Chapter 13 Assessment and Care of Patients with Fluid and Electrolyte Imbalances
Safe and Effective Care Environment
1. Assess the patient with a fluid or electrolyte imbalance for falls, especially older adults.
2. Use safety precautions to prevent injury or death when administering parenteral potassium-containing solutions.
3. Supervise the oral fluid therapy and intake and output measurement aspects of care delegated to unlicensed assistive personnel.
Health Promotion and Maintenance
4. Teach healthy adults and patients how to prevent dehydration.
5. Assess patients for factors that increase the risk for fluid and electrolyte imbalances, especially for older adults.
6. Teach patients at risk for fluid or electrolyte imbalances as a result of drug therapy about the manifestations of the imbalance.
7. Explain the relationship between weight gain or loss and fluid imbalances.
8. Apply knowledge of the anatomic and physiologic responses to aging when assessing hydration status of an older adult.
9. Use laboratory data and clinical manifestations to determine the presence of fluid or electrolyte imbalances.
10. Interpret blood chemistry laboratory results to determine whether the patient has a fluid or electrolyte imbalance and to determine effectiveness of interventions.
11. Assess the breathing effectiveness of any patient with skeletal muscle weakness from an electrolyte imbalance.
12. Prioritize interventions for patients who have dehydration or fluid overload.
13. Prioritize interventions for patients who have specific electrolyte imbalances.
http://evolve.elsevier.com/Iggy/
Answer Key for NCLEX Examination Challenges and Decision-Making Challenges
Fluid and Electrolyte Tutorial
Review Questions for the NCLEX® Examination
Homeostasis
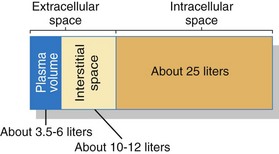
An important area for homeostasis is maintaining the body’s normal fluid volume and composition. Water is the most common substance in the body, making up about 55% to 60% of total body weight for healthy younger adults and 50% to 55% of total body weight for healthy older adults. This water (fluid) is divided into two main spaces or compartments—the fluid outside the cells, which is the extracellular fluid (ECF); and the fluid inside the cells, which is the intracellular fluid (ICF). The ECF space contains about one third (about 15 L) of the total body water. The ECF includes interstitial fluid (fluid between cells, sometimes called the “third space”); blood, lymph, bone, and connective tissue water; and the transcellular fluids. Transcellular fluids are the fluids in special body spaces and include cerebrospinal fluid, synovial fluid, peritoneal fluid, and pleural fluid. ICF contains the remaining two thirds (about 25 L) of total body water. Fig. 13-1 shows the normal distribution of total body water.
Anatomy and Physiology Review
Physiologic Influences on Fluid and Electrolyte Balance
Filtration
Physiologic Action
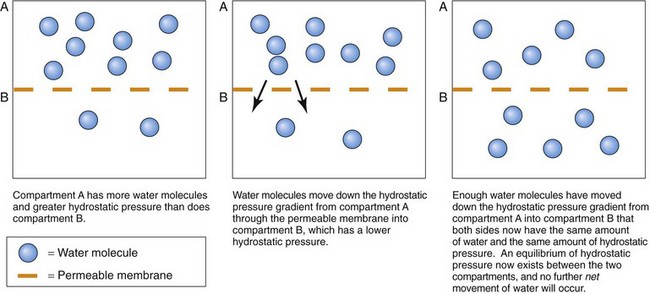
Fluid weight in a confined space is related to the amount of fluid present in that area. Water molecules in a confined space constantly press outward against the confining walls. This pressing of water molecules is hydrostatic pressure. It is a “water-pushing” pressure, because it is the force that pushes water outward from a confined space through a membrane (Fig. 13-2).
The hydrostatic pressures of two fluid spaces can be compared whenever a porous (permeable) membrane separates the two spaces. If the hydrostatic pressure is the same in both fluid spaces, there is no pressure difference between the two spaces and the hydrostatic pressure is now at equilibrium. If the hydrostatic pressure is not the same in both spaces, disequilibrium exists. This means that the two spaces have a graded difference (gradient) for hydrostatic pressure: one space has a higher hydrostatic pressure than the other. The human body constantly seeks equilibrium. When a gradient exists, water movement (filtration) occurs until the hydrostatic pressure is the same in both spaces (see Fig. 13-2).
Diffusion
Physiologic Action
A concentration gradient exists when two fluid spaces have different amounts of the same type of particles. Particle collisions cause them to move down the concentration gradient. Any membrane that separates two spaces is struck repeatedly by particles. When the particle strikes a pore in the membrane that is large enough for it to pass through, diffusion occurs (Fig. 13-4). The chance of any single particle hitting the membrane and going through a pore is much greater on the side of the membrane with a higher solute particle concentration.
Osmosis
Physiologic Action
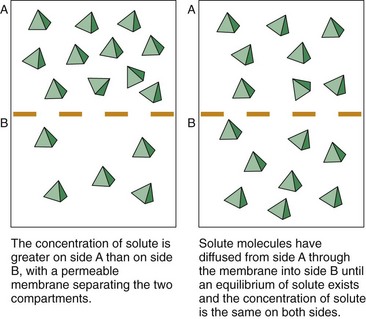
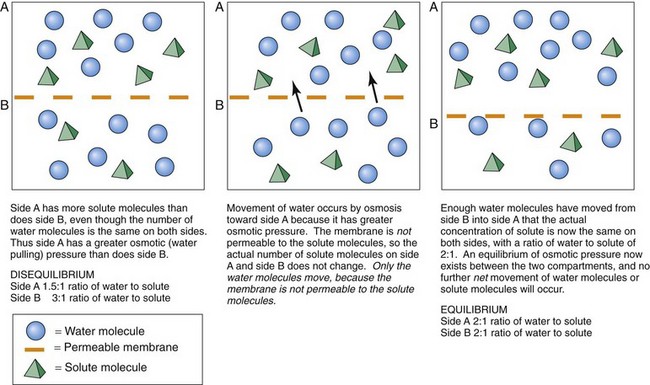
For the fluid spaces to have equal concentrations of the particle, the water molecules move down their concentration gradient from the side with the higher concentration of water molecules (and thus a lower concentration of particles along with a greater hydrostatic pressure) to the side with the lower concentration of water molecules (and a higher concentration of particles along with a lower hydrostatic pressure). This movement continues until both spaces contain the same proportions of particles to water. Dilute (less concentrated) fluid has fewer particles and more water molecules than the more concentrated fluid. Thus water moves by osmosis down its hydrostatic pressure gradient from the dilute fluid to the more concentrated fluid until a concentration equilibrium occurs (Fig. 13-5).
Fluid Balance
Body Fluids
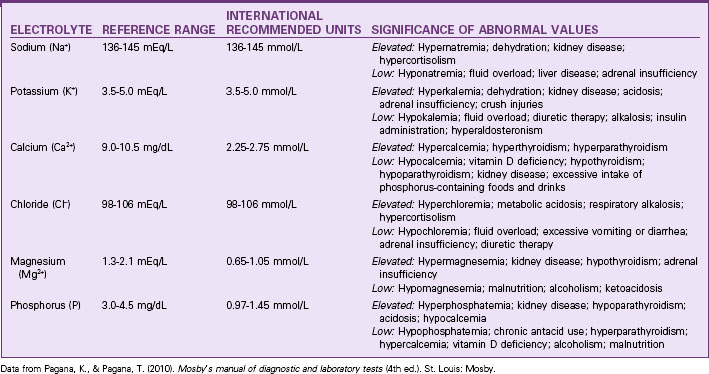
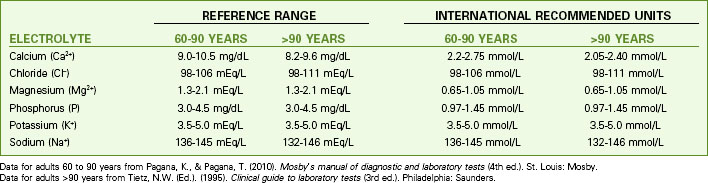
Fluid balance is closely linked to and affected by electrolyte concentrations. Table 13-1 lists the normal ranges of the major serum electrolytes. Chart 13-1 lists the normal electrolyte values for people older than 60 years.
Chart 13-1 Nursing Focus on the Older Adult
Normal Plasma Electrolyte Values for People Older Than 60 Years
A person’s age, gender, and amount of fat affect the amount and distribution of body fluids. An older adult has less total body water than a younger adult. Chart 13-2 discusses age-related changes in fluid balance. An obese person has less total water than a lean person of the same weight because fat cells contain almost no water.
Chart 13-2 Nursing Focus on the Older Adult
Impact of Age-Related Changes on Fluid Balance
| SYSTEM | CHANGE | RESULT |
|---|---|---|
| Skin | ||
| Kidney | ||
| Muscular | ||
| Neurologic | ||
| Endocrine |
Fluid intake is regulated through the thirst drive. Fluids enter the body mainly as liquids (Table 13-2). Solid foods also contain up to 85% water, allowing some fluid to enter the body with ingested solid foods.
TABLE 13-2 ROUTES OF FLUID INGESTION AND EXCRETION
| INTAKE | OUTPUT |
|---|---|
| Measurable | |
| Oral fluids | Urine |
| Parenteral fluids | Emesis |
| Enemas* | Feces |
| Irrigation fluids* | Drainage from body cavities |
| Not Measurable | |
| Solid foods | Perspiration |
| Metabolism | Vaporization through the lungs |
* Measured by subtracting the amount returned from the amount instilled.
Fluid loss occurs through several routes (see Table 13-2). Of all the water loss pathways, the kidney is the most important and the most sensitive. Water loss by the kidney is regulated and is adjustable. The volume of urine excreted daily varies depending on the amount of fluid taken in and the body’s need to conserve fluids.
Physiological Integrity
Significance of Fluid Balance
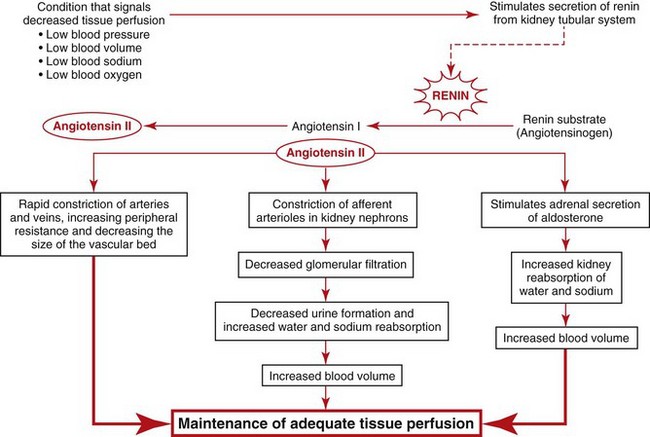
The Renin-Angiotensin II Pathway
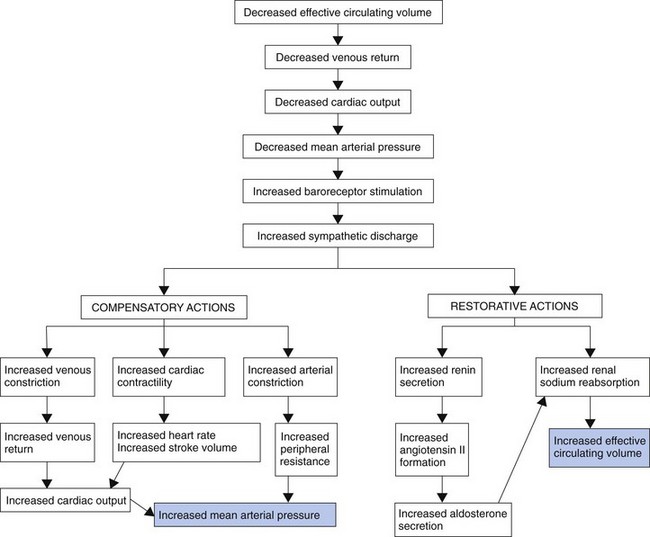
Because the kidney is a major regulator of water and sodium balance to maintain blood pressure and perfusion to all tissues and organs, the kidneys monitor blood pressure, blood volume, blood oxygen levels, and blood osmolarity (related to sodium concentration). When the kidneys sense that any one of these parameters is getting low, they begin to secrete a substance called renin that sets into motion a group of hormonal and blood vessel responses to ensure that blood pressure is raised back up to normal. Fig. 13-6 summarizes these responses.
Fluid Imbalances
Dehydration
Pathophysiology
In dehydration, fluid intake or fluid retention is less than what is needed to meet the body’s fluid needs, resulting in a fluid volume deficit, especially a plasma volume deficit. It is a condition rather than a disease and can be caused by many factors (Table 13-3). Dehydration may be an actual decrease in total body water caused by either too little intake of fluid or too great a loss of fluid. It also can occur without an actual loss of total body water, such as when water shifts from the plasma into the interstitial space. This condition is called relative dehydration.
TABLE 13-3 COMMON CAUSES OF FLUID IMBALANCES
| DEHYDRATION | FLUID OVERLOAD |
|---|---|
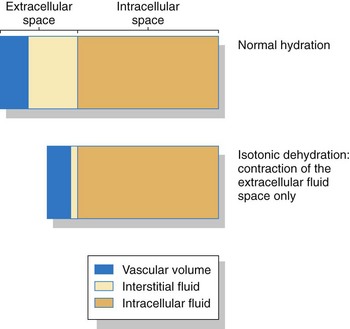
Dehydration may occur with just water loss or with water and electrolyte loss (isotonic dehydration). Isotonic dehydration is the most common type of fluid loss problem. Fluid is lost only from the extracellular fluid (ECF) space, including both the plasma and the interstitial spaces. There is no shift of fluids between spaces, so the intracellular fluid (ICF) volume remains normal (Fig. 13-7). Circulating blood volume is decreased (hypovolemia) and leads to inadequate tissue perfusion. The body’s defenses adapt (compensate) during dehydration to maintain adequate blood flow to vital organs in spite of hypovolemia (Fig. 13-8).
Patient-Centered Collaborative Care
Assessment
History
One way of organizing history data to assess the patient’s fluid status is to use Gordon’s Functional Health Patterns (Gordon, 2010). The patterns that most affect fluid status are the Nutritional-Metabolic Pattern and the Elimination Pattern (Chart 13-3).
Fluid and Electrolyte Assessment
Using Gordon’s Functional Health Patterns
Nutritional-Metabolic Pattern
• What is your typical daily food intake? Describe a day’s meals, snacks, and vitamins.
• How much salt do you typically add to your food? Do you use salt substitutes?
• Do you have any difficulty chewing or swallowing?
• What is your typical daily fluid intake? What types of fluids (water, juices, soft drinks, coffee, tea, beer, other alcoholic drinks)? How much?
• Have you had any recent change in your weight? Weight gain? Weight loss? How much?
• Have you noticed a change in tightness of your rings or shoes? Tighter? Looser?
Elimination Pattern
Based on Gordon, M. (2010). Manual of nursing diagnosis (12th ed.). Boston: Jones & Bartlett.
Physical Assessment/Clinical Manifestations
• How easily the skin over the back of the hand and arm can be gently pinched between the thumb and the forefinger to form a “tent”
• How soon the pinched skin resumes its normal position after release
Considerations for Older Adults
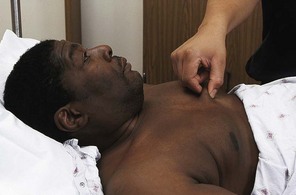
Assess skin turgor in an older adult by pinching the skin over the sternum or on the forehead, rather than the back of the hand, because these areas more reliably indicate hydration (Fig. 13-9). As a person ages, the skin loses elasticity and tents on hands and arms even when the person is well hydrated.
Neurologic changes with dehydration include alterations of mental status and body temperature status because blood flow in the brain is reduced. Mental status changes, especially confusion, are more common among older adults and may be the first indication of a fluid balance problem. Check to determine whether the patient is alert and oriented. Chapter 43 provides more information about assessment of mental status.
Laboratory Assessment
No single laboratory test result confirms or rules out dehydration. Instead, dehydration is determined by laboratory findings along with clinical manifestations (see Table 13-1). Usually, laboratory findings with dehydration show elevated levels of hemoglobin, hematocrit, serum osmolarity, glucose, protein, blood urea nitrogen, and various electrolytes because more water is lost and other substances remain, increasing the osmolarity or concentration of the blood (hemoconcentration). Hemoconcentration is not present when dehydration is caused by hemorrhage, because loss of all blood and plasma products occurs together.
Interventions
Patient safety issues and strategies are priorities of care before and during other therapies for dehydration. Monitor vital signs, especially heart rate and blood pressure. The patient with dehydration is at risk for falls because of the accompanying orthostatic hypotension, dysrhythmia, muscle weakness, and possible confusion. Assess his or her muscle strength, gait stability, and level of alertness. Instruct the patient to get up slowly from a lying or sitting position and to immediately sit down if he or she feels light-headed. Implement the falls precautions listed in Chart 13-4.
Chart 13-4 Best Practice for Patient Safety & Quality Care
Falls Precautions
• Assess for orthostatic hypotension.
• Assess muscle strength in legs.
• Orient the patient to the environment.
• Remind the patient to call for help before getting out of bed or a chair.
• Help the patient get out of bed or a chair.
• Provide, or remind the patient to use, a walker or cane for ambulating.
• Help the incontinent patient toilet every 1 to 2 hours.
• Clean up spills immediately.
• Provide adequate lighting at all times, especially at night.
• Keep the call light within reach, and ensure that the patient can use it.
• Place the bed in the lowest position with the brakes locked.
• Place objects that the patient needs within reach.
• Ensure that adequate handrails are present in the patient’s room, bathroom, and hall.
• Encourage family members or significant other to stay with the patient.
Fluid replacement is key to correcting dehydration and preventing death from reduced perfusion. Best practices for nursing care of the patient with dehydration are listed in Chart 13-5. Mild to moderate dehydration is corrected with oral fluid replacement if the patient is alert enough to swallow and can tolerate oral fluids. Verify that he or she does not have a health problem that requires restriction of fluid volume or fluid types. Urge fluid intake, and measure the amount ingested.
Chart 13-5 Best Practice for Patient Safety & Quality Care
The Patient with Dehydration
• When possible, provide oral fluids that meet the patient’s dietary restrictions (e.g., sugar-free, low-sodium, thickened).
• Collaborate with other members of the health care team to determine the amount of fluids needed during a 24-hour period.
• Ensure the fluids are offered and ingested on an even schedule at least every 2 hours throughout 24 hours.
• Teach unlicensed assistive personnel to actively participate in the hydration therapy and not to withhold fluids to prevent incontinence.
• Administer prescribed IV fluids at a rate consistent with hydration needs and any known cardiac, pulmonary, or kidney problems.
• Monitor the patient’s response to fluid therapy at least every 2 hours for indicators of adequate rehydration or the need for continuing therapy, especially:
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree