27 Assessment and monitoring of the perianesthesia patient
Alveolar Artery Carbon Dioxide Differences: The difference between the PaCO2 and the ETCO2 level is referred to as the alveolar-arterial CO2 difference (a-ADCO2).
Alveolar Dead Space: Alveoli that do not participate in gas exchange because of lack of blood flow.
Anatomic Dead Space: Areas of the tracheobronchial tree not involved in gas exchange.
Capnography: Measurement of end-tidal carbon dioxide at the patient’s airway that allows continuous assessment of the adequacy of alveolar ventilation.
Dead Space Ventilation: Includes anatomic, alveolar, and physiologic (total) dead space.
End-Tidal Carbon Dioxide (ETCO2): At the end of exhalation, the peak carbon dioxide occurs, which in the normal lung is the best approximation of alveolar carbon dioxide levels.
Flow-Directed Pulmonary Artery Catheter (FDPAC): Pulmonary artery thermodilution catheter used in hemodynamic monitoring.
Hemodynamic Monitoring (Invasive Monitoring): The monitoring of blood flow through the use of invasive catheters to provide pressure measurements in the systemic and pulmonary circulations, central veins, pulmonary capillary bed, and the right or left atrium, as well as cardiac output.
Hyperthermia: A core temperature greater than 38° C (100.4° F).
Hypothermia: A core temperature less than 36° C (96.8° F).
Left Atrial Pressure: Measured with a catheter placed directly in the left atrium. Usually monitored only in open heart cases when direct access to the left atrium can be reached. In the absence of mitral valve disease or left atrial tumor, left atrial pressure reflects left ventricular end-diastolic pressure and left ventricle preload.
Obstructive Sleep Apnea: Repeated episodes of obstructive apnea during sleep together with daytime sleepiness, mood changes, and altered function.1
Physiologic Dead Space: The sum of anatomic and alveolar dead space.
Pulmonary Artery Pressure: Pressure in the pulmonary artery.
Pulmonary Capillary Wedge Pressure (PCWP): Also known as the pulmonary capillary occlusive pressure; reflects the pressure in the left atrium.
Pulmonary Vascular Resistance: The resistance, impedance, or pressure that the right ventricle must overcome to eject the blood into the pulmonary artery.
Pulse Oximetry: Pulse oximetry (SpO2) is used for noninvasive measurement of arterial oxygen saturation (SaO2) in the blood.
Right Atrial Pressure: Reflects venous return to the right side of the heart and right ventricular end diastolic pressure (preload).
Systemic Vascular Resistance: The resistance, impedance, or pressure the left ventricle must overcome to eject the blood from the left ventricle.
Temporal Artery Temperatures: Scanning of the forehead over the temporal artery with a noninvasive thermometer.
The primary purpose of the postanesthesia care unit (PACU) is the critical evaluation and stabilization of patients after procedures, with an emphasis on the anticipation and prevention of complications that result from anesthesia or the operative or interventional procedure. A knowledgeable, skillful perianesthesia nurse must fully assess the condition of each patient not only at admission and at discharge but also at frequent intervals throughout the postanesthesia period. Assessment must be a continuous and complete process that leads to sound nursing judgment and the implementation of therapeutic care. Assessment includes the gathering of information from direct observation of the patient (the primary source), from the physician, other health care personnel, and from the medical record and the care plan. Traditionally perianesthesia nurses have, with only limited information, performed the role of caring for the surgical and interventional patients in the vulnerable postanesthesia state. However, for assessment of the perianesthesia patient and plan and implementation of appropriate care, preoperative information must be available as a basis for comparison with postoperative data. The perianesthesia nurse has a professional obligation to consider the patient’s history, clinical status, and psychosocial state. The necessary data may be gathered with chart review, personal preoperative visit, and consultation with other health care members who provide care to the patient. The collection of such information should be a coordinated effort with all involved members of the health care team. This chapter discusses the assessment of postprocedure patients and their common needs. Specific assessments related to patient age, the type of procedure, and problems that result from complicated diagnoses are addressed in following chapters. The assessment and management of postoperative pain is presented in Chapter 31.
Preoperative assessments
The preoperative evaluation of both the physical and the emotional status of the surgical patient is extremely important, and nursing brings a unique perspective to this assessment. The scope of perianesthesia nursing practice involves the age-specific assessment, diagnosis, intervention, and evaluation of patients within the perianesthesia continuum. The scope identifies risks for problems that can result from the administration of sedation/analgesia or anesthetic agents for surgical, diagnostic, or therapeutic procedures.2 Nurses in a number of subspecialties, including perianesthesia nurses, perioperative nurses, and general unit nurses, have advocated this assessment. A preoperative visit from each nurse who will care for the patient seems redundant and can be overwhelming for the patient. More appropriately, nurses should treat each other as colleagues who communicate needs for specific information, coordinate the collection of such information, and document data to be used for planning care. Multidisciplinary communications are instrumental in the education of all those who care for the patient and in the development of communication patterns.
Admission observations
Physical assessment of the perianesthesia patient must begin immediately on admission to the PACU. The patient is accompanied from the procedure room to the PACU by the anesthesia provider or monitoring nurse, who reports to the receiving nurse on the patient’s general condition, the procedure performed, and the type of anesthesia or sedation used. In addition, the nurse should be informed of any problems or complications encountered during the procedure and anesthesia or sedation (see Chapter 26). Because all anesthetics are depressants, postoperative assessment and care generally are the same, regardless of the specific agent used. For special precautions required for certain agents, review the chapters on anesthesia (see Chapters 19 through 25).
Respiratory function
Respiratory assessment is coupled with the related responses of the cardiovascular and neurologic systems for total evaluation of the adequacy of gas exchange and ventilatory efficiency. Respiratory function is evaluated with clinical assessment. Pulse oximetry is used for assessment of arterial oxygenation, and capnography is used in evaluation of the adequacy of alveolar ventilation. Arterial blood gas measurements may be a part of the respiratory assessment (see Chapters 12, 29, and 30).
Clinical assessment
Inspection
The resting respiratory rate of a normal adult is approximately 12 to 20 beats/min. Infants and children have a higher respiratory rate and a lower tidal volume than adults (see Chapter 49). Respirations should be quiet and easy and have a regular rate and rhythm. The chest should move freely as a unit, and expansion should be equal bilaterally. Alterations in symmetry can be caused by many factors, including pain, that may cause splinting at the incision site, consolidation, and pneumothorax. The nurse should note the character of the respirations; intercostal retractions, bulging, nasal flaring, or use of the accessory respiratory muscles, which are signs of respiratory distress. The depth of respiration is as important as the rate. Shallow respirations are the cardinal sign of continuing depression from anesthesia or preoperative medications, but can be caused by many other factors, including incisional pain, obesity, tight binders, and dressings that restrict movements of the thoracic cage or abdomen. Shallow respirations and use of the neck and diaphragmatic muscles may also indicate reparalyzation from the use of skeletal muscle relaxants such as succinylcholine, atracurium, pancuronium, and vecuronium. The presence of chest movements alone does not provide evidence that adequate gas exchange is occurring.
Listening and auscultation
First, the perianesthesia nurse should listen unaided to the patient’s respirations. Normal respiration should be quiet; noisy breathing indicates a problem. Extraneous sounds always indicate some kind of obstruction; however, quiet breathing does not always indicate the absence of problems. An accumulation of mucus or other secretions, evidenced by gurgling in any of the respiratory passages, can cause airway obstruction and should be removed immediately. Purposeful coughing with good expiratory airflow is the most effective method of clearing secretions. If the patient is not yet reactive enough to do this alone, the secretions must be suctioned orally and nasally. Nasotracheal suctioning may be useful to clear secretions and to stimulate cough, but the catheter is ineffective for reaching secretions distal to the carina. Obstruction can also occur from poor oropharyngeal muscle tone caused by the muscle-relaxant effect of general anesthesia plus the rolling back of the tongue. Patients with obstructive sleep apnea are prone to airway obstruction and should not undergo extubation until they are fully awake. Tracheal extubation should be performed only when the patient is breathing spontaneously with adequate tidal volumes, oxygenation, and ventilation.1,3 To relieve airway obstruction, use the jaw thrust maneuver by providing anterior pressure support on the angle of the jaw to open the air passages.
Monitoring of oxygenation with pulse oximetry
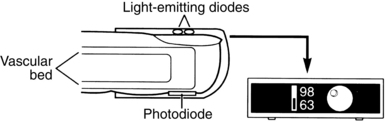
A pulse oximeter is used for noninvasive measurement of arterial oxygen saturation (SaO2) in the blood (SpO2 when measured with pulse oximetry) and is a valuable adjunct to the clinical assessment of oxygenation. Many clinical indicators, such as the patient’s color and the characteristics of the respirations, are subjective, and the physical signs of cyanosis are not evident until hypoxia is severe. Pulse oximetry monitoring is objective and continuous and provides an early warning of developing hypoxemia, thus allowing intervention before signs of hypoxia appear. Consequently, pulse oximetry has been widely adopted in the PACU as a tool for both safety monitoring and patient management. As a confirmation of the importance of pulse oximetry, the American Society of PeriAnesthesia Nurses (ASPAN) PeriAnesthesia Nursing Standards and Practice Recommendations 2010-2012 recommends evaluation of all PACU patients with pulse oximetry at admission and discharge, and ASPAN recommends a pulse oximeter for every patient in all phases of perianesthesia nursing levels of care.2
A pulse oximeter consists of a microprocessor-based monitor and a sensor (Fig. 27-1). In addition to a SpO2 display, most oximeters display the pulse rate and have an adjustable alarm system that sounds when values register outside a designated range. A variety of sensors is available, each intended for application to specific sites and for use on patients of various sizes (the manufacturer’s instructions describe these requirements). The sensor is applied to a site with a good arterial supply. The most common application site is a finger or toe (hand or foot in neonates); other sites include the nose, the forehead, the earlobe, or the temple. Both reusable sensors and disposable adhesive sensors are available, and disposable sensors allow for patient-dedicated monitoring when infection control concerns are present.
Technology overview
Interpretation of pulse oximetry measurements
Consideration of the mechanisms of oxygen transport is essential for adequate interpretation of SpO2. Approximately 98% of the oxygen in blood is bound to hemoglobin; SaO2 and SpO2 reflect this blood oxygen. The remaining blood oxygen is dissolved in plasma; blood gas analysis measures the partial pressure exerted by this oxygen dissolved in plasma (PaO2 = 80 – 100 mm Hg at sea level). The dissolved oxygen is used to meet immediate metabolic needs. The oxygen bound to hemoglobin serves as the reservoir that replenishes the pool of dissolved oxygen (see Chapter 12).
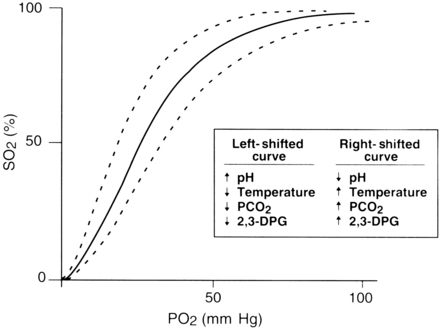
The rate at which oxygen binds to hemoglobin is primarily controlled by two factors: the PaO2 and the affinity of hemoglobin for oxygen. This relationship between SaO2 and PaO2 is represented by the oxyhemoglobin dissociation curve. The curve is sigmoid in shape, and its position is affected by a number of physiologic variables that change the affinity of hemoglobin for oxygen (Fig. 27-2).
Clinical issues
As with any technology, important clinical issues must be considered for appropriate use of pulse oximetry. Shifts in the oxyhemoglobin dissociation curve that are caused by abnormal values of pH, temperature, partial pressure of carbon dioxide (PCO2), and 2,3-diphosphoglycerate must be considered. Consideration of the patient’s hemoglobin level is also important because a pulse oximeter cannot detect depletion in the total amount of hemoglobin. When pulse oximetry is used on a postoperative patient with a low hemoglobin level, a high SpO2 value might not reflect adequate oxygenation. The amount of hemoglobin, although it is well saturated with oxygen, may be inadequate to meet tissue needs because fewer carriers are available to transport oxygen.
Patient movement can produce false signals that interfere with the ability of the pulse oximeters to identify the true pulse, thus leading to unreliable SpO2 and pulse rate readings. The sensor should be properly and securely applied; a sensor that is loosely attached or incorrectly positioned can magnify the effect of motion. If the problem persists, consideration should be given to moving the sensor to a less active site. Pulse oximeters that use the electrocardiographic signal as an aid in identification of the pulse can have an enhanced ability to distinguish between the true pulse and artifacts produced by motion. The result is more reliable SpO2 readings.
Monitoring of ventilation with capnography
Technology overview
Sidestream capnographs incorporate moisture-control features that are designed to minimize clogging of the sample tube, protect the measurement chamber from moisture-induced damage, and minimize the risk of cross contamination. The design of these moisture-control systems significantly affects a monitor’s ease of use. Most systems rely on water traps, which must be emptied routinely. A new technology uses a special system of filters and tubing to dehumidify the sample and thus eliminate the need for water traps.
The normal capnogram
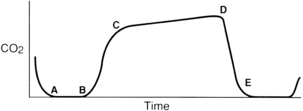
For effective use of capnography, it is important to understand the components of the normal CO2 waveform (capnogram)—a square wave pattern with a plateau (Fig. 27-3). Early in exhalation, air from the anatomic dead space, which is virtually CO2 free, is measured with the instrument. As exhalation continues, alveolar gas reaches the sampling site, and the CO2 level increases rapidly. The CO2 concentration continues to increase throughout exhalation and reaches the alveolar plateau because alveolar gas dominates the sample. At the end of exhalation, the ETCO2 occurs, which in the normal lung is the best approximation of alveolar CO2 levels. The CO2 concentration then drops rapidly as the next inhalation of CO2-free gas begins.
End-tidal versus arterial carbon dioxide
In normal conditions, when ventilation and perfusion are well matched, ETCO2 closely approximates arterial CO2 (PaCO2). The difference between the PaCO2 and the ETCO2 level is the alveolar-arterial CO2 difference (a-ADCO2). ETCO2 is usually as much as 5 mm Hg lower than PaCO2. When the two measurements differ significantly, an anomaly in the patient’s physiology, the breathing circuit, or the capnograph is usually present. Significant divergence between ETCO2 and PaCO2 is often attributable to increased alveolar dead space. CO2-free gas from nonperfused alveoli mixes with gas from perfused regions, thus decreasing the ETCO2 measurement. Clinical conditions that cause increased dead space, such as pulmonary hypoperfusion, cardiac arrest, and embolic conditions (e.g., air, fat thrombus, amniotic fluid), can increase the a-ADCO2. Changes in the a-ADCO2 can be used in assessing the efficacy of the treatment; as the patient’s dead space improves, the partial pressure of the alveolar carbon dioxide less the partial pressure of arterial carbon dioxide (PACO2 – PaCO2) narrows. Increases in dead space ventilation lower ETCO2 and therefore increase the PaCO2 – ETCO2 gradient. Widened PaCO2 – ETCO2 examples include embolic phenomena, hypoperfusion, and chronic obstructive pulmonary disease. Alternatively, a significant PACO2 – PaCO2 value can indicate incomplete alveolar emptying (e.g., with reactive airway disease), a leak in the gas-sampling system that allows loss of respiratory gas, and contamination of respiratory gas with fresh gas.4
Interpretation of changes in the capnogram
A sudden decrease in ETCO2 to a near-zero level indicates that the monitor is no longer detecting CO2 in exhaled gases (Fig. 27-4). Immediate action is crucial for detection and correction of the cause of this loss of ventilation. Possible causes include a completely blocked endotracheal tube, esophageal intubation, a disconnection in the breathing circuit, and inadvertent extubation. The latter three possibilities are particularly likely if the decrease in ETCO2 coincides with movement of the patient’s head. First, after elimination of possible clinical causes for this decrease in ETCO2, a clogged sampling tube or instrument malfunction is investigated as the cause of the problem.
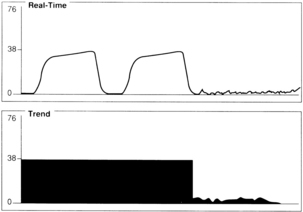
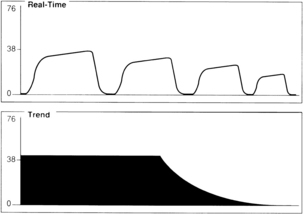
An exponential decrease in ETCO2 over a small number of breaths usually signals a life-threatening cardiopulmonary event that has dramatically increased dead space ventilation (Fig. 27-5). Sudden hypotension, pulmonary embolism, and circulatory arrest with continued ventilation must be considered.
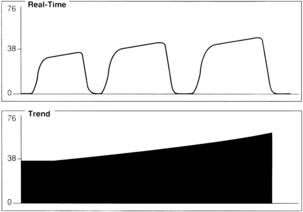
A gradual increase in the ETCO2 level while the capnogram retains its normal shape usually indicates that ventilation is inadequate to eliminate the CO2 that is produced (Fig. 27-6). This situation can be the result of a small ventilator leak or a partial airway obstruction that reduces minute ventilation. It can also reflect increased CO2 production associated with increased body temperature, the onset of sepsis, or shivering. Of particular importance, a large increase in ETCO2 can be one of the earliest signs of malignant hyperthermia, which may not begin until after emergence from anesthesia.
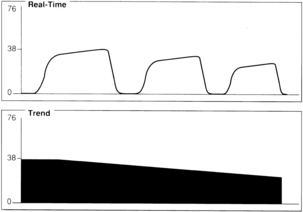
A gradual decrease in the ETCO2 level commonly occurs in the patient who is anesthetized, narcotized, hyperventilated, or hypothermic (Fig. 27-7).
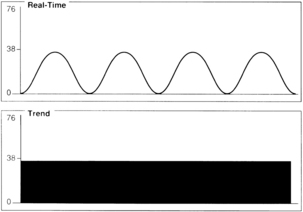
Assessment of the capnogram can reveal information about the quality of alveolar emptying. For example, the patient with bronchospasm is unable to completely empty the alveoli, and the resulting capnogram does not have an alveolar plateau (Fig. 27-8). The ETCO2 reported by the capnograph in this instance is not a good estimate of alveolar CO2. Effective administration of bronchodilator therapy commonly improves alveolar emptying and results in a more normal capnogram.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree