Bacteria are single-celled prokaryote organisms that reproduce by binary fission, a process that involves duplication of the chromosome and subsequent fission (splitting in half) of the cell. This process of asexual reproduction results in tremendous numbers of bacteria from a single cell and explains why bacterial infections can quickly overwhelm a person’s immune system. Some bacteria reproduce in as little as 14 minutes, whereas others take days to divide. Theoretically, a single E. coli cell, which has a reproduction time of about 30 minutes, produces 351,843,724,088,831 offspring in 24 hours if it is able to enter the urinary bladder.
Bacteria often are classified according to their shape, their staining characteristics, and the environmental conditions in which they thrive. Both shape and staining characteristics are direct results of the cell wall composition. Three types of cell wall structures are found among pathogenic bacteria: gram positive, gram negative, and acid fast. These designations are based on reactions in specialized stains used to see the bacteria under the microscope. The bacterial cell wall is composed of peptidoglycan (PG), a molecule composed of carbohydrate and protein. The gram-positive cell has a thick layer of PG with no lipid layer surrounding it; the gram-negative cell has a thin layer of PG with a lipid layer surrounding it; and the acid-fast cell has a thin layer of PG surrounded by a thick layer of waxlike lipids. Acid-fast bacteria do not stain well with Gram stain, and gram-positive and gram-negative bacteria both stain negative with the acid-fast stain. With Gram stain, gram-positive bacteria stain purple, and gram-negative bacteria stain pink. With the acid-fast stain, acid-fast–positive bacteria (AFB) stain pink and acid-fast–negative bacteria stain blue.
Bacterial Shapes
Pathogenic bacteria assume three different morphologic shapes. Spheric bacteria are called cocci (singular, coccus), rod-shaped bacteria are bacilli (singular, bacillus), and spiral bacteria are spirilla (singular, spirillum). Tightly coiled spirilla are called spirochetes. Certain arrangements are also seen in different genera and species. When bacteria are in a chain formation, the prefix strepto- is used. When bacteria are found in pairs, the prefix diplo- is used, and when they are found in grapelike clusters, the prefix staphylo- is used. Cocci in packets of four are called tetrads and in packets of eight or 16 are called sarcinae (Figure 55-4).
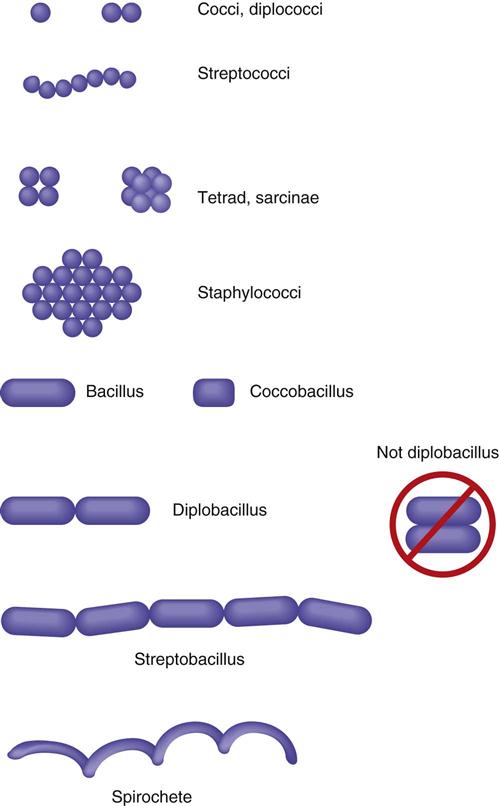
Bacterial Oxygen Requirements
Bacteria are also classified according to oxygen requirements. As mentioned, those that require oxygen to live are called aerobes; those that die in the presence of oxygen are anaerobes. Some bacteria are flexible concerning oxygen requirements and, although they are anaerobes, can survive in the presence of oxygen. These organisms are called facultative anaerobes. Mycobacterium tuberculosis thrives in white blood cells in the lungs, causing tuberculosis; it is an aerobe. Bacteroides fragilis is the predominant bacterium found in the intestines. This gram-negative bacillus is an anaerobe. E. coli, also an inhabitant of the intestines and the most common cause of urinary tract infections, is a facultative anaerobe.
Bacterial Physical Structures
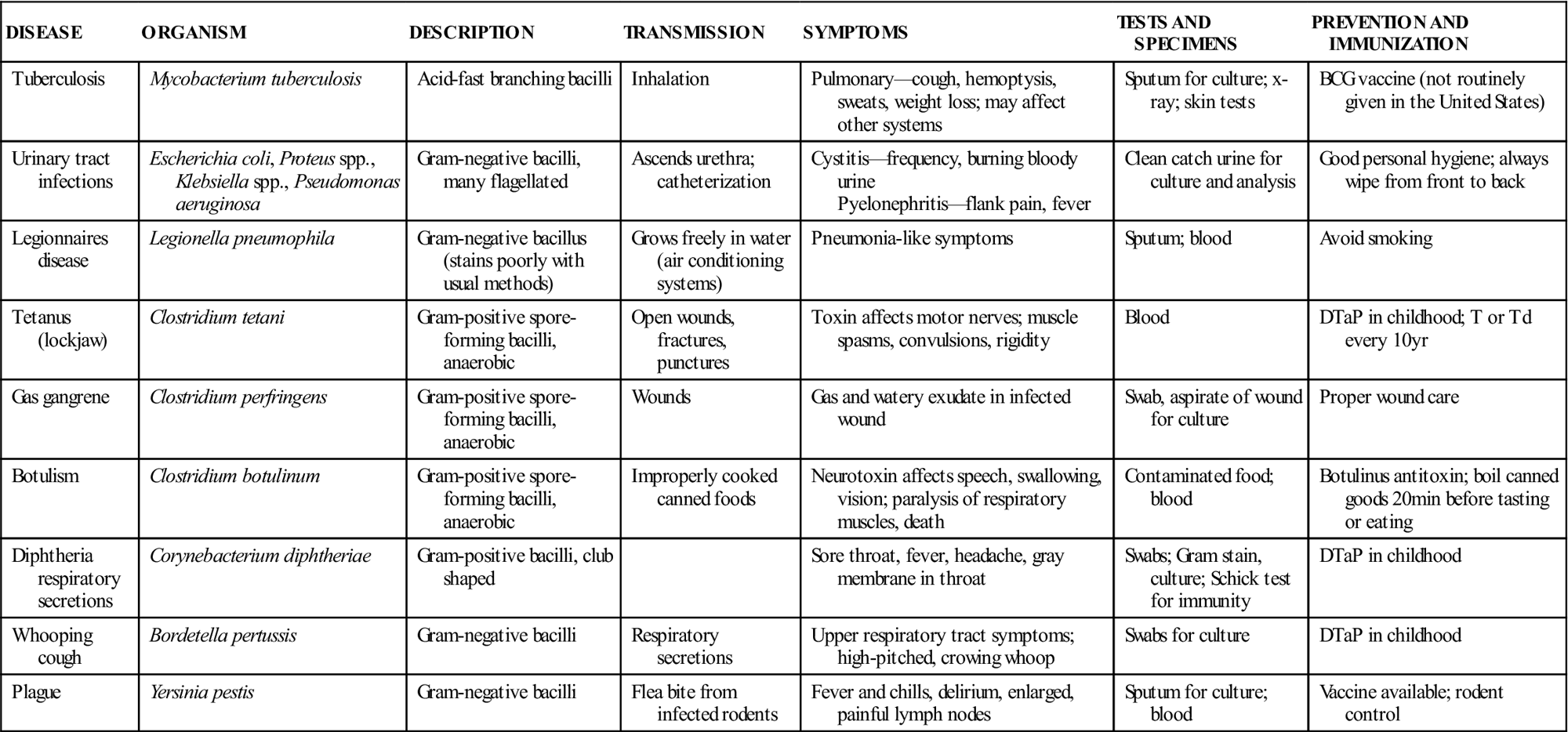
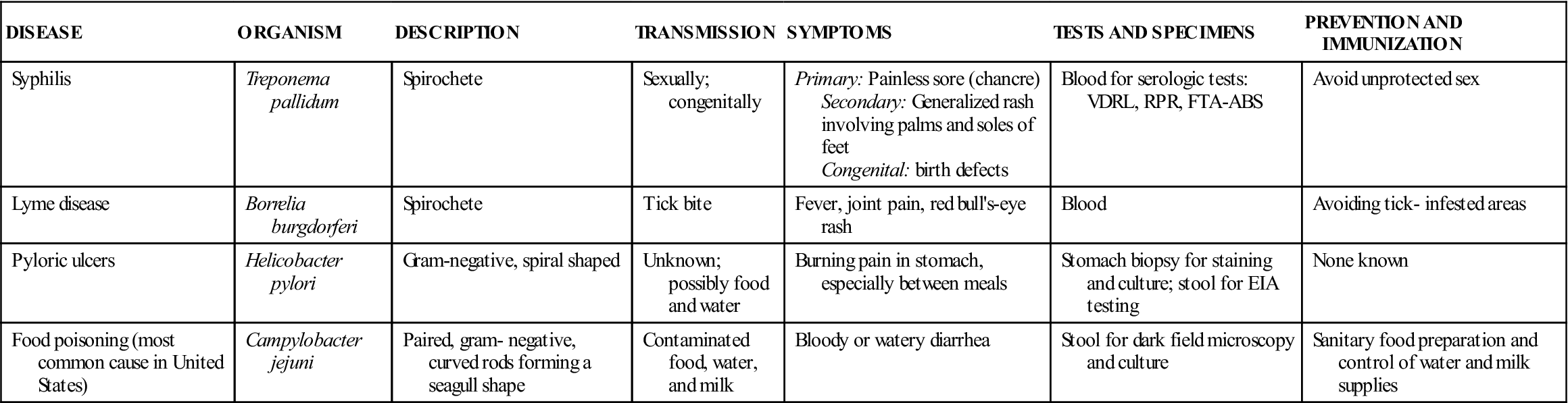
Bacteria can be classified and identified according to physical structures. Some bacteria have thin, long structures called flagella that aid in propulsion. Proteus vulgaris is a gram-negative bacillus with many flagella surrounding the cell. It can propel itself into the bladder and is the primary cause of nosocomial urinary tract infections. Some bacteria may have thick, gelatinous coats surrounding the cell wall; these are called capsules. Streptococcus pneumoniae is nonpathogenic if it is not producing a capsule; however, it is the most common cause of pneumonia in older adults when it is encapsulated. The Pneumovax vaccine, which is given to older patients and those at high risk for respiratory complications (e.g., patients with asthma), is composed of highly purified capsular polysaccharides from 23 strains of S. pneumoniae. Certain bacteria are able to form intracellular structures called endospores that allow the cell to remain viable when environmental conditions are not favorable. Bacillus anthracis produces such spores, as does Clostridium tetani. If spores of C. tetani enter a wound and germinate, they cause the disease known as tetanus. Tables 55-3 to 55-5 list some important infectious diseases caused by typical pathogenic bacteria.
TABLE 55-3
Common Diseases Caused by Bacilli
| DISEASE | ORGANISM | DESCRIPTION | TRANSMISSION | SYMPTOMS | TESTS AND SPECIMENS | PREVENTION AND IMMUNIZATION |
| Tuberculosis | Mycobacterium tuberculosis | Acid-fast branching bacilli | Inhalation | Pulmonary—cough, hemoptysis, sweats, weight loss; may affect other systems | Sputum for culture; x-ray; skin tests | BCG vaccine (not routinely given in the United States) |
| Urinary tract infections | Escherichia coli, Proteus spp., Klebsiella spp., Pseudomonas aeruginosa | Gram-negative bacilli, many flagellated | Ascends urethra; catheterization | Cystitis—frequency, burning bloody urine Pyelonephritis—flank pain, fever | Clean catch urine for culture and analysis | Good personal hygiene; always wipe from front to back |
| Legionnaires disease | Legionella pneumophila | Gram-negative bacillus (stains poorly with usual methods) | Grows freely in water (air conditioning systems) | Pneumonia-like symptoms | Sputum; blood | Avoid smoking |
| Tetanus (lockjaw) | Clostridium tetani | Gram-positive spore-forming bacilli, anaerobic | Open wounds, fractures, punctures | Toxin affects motor nerves; muscle spasms, convulsions, rigidity | Blood | DTaP in childhood; T or Td every 10 yr |
| Gas gangrene | Clostridium perfringens | Gram-positive spore-forming bacilli, anaerobic | Wounds | Gas and watery exudate in infected wound | Swab, aspirate of wound for culture | Proper wound care |
| Botulism | Clostridium botulinum | Gram-positive spore-forming bacilli, anaerobic | Improperly cooked canned foods | Neurotoxin affects speech, swallowing, vision; paralysis of respiratory muscles, death | Contaminated food; blood | Botulinus antitoxin; boil canned goods 20 min before tasting or eating |
| Diphtheria respiratory secretions | Corynebacterium diphtheriae | Gram-positive bacilli, club shaped | Sore throat, fever, headache, gray membrane in throat | Swabs; Gram stain, culture; Schick test for immunity | DTaP in childhood | |
| Whooping cough | Bordetella pertussis | Gram-negative bacilli | Respiratory secretions | Upper respiratory tract symptoms; high-pitched, crowing whoop | Swabs for culture | DTaP in childhood |
| Plague | Yersinia pestis | Gram-negative bacilli | Flea bite from infected rodents | Fever and chills, delirium, enlarged, painful lymph nodes | Sputum for culture; blood | Vaccine available; rodent control |
Courtesy Kathleen Moody.
TABLE 55-4
Common Diseases Caused by Cocci
| DISEASE | ORGANISM | DESCRIPTION | TRANSMISSION | SYMPTOMS | SPECIMENS | TESTS | PREVENTION |
| Pneumonia | Streptococcus pneumoniae | Gram-positive encapsulated cocci in pairs | Direct contact, droplets | Productive cough, fever, chest pain | Sputum; bronchoscopy secretions | Culture, Gram stain | Vaccine |
| Strep throat | Streptococcus pyogenes (group A streptococcus) | Gram-positive cocci in chains | Direct contact, droplets, fomites | Severe sore throat, fever, malaise | Direct swab | Rapid strep test, throat culture | Good personal hygiene |
| Wound infection, abscesses, boils | Staphylococcus aureus | Gram-positive cocci in clusters | Direct contact, fomites, carriers; poor hand washing | Area red, warm, swollen; pus; pain; ulceration or sinus formation | Deep swab; aspirate of drainage | Culture and sensitivity (aerobic and anaerobic) | Good personal hygiene |
| Staphylococcal food poisoning | Staphylococcus aureus | Gram-positive cocci in clusters | Poor hygiene and improper refrigeration of foods | Vomiting, abdominal cramps, diarrhea | Suspected food, stool | Culture of food (organism is not found in stool) | Refrigerate food to prevent toxin production |
| Toxic shock | Staphylococcus aureus | Gram-positive cocci in clusters | Use of absorbent packing materials (e.g., tampons, nasal packs) | Fever, headache, nausea, vomiting, delirium, low blood pressure | Swab, blood | Culture and serology | Change tampon, packing material often |
| Gonorrhea | Neisseria gonorrhoeae | Gram-negative cocci in pairs; intracellular in white blood cells | Sexually transmitted | Females: Pelvic pain, discharge; may be asymptomatic Males: Urethral drip, pain on urination | Swab of cervix, urethra; rectal and pharyngeal swabs in homosexual men | Gram stain; culture | Avoid unprotected sex |
| Meningococcal meningitis | Neisseria meningitidis | Gram-negative diplococci | Respiratory tract secretions | High fever, headache, projectile vomiting, delirium, neck and back rigidity, convulsions, petechial rash | Nasopharyngeal swabs, cerebrospinal fluid, blood | Gram stain; culture; cell counts and chemistries | Vaccine; prophylactic antibiotics |
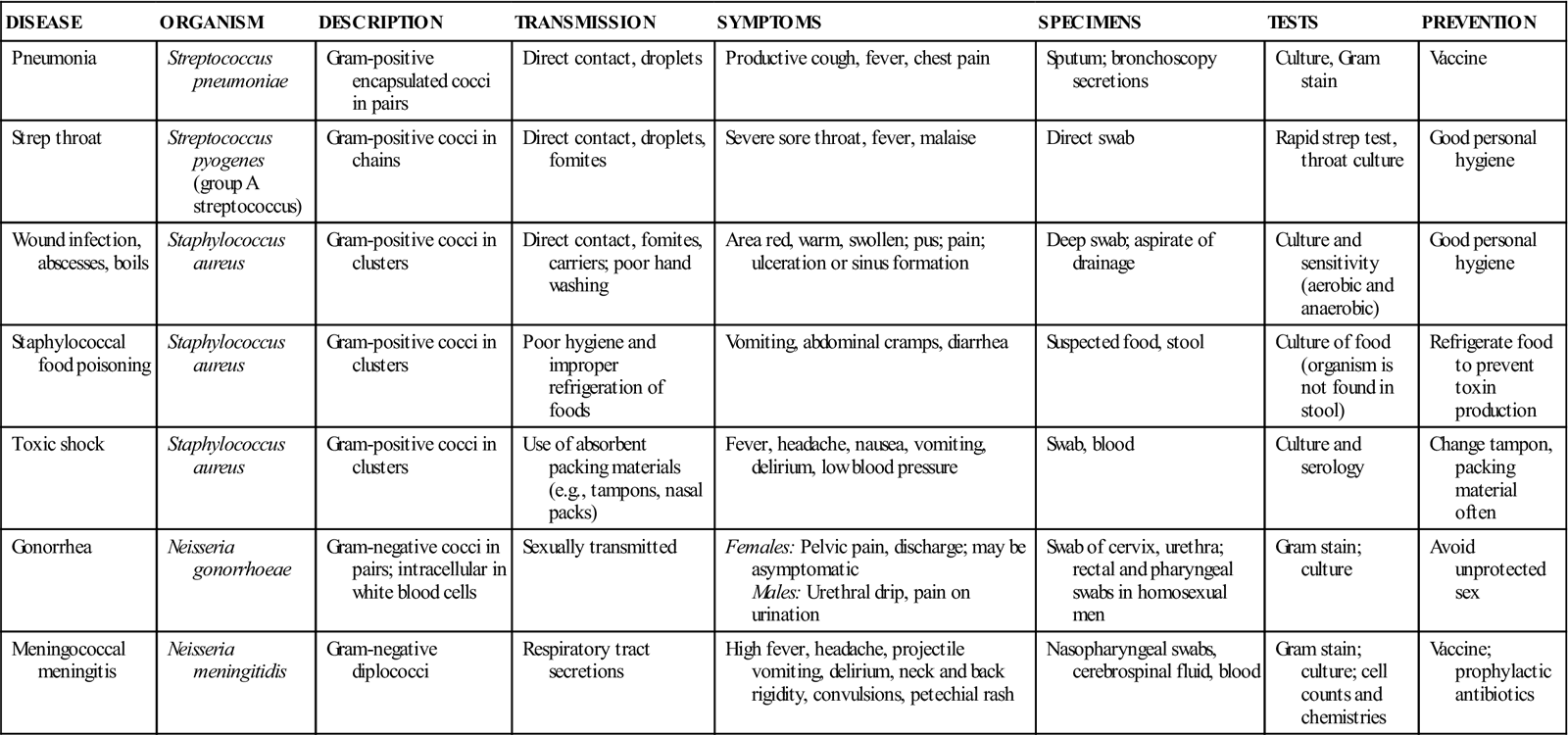
TABLE 55-5
Common Diseases Caused by Spirilla
| DISEASE | ORGANISM | DESCRIPTION | TRANSMISSION | SYMPTOMS | TESTS AND SPECIMENS | PREVENTION AND IMMUNIZATION |
| Syphilis | Treponema pallidum | Spirochete | Sexually; congenitally | Primary: Painless sore (chancre) Secondary: Generalized rash involving palms and soles of feet Congenital: birth defects | Blood for serologic tests: VDRL, RPR, FTA-ABS | Avoid unprotected sex |
| Lyme disease | Borrelia burgdorferi | Spirochete | Tick bite | Fever, joint pain, red bull’s-eye rash | Blood | Avoiding tick- infested areas |
| Pyloric ulcers | Helicobacter pylori | Gram-negative, spiral shaped | Unknown; possibly food and water | Burning pain in stomach, especially between meals | Stomach biopsy for staining and culture; stool for EIA testing | None known |
| Food poisoning (most common cause in United States) | Campylobacter jejuni | Paired, gram- negative, curved rods forming a seagull shape | Contaminated food, water, and milk | Bloody or watery diarrhea | Stool for dark field microscopy and culture | Sanitary food preparation and control of water and milk supplies |
Courtesy Kathleen Moody.
Unusual Pathogenic Bacteria: Chlamydiae, Mycoplasmas, and Rickettsiae
In the small scale used to measure microorganisms, viruses range from 10 to 100 nanometers (nm). Typical pathogenic bacteria measure 1,000 to 5,000 nm. Chlamydiae, mycoplasmas, and rickettsiae are tiny, unusual bacteria that fall between the size ranges of viruses and typical pathogenic bacteria.
The rickettsiae are tiny gram-negative bacteria that are transmitted by blood-sucking arthropods. They cannot multiply outside a host cell, and once inside the host cell, they are able to perform only some of the life-sustaining metabolic reactions on their own. Chlamydiae also are tiny bacteria that require host cells for growth and once were considered viruses. Unlike rickettsiae, chlamydiae are not transmitted by arthropod vectors.
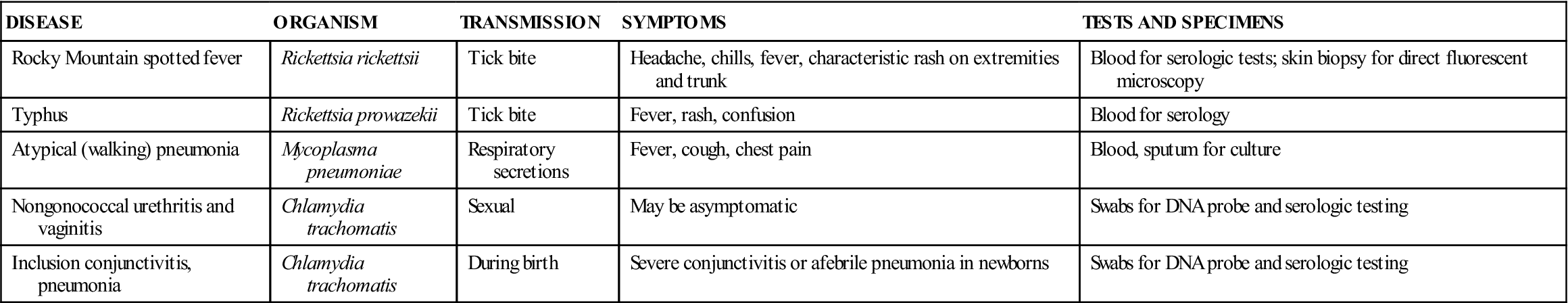
Mycoplasmas are unusual in that they have no PG in the cell wall, but they are not obligate parasites as are rickettsiae and chlamydiae. Rickettsiae and chlamydiae do not grow on artificial media in the laboratory; tissue culture or serologic testing is required to identify them. Mycoplasmas can be cultivated from a patient specimen in the laboratory (Table 55-6).
TABLE 55-6
Diseases Caused by Rickettsiae, Mycoplasmas, and Chlamydiae
| DISEASE | ORGANISM | TRANSMISSION | SYMPTOMS | TESTS AND SPECIMENS |
| Rocky Mountain spotted fever | Rickettsia rickettsii | Tick bite | Headache, chills, fever, characteristic rash on extremities and trunk | Blood for serologic tests; skin biopsy for direct fluorescent microscopy |
| Typhus | Rickettsia prowazekii | Tick bite | Fever, rash, confusion | Blood for serology |
| Atypical (walking) pneumonia | Mycoplasma pneumoniae | Respiratory secretions | Fever, cough, chest pain | Blood, sputum for culture |
| Nongonococcal urethritis and vaginitis | Chlamydia trachomatis | Sexual | May be asymptomatic | Swabs for DNA probe and serologic testing |
| Inclusion conjunctivitis, pneumonia | Chlamydia trachomatis | During birth | Severe conjunctivitis or afebrile pneumonia in newborns | Swabs for DNA probe and serologic testing |
Courtesy Kathleen Moody.
Fungi
Mycology is the study of fungi and the diseases they cause. Fungi (singular, fungus) are eukaryotes that are larger than bacteria; they include unicellular yeasts and multicellular molds. Fungi are present in the soil, air, and water, but only a few species cause disease. They are transmitted by direct contact with infected persons, by prolonged exposure to a moist environment, and by inhalation of contaminated dust or soil. Fungal infections may be superficial, affecting only the skin, hair, or nails. However, some fungi can penetrate the tissues of the internal body structures and produce serious diseases of the mucous membranes, heart, lungs, and other organs. Fungal infections are resistant to the antibiotics used in the treatment of bacterial infections, and fungi must be treated with drugs active against their unusual cell walls.
A superficial fungal infection often is referred to as a tinea (Latin for “ringworm”). Tinea pedis, for example, is athlete’s foot; tinea barbae is a fungal infection of the facial hair follicles. The term ringworm arose because the infected area is often circular and appears wrinkled in the center as a result of the healing process. Diagnosis of fungal infections usually is based on culturing or microscopic observation of skin scrapings, hair samples, or samples of sputum or mucous membranes. Usually the samples are treated with potassium hydroxide before microscopic observation to dissolve away nonfungal material, making the fungal elements easier to observe (Table 55-7).
TABLE 55-7
Common Diseases Caused by Fungi
| DISEASE | ORGANISM | PREDISPOSING CONDITIONS AND TRANSMISSION | SYMPTOMS | TESTS AND SPECIMENS |
| Thrush (oral yeast), vulvovaginal candidiasis, or monilia (vaginal yeast) | Candida spp. (yeast) | Oral: During birth Other: After antibiotic therapy, oral birth control, severe diabetes | White, cheesy growth | Swab for KOH prep, culture |
| Athlete’s foot, jock itch, ringworm (tinea) | Trichophyton spp., Microsporum spp., and others (skin fungi) | Opportunist; direct contact; clothing; prolonged exposure to moist environment | Hair loss, thickening of skin, nails; itching; red, scaly patches | Skin scraping for KOH prep; skin, hair for culture |
| Histoplasmosis | Histoplasma capsulatum | Inhalation of dust contaminated with bird or bat droppings | Mild, flulike to systemic | Serologic; culture of biopsy material |
| Cryptococcosis | Cryptococcus neoformans | Contact with poultry droppings | Cough, fever, malaise; can become systemic | Sputum culture; cerebrospinal fluid culture, India ink direct examination |
| Sporotrichosis | Sporothrix schenckii | Farmers, florists, people exposed to soil | Skin lesions that spread along lymphatics; can become systemic | Skin scraping for KOH prep; serologic |
| Pneumocystis pneumonia | Pneumocystis carinii | Widely prevalent in animals; occurs in debilitated or immunosuppressed individuals; common in patients with AIDS | Pneumonia-like | Biopsy of lung tissue with microscopic examination |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree