CHAPTER 31 Schizophrenia is a chronic psychotic illness characterized by disordered thinking and a reduced ability to comprehend reality. Symptoms usually emerge during adolescence or early adulthood. In the United States, about 3.2 million people are affected. Diagnostic criteria for schizophrenia are presented in Table 31–1. TABLE 31–1 DSM-5 Diagnostic Criteria for Schizophrenia Modified from the proposed diagnostic criteria for Schizophrenia, to be published in Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Washington, DC: American Psychiatric Association. Expected publication date: May 2013. Copyright © American Psychiatric Association. The proposed criteria are from the DSM-5 web site—–www.DSM5.org–—accessed on June 20, 2011. Symptoms of schizophrenia can be divided into three groups: positive symptoms, negative symptoms, and cognitive symptoms. Positive and negative symptoms are summarized in Table 31–2. Table 31–2 Positive and Negative Symptoms of Schizophrenia First-generation antipsychotics can be classified as low potency, medium potency, or high potency (Table 31–3). The low-potency drugs, represented by chlorpromazine, and the high-potency drugs, represented by haloperidol, are of particular interest. Table 31–3 Antipsychotic Drugs: Relative Potency and Incidence of Selected Side Effects *Doses listed are the therapeutic equivalent of 100 mg of oral chlorpromazine. †Incidence here refers to early extrapyramidal reactions (acute dystonia, parkinsonism, akathisia). The incidence of late reactions (tardive dyskinesia) is the same for all traditional antipsychotics. The FGAs fall into five major chemical categories (Table 31–4). One of these categories, the phenothiazines, has three subgroups. Drugs in all groups are equivalent with respect to antipsychotic actions, and hence chemical classification is not emphasized in this chapter. Table 31–4 Antipsychotic Drugs: Routes and Dosages The antipsychotic drugs block several kinds of receptors, and hence produce an array of side effects. Side effects associated with blockade of specific receptors are summarized in Table 31–5. Table 31–5 Receptor Blockade and Side Effects of Antipsychotic Drugs Four types of EPS occur. They differ with respect to time of onset and management. Three of these reactions—acute dystonia, parkinsonism, and akathisia—occur early in therapy and can be managed with a variety of drugs. The fourth reaction—tardive dyskinesia—occurs late in therapy and has no satisfactory treatment. Characteristics of EPS are summarized in Table 31–6. Table 31–6 Extrapyramidal Side Effects of Antipsychotic Drugs Treatment consists of supportive measures, drug therapy, and immediate withdrawal of antipsychotic medication. Hyperthermia should be controlled with cooling blankets and antipyretics (eg, aspirin, acetaminophen). Hydration should be maintained with fluids. Benzodiazepines may relieve anxiety and help reduce blood pressure and tachycardia. Two drugs—dantrolene and bromocriptine—may be especially helpful. Dantrolene is a direct-acting muscle relaxant (see Chapter 25). In patients with NMS, this drug reduces rigidity and hyperthermia. Bromocriptine is a dopamine receptor agonist (see Chapter 21) that may relieve CNS toxicity. First-generation agents produce varying degrees of muscarinic cholinergic blockade (see Table 31–3), and can elicit the full spectrum of anticholinergic responses (dry mouth, blurred vision, photophobia, urinary hesitancy, constipation, tachycardia). Patients should be informed about these responses and taught how to minimize danger and discomfort. As indicated in Table 31–3, anticholinergic effects are more likely with low-potency FGAs than with high-potency FGAs. Anticholinergic effects and their management are discussed in detail in Chapter 14. Antipsychotic drugs promote orthostatic hypotension by blocking alpha1-adrenergic receptors on blood vessels. Alpha-adrenergic blockade prevents compensatory vasoconstriction when the patient stands, thereby causing blood pressure to fall. Patients should be informed about signs of hypotension (lightheadedness, dizziness) and advised to sit or lie down if these occur. In addition, patients should be informed that hypotension can be minimized by moving slowly when assuming an erect posture. With hospitalized patients, blood pressure and pulses should be checked before dosing and 1 hour after. Measurements should be made while the patient is lying down and again after the patient has been sitting or standing for 1 to 2 minutes. If blood pressure is low, or if pulse rate is high, the dose should be withheld and the prescriber consulted. Hypotension is more likely with low-potency FGAs than with the high-potency FGAs (see Table 31–3). Tolerance to hypotension develops in 2 to 3 months. Four FGAs—chlorpromazine, haloperidol, thioridazine, and pimozide—pose a risk of fatal cardiac dysrhythmias. The mechanism is prolongation of the QT interval, an index of cardiac function that can be measured with an electrocardiogram (ECG). As discussed in Chapter 7 (Adverse Drug Reactions and Medication Errors), drugs that prolong the QT interval increase the risk of torsades de pointes, a dysrhythmia than can progress to fatal ventricular fibrillation. To reduce the risk of dysrhythmias, patients should undergo an ECG and serum potassium determination prior to treatment and periodically thereafter. In addition, they should avoid other drugs that cause QT prolongation (see Chapter 7, Table 7–2), as well as drugs that can increase levels of the drugs under consideration.
Antipsychotic agents and their use in schizophrenia
Schizophrenia: clinical presentation and etiology
Clinical presentation


Three types of symptoms

Positive Symptoms
Negative Symptoms
Hallucinations
Social withdrawal
Delusions
Emotional withdrawal
Disordered thinking
Lack of motivation
Disorganized speech
Poverty of speech
Combativeness
Blunted affect
Agitation
Poor insight
Paranoia
Poor judgment
Poor self-care
First-generation (conventional) antipsychotics
Group properties
Classification
Classification by potency

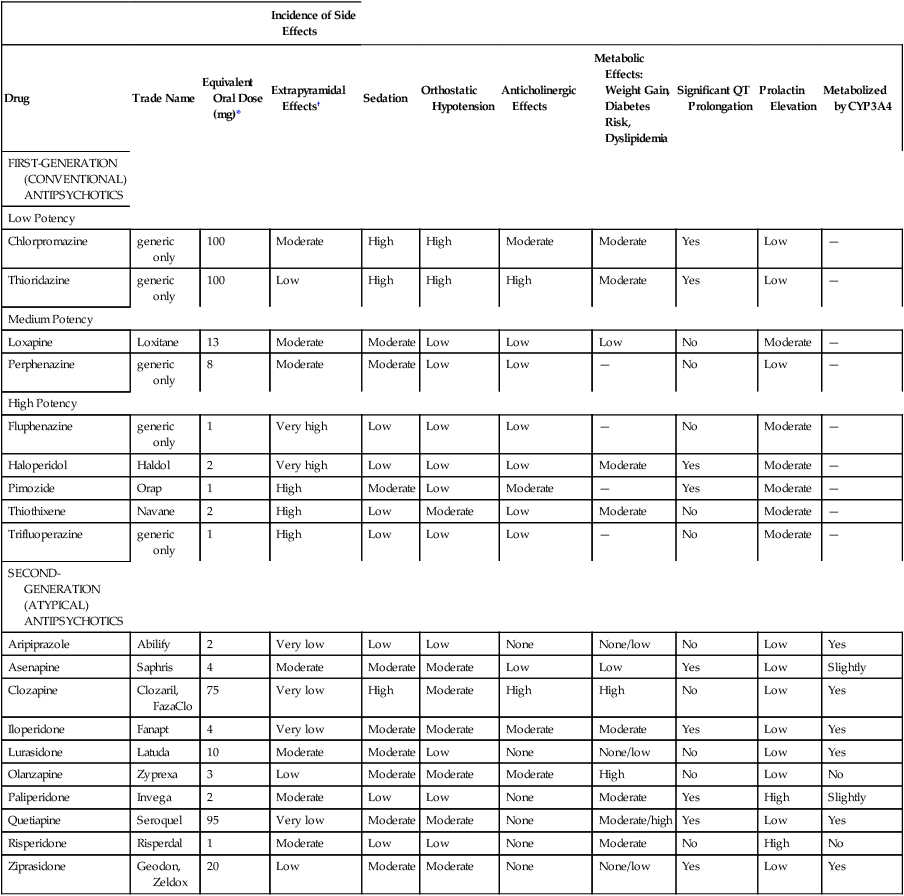
Incidence of Side Effects
Drug
Trade Name
Equivalent Oral Dose (mg)*
Extrapyramidal Effects†
Sedation
Orthostatic Hypotension
Anticholinergic Effects
Metabolic Effects: Weight Gain, Diabetes Risk, Dyslipidemia
Significant QT Prolongation
Prolactin Elevation
Metabolized by CYP3A4
FIRST-GENERATION (CONVENTIONAL) ANTIPSYCHOTICS
Low Potency
Chlorpromazine
generic only
100
Moderate
High
High
Moderate
Moderate
Yes
Low
—
Thioridazine
generic only
100
Low
High
High
High
Moderate
Yes
Low
—
Medium Potency
Loxapine
Loxitane
13
Moderate
Moderate
Low
Low
Low
No
Moderate
—
Perphenazine
generic only
8
Moderate
Moderate
Low
Low
—
No
Low
—
High Potency
Fluphenazine
generic only
1
Very high
Low
Low
Low
—
No
Moderate
—
Haloperidol
Haldol
2
Very high
Low
Low
Low
Moderate
Yes
Moderate
—
Pimozide
Orap
1
High
Moderate
Low
Moderate
—
Yes
Moderate
—
Thiothixene
Navane
2
High
Low
Moderate
Low
Moderate
No
Moderate
—
Trifluoperazine
generic only
1
High
Low
Low
Low
—
No
Moderate
—
SECOND-GENERATION (ATYPICAL) ANTIPSYCHOTICS
Aripiprazole
Abilify
2
Very low
Low
Low
None
None/low
No
Low
Yes
Asenapine
Saphris
4
Moderate
Moderate
Moderate
Low
Low
Yes
Low
Slightly
Clozapine
Clozaril, FazaClo
75
Very low
High
Moderate
High
High
No
Low
Yes
Iloperidone
Fanapt
4
Very low
Moderate
Moderate
Moderate
Moderate
Yes
Low
Yes
Lurasidone
Latuda
10
Moderate
Moderate
Low
None
None/low
No
Low
Yes
Olanzapine
Zyprexa
3
Low
Moderate
Moderate
Moderate
High
No
Low
No
Paliperidone
Invega
2
Moderate
Low
Low
None
Moderate
Yes
High
Slightly
Quetiapine
Seroquel
95
Very low
Moderate
Moderate
None
Moderate/high
Yes
Low
Yes
Risperidone
Risperdal
1
Moderate
Low
Low
None
Moderate
No
High
No
Ziprasidone
Geodon, Zeldox
20
Low
Moderate
Moderate
None
None/low
Yes
Low
Yes
Chemical classification

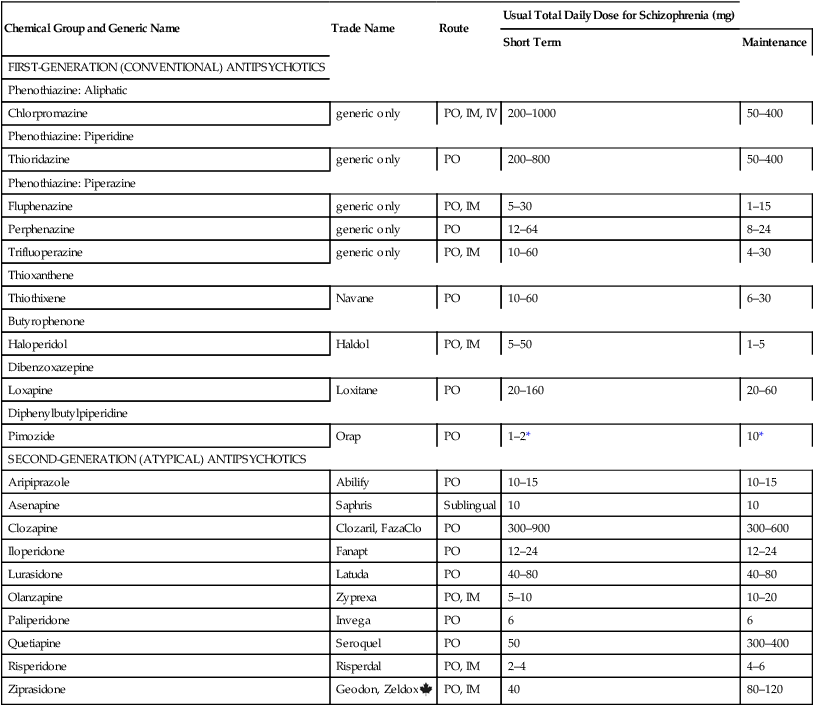
Chemical Group and Generic Name
Trade Name
Route
Usual Total Daily Dose for Schizophrenia (mg)
Short Term
Maintenance
FIRST-GENERATION (CONVENTIONAL) ANTIPSYCHOTICS
Phenothiazine: Aliphatic
Chlorpromazine
generic only
PO, IM, IV
200–1000
50–400
Phenothiazine: Piperidine
Thioridazine
generic only
PO
200–800
50–400
Phenothiazine: Piperazine
Fluphenazine
generic only
PO, IM
5–30
1–15
Perphenazine
generic only
PO
12–64
8–24
Trifluoperazine
generic only
PO, IM
10–60
4–30
Thioxanthene
Thiothixene
Navane
PO
10–60
6–30
Butyrophenone
Haloperidol
Haldol
PO, IM
5–50
1–5
Dibenzoxazepine
Loxapine
Loxitane
PO
20–160
20–60
Diphenylbutylpiperidine
Pimozide
Orap
PO
1–2*
10*
SECOND-GENERATION (ATYPICAL) ANTIPSYCHOTICS
Aripiprazole
Abilify
PO
10–15
10–15
Asenapine
Saphris
Sublingual
10
10
Clozapine
Clozaril, FazaClo
PO
300–900
300–600
Iloperidone
Fanapt
PO
12–24
12–24
Lurasidone
Latuda
PO
40–80
40–80
Olanzapine
Zyprexa
PO, IM
5–10
10–20
Paliperidone
Invega
PO
6
6
Quetiapine
Seroquel
PO
50
300–400
Risperidone
Risperdal
PO, IM
2–4
4–6
Ziprasidone
Geodon, Zeldox ![]()
PO, IM
40
80–120
Adverse effects

Receptor Type
Consequence of Blockade
D2 dopaminergic
EPS; prolactin release
H1 histaminergic
Weight gain, sedation
Muscarinic cholinergic
Dry mouth, blurred vision, urinary retention, constipation, tachycardia
Alpha1-adrenergic
Orthostatic hypotension; reflex tachycardia
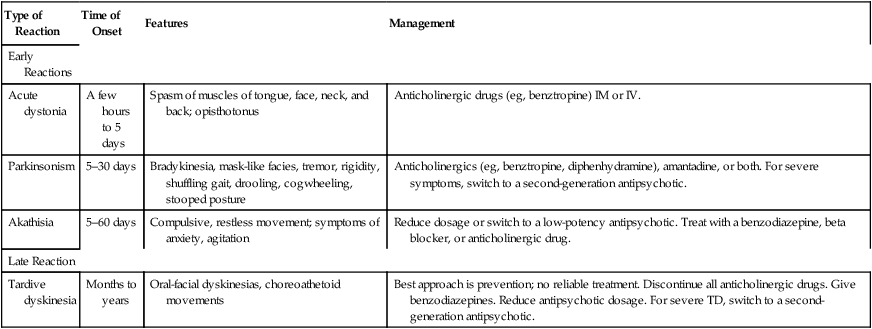
Extrapyramidal symptoms

Type of Reaction
Time of Onset
Features
Management
Early Reactions
Acute dystonia
A few hours to 5 days
Spasm of muscles of tongue, face, neck, and back; opisthotonus
Anticholinergic drugs (eg, benztropine) IM or IV.
Parkinsonism
5–30 days
Bradykinesia, mask-like facies, tremor, rigidity, shuffling gait, drooling, cogwheeling, stooped posture
Anticholinergics (eg, benztropine, diphenhydramine), amantadine, or both. For severe symptoms, switch to a second-generation antipsychotic.
Akathisia
5–60 days
Compulsive, restless movement; symptoms of anxiety, agitation
Reduce dosage or switch to a low-potency antipsychotic. Treat with a benzodiazepine, beta blocker, or anticholinergic drug.
Late Reaction
Tardive dyskinesia
Months to years
Oral-facial dyskinesias, choreoathetoid movements
Best approach is prevention; no reliable treatment. Discontinue all anticholinergic drugs. Give benzodiazepines. Reduce antipsychotic dosage. For severe TD, switch to a second-generation antipsychotic.
Other adverse effects
Neuroleptic malignant syndrome.
Anticholinergic effects.
Orthostatic hypotension.
Severe dysrhythmias.
Get Clinical Tree app for offline access

Antipsychotic agents and their use in schizophrenia
Get Clinical Tree app for offline access


