CHAPTER 93 Antiviral drugs are discussed in this chapter and the one that follows. In this chapter, we consider drugs used to treat infections caused by viruses other than HIV. In Chapter 94, we consider drugs used against HIV infection. Drugs for non-HIV infections are summarized in Table 93–1. TABLE 93–1 Major Drugs for Non-HIV Viral Infections Herpes simplex virus (HSV) and varicella-zoster virus (VZV) are members of the herpesvirus group. HSV causes infection of the genitalia, mouth, face, and other sites. VZV is the cause of varicella (chickenpox) and herpes zoster (shingles), a painful condition resulting from reactivation of VZV that had been dormant within sensory nerve roots. Both conditions are discussed further in Chapter 68, along with the vaccine used to prevent chickenpox. Drugs for infection with HSV and VZV are summarized in Table 93–2. Genital herpes is discussed in Chapter 95. TABLE 93–2 Treatment of Herpes Simplex Virus and Varicella-Zoster Virus Infections Cytomegalovirus (CMV) is a member of the herpesvirus group, which includes herpes simplex virus types 1 and 2, varicella-zoster virus (the cause of chickenpox), and Epstein-Barr virus (the cause of infectious mononucleosis). Transmission of CMV occurs person to person—through direct contact with saliva, urine, blood, tears, breast milk, semen, and other body fluids. Infection can also be acquired by way of blood transfusion or organ transplantation. Infection with CMV is very common: Between 50% and 85% of Americans age 40 and older harbor the virus. After the initial infection, which has minimal symptoms in healthy people, the virus remains dormant within cells for life, without causing detectable injury or clinical illness. Hence, for most healthy people, CMV infection is of little concern. By contrast, people who are immunocompromised—owing to HIV infection, cancer chemotherapy, or use of immunosuppressive drugs—are at high risk of serious morbidity and even death, both from initial CMV infection and from reactivation of dormant CMV. Common sites for infection are the lungs, eyes, and GI tract. Among people with AIDS, CMV retinitis is the principal reason for loss of vision (see Chapter 94). The four drugs used against CMV are discussed below. Granulocytopenia and thrombocytopenia. The adverse effect of greatest concern is bone marrow suppression, which can result in granulocytopenia (40%) and thrombocytopenia (20%). These effects, which are usually reversible, are more likely with IV therapy than with oral therapy. These hematologic responses can be exacerbated by concurrent therapy with zidovudine. Conversely, granulocytopenia can be reduced with granulocyte colony-stimulating factors (see Chapter 56). Because of the risk of adverse hematologic effects, blood cell counts must be monitored. Treatment should be interrupted if the absolute neutrophil count falls below 500/mm3 or if the platelet count falls below 25,000/mm3. Cell counts usually begin to recover within 3 to 5 days. Ganciclovir should be used with caution in patients with pre-existing cytopenias, in those with a history of cytopenic reactions to other drugs, and in those taking other bone marrow suppressants (eg, zidovudine, trimetrexate). Most cases (90%) of chronic hepatitis are caused by either hepatitis B virus (HBV) or hepatitis C virus (HCV). Accordingly, our discussion focuses on hepatitis B and hepatitis C. About 1.5% of Americans are infected with HBV or HCV, which is 5 times more than the number infected with HIV. Comparisons between hepatitis A, B, and C are summarized in Table 93–3. Vaccines for hepatitis A and B are discussed in Chapter 68. Drugs for hepatitis B and C are discussed below. TABLE 93–3 Characteristics of Hepatitis A, Hepatitis B, and Hepatitis C
Antiviral agents I: drugs for non-HIV viral infections

Drug
Antiviral Spectrum
Drugs for Herpes Simplex Virus and Varicella-Zoster Virus Infections
Systemic Drugs
Acyclovir
HSV, VZV
Famciclovir
HSV, VZV
Foscarnet
HSV, VZV
Valacyclovir
HSV, VZV
Topical Drugs
Vidarabine
HSV, VZV
Penciclovir
HSV
Trifluridine
HSV keratitis
Docosanol
HSV keratitis
Ganciclovir
HSV keratitis
Drugs for Hepatitis
Alfa Interferons
Interferon alfa-2b
HCV, HBV
Interferon alfacon-1
HCV
Peginterferon alfa-2a
HCV, HBV
Peginterferon alfa-2b
HCV
Protease Inhibitors
Boceprevir
HCV
Telaprevir
HCV
Nucleoside Analogs
Ribavirin (oral)
HCV
Adefovir
HBV*
Entecavir
HBV*
Lamivudine
HBV*
Telbivudine
HBV
Tenofovir
HBV*
Drugs for Cytomegalovirus Infection
Ganciclovir
CMV
Valganciclovir
CMV
Cidofovir
CMV
Foscarnet
CMV
Drugs for Influenza
Oseltamivir
Influenza A and B
Zanamivir
Influenza A and B
Drugs for Respiratory Syncytial Virus Infection
Ribavirin (inhaled)
RSV
Palivizumab
RSV

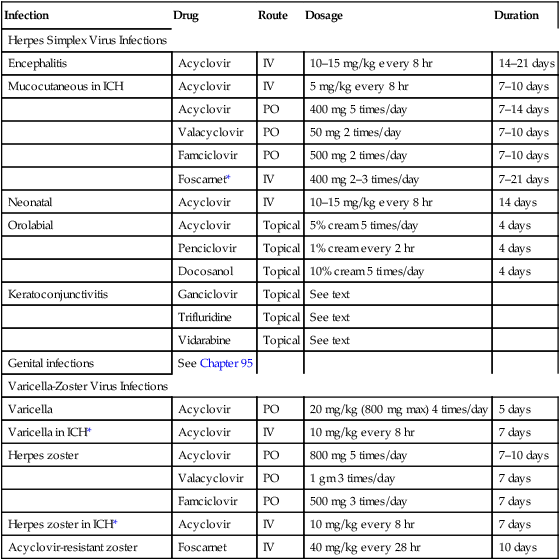
Drugs for infection with herpes simplex viruses and varicella-zoster virus

Infection
Drug
Route
Dosage
Duration
Herpes Simplex Virus Infections
Encephalitis
Acyclovir
IV
10–15 mg/kg every 8 hr
14–21 days
Mucocutaneous in ICH
Acyclovir
IV
5 mg/kg every 8 hr
7–10 days
Acyclovir
PO
400 mg 5 times/day
7–14 days
Valacyclovir
PO
50 mg 2 times/day
7–10 days
Famciclovir
PO
500 mg 2 times/day
7–10 days
Foscarnet*
IV
400 mg 2–3 times/day
7–21 days
Neonatal
Acyclovir
IV
10–15 mg/kg every 8 hr
14 days
Orolabial
Acyclovir
Topical
5% cream 5 times/day
4 days
Penciclovir
Topical
1% cream every 2 hr
4 days
Docosanol
Topical
10% cream 5 times/day
4 days
Keratoconjunctivitis
Ganciclovir
Topical
See text
Trifluridine
Topical
See text
Vidarabine
Topical
See text
Genital infections
See Chapter 95
Varicella-Zoster Virus Infections
Varicella
Acyclovir
PO
20 mg/kg (800 mg max) 4 times/day
5 days
Varicella in ICH*
Acyclovir
IV
10 mg/kg every 8 hr
7 days
Herpes zoster
Acyclovir
PO
800 mg 5 times/day
7–10 days
Valacyclovir
PO
1 gm 3 times/day
7 days
Famciclovir
PO
500 mg 3 times/day
7 days
Herpes zoster in ICH*
Acyclovir
IV
10 mg/kg every 8 hr
7 days
Acyclovir-resistant zoster
Foscarnet
IV
40 mg/kg every 28 hr
10 days
Drugs for cytomegalovirus infection
Ganciclovir
Adverse effects.
Drugs for hepatitis

Point of Comparison
Hepatitis A
Hepatitis B
Hepatitis C
Causative agent
Hepatitis A virus
Hepatitis B virus
Hepatitis C virus
Percent of Americans ever infected
29–34
4.3–5.6
1.3–1.9
Percent of infections that become chronic
0
3–5
Over 70
New acute infections in the United States (2009)
21,000
38,000
16,000
U.S. residents with chronic infection
None
0.8–1.4 million
2.7–3.9 million
Annual deaths in the United States from chronic infection
None
1800
15,000
People worldwide with chronic infection
None
350 million
170 million
Method of prevention
Hepatitis A vaccine
Hepatitis B vaccine
None available
Preferred treatment
None
Interferon alfa or lamivudine
Peginterferon alfa plus ribavirin plus either boceprevir or telaprevir ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Antiviral agents I: drugs for non-HIV viral infections
Only gold members can continue reading. Log In or Register to continue
