CHAPTER 90 Diagnostic testing is indicated for (1) individuals with clinical manifestations that suggest TB and (2) individuals with a positive skin test or blood test (see below under Diagnosis and Treatment of Latent Tuberculosis), who are at high risk of developing active disease. A definitive diagnosis is made with a chest radiograph and microbiologic evaluation of sputum. A chest radiograph should be ordered for all persons suspected of active infection. • Resistance in M. tuberculosis occurs because of spontaneous mutations. • Each mutational event confers resistance to only one drug. • Mutations conferring resistance to a single drug occur in about 1 of every 100 million (108) bacteria. • The bacterial burden in active TB is well above 108 organisms but far below 1016. • M. tuberculosis grows slowly, hence treatment is prolonged. In Chapter 83 (Basic Principles of Antimicrobial Therapy), we noted that treatment with multiple antibiotics broadens the spectrum of antimicrobial coverage, thereby increasing the risk of suprainfection. This is not the case with multidrug therapy of TB. The major drugs used against M. tuberculosis are selective for this organism. As a result, these drugs, even when used in combination, do not kill off other microorganisms, and therefore do not create the conditions that lead to suprainfection. Several regimens may be employed for active TB. Drug selection is based largely on the susceptibility of the infecting organism and the immunocompetence of the host. Therapy is usually initiated with a four-drug regimen; isoniazid and rifampin are almost always included. In the event of suspected or proved resistance, more drugs are added; the total may be as high as seven. Representative regimens are shown in Table 90–1 and discussed below. TABLE 90–1 Representative Antituberculosis Regimens *Drugs used in these regimens: E = ethambutol, I = isoniazid, P = pyrazinamide, R = rifampin, S = streptomycin. †PI = HIV-protease inhibitor, NNRTI = non-nucleoside reverse transcriptase inhibitor. If the infecting organisms are not resistant to isoniazid or rifampin, treatment is relatively simple. As indicated in Table 90–1, the induction phase, which lasts 2 months, consists of four drugs: isoniazid, rifampin, pyrazinamide, and ethambutol. Dosing may be done daily, twice weekly, or thrice weekly. The continuation phase, which lasts 4 months, consists of two drugs—isoniazid and rifampin—administered daily, twice weekly, or thrice weekly. Note that the entire course of treatment is prolonged, making adherence a significant problem. Testing should be limited to people who are at high risk of either (1) having acquired the infection recently or (2) progressing from latent TB to active TB. Included in this group are people with HIV infection, people receiving immunosuppressive drugs, recent contacts of TB patients, and people with high-risk medical conditions, such as diabetes, silicosis, or chronic renal failure. A complete list of candidates for testing is given in Table 90–2. Routine testing of low-risk individuals is not recommended. TABLE 90–2 Candidates for Targeted Tuberculosis Testing The decision to treat latent TB is based on two factors: (1) the risk category of the individual and (2) the size of the region of induration produced by the TST (Table 90–3). For individuals at high risk, treatment is recommended if the region of induration is relatively small (5 mm). For individuals at moderate risk, treatment is indicated when the region of induration is larger (10 mm). And for individuals at low risk (who should not be routinely tested), the region must be larger still (15 mm) to justify treatment. TABLE 90–3
Antimycobacterial agents: drugs for tuberculosis, leprosy, and mycobacterium avium complex infection
Drugs for tuberculosis
Clinical considerations
Pathogenesis
Diagnosis and treatment of active tuberculosis
Diagnosis
The prime directive: always treat tuberculosis with two or more drugs
Treatment regimens

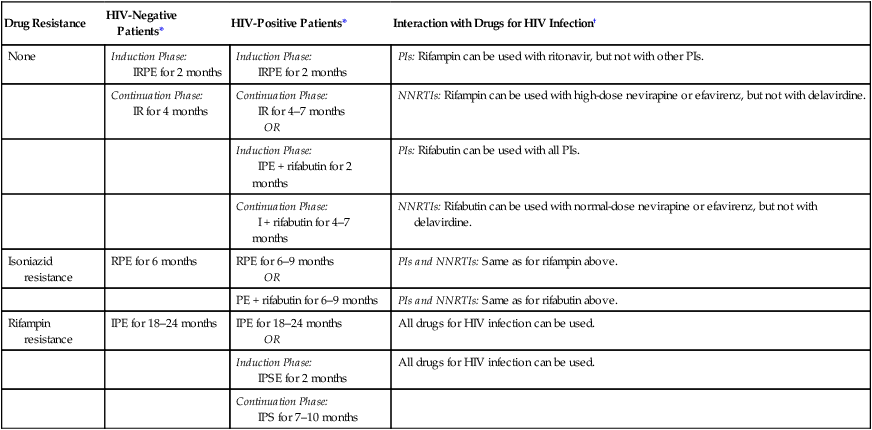
Drug Resistance
HIV-Negative Patients*
HIV-Positive Patients*
Interaction with Drugs for HIV Infection†
None
Induction Phase:
IRPE for 2 months
Induction Phase:
IRPE for 2 months
PIs: Rifampin can be used with ritonavir, but not with other PIs.
Continuation Phase:
IR for 4 months
Continuation Phase:
IR for 4–7 months
OR
NNRTIs: Rifampin can be used with high-dose nevirapine or efavirenz, but not with delavirdine.
Induction Phase:
IPE + rifabutin for 2 months
PIs: Rifabutin can be used with all PIs.
Continuation Phase:
I + rifabutin for 4–7 months
NNRTIs: Rifabutin can be used with normal-dose nevirapine or efavirenz, but not with delavirdine.
Isoniazid resistance
RPE for 6 months
RPE for 6–9 months
OR
PIs and NNRTIs: Same as for rifampin above.
PE + rifabutin for 6–9 months
PIs and NNRTIs: Same as for rifabutin above.
Rifampin resistance
IPE for 18–24 months
IPE for 18–24 months
OR
All drugs for HIV infection can be used.
Induction Phase:
IPSE for 2 months
All drugs for HIV infection can be used.
Continuation Phase:
IPS for 7–10 months
Drug-sensitive tuberculosis.
Diagnosis and treatment of latent tuberculosis
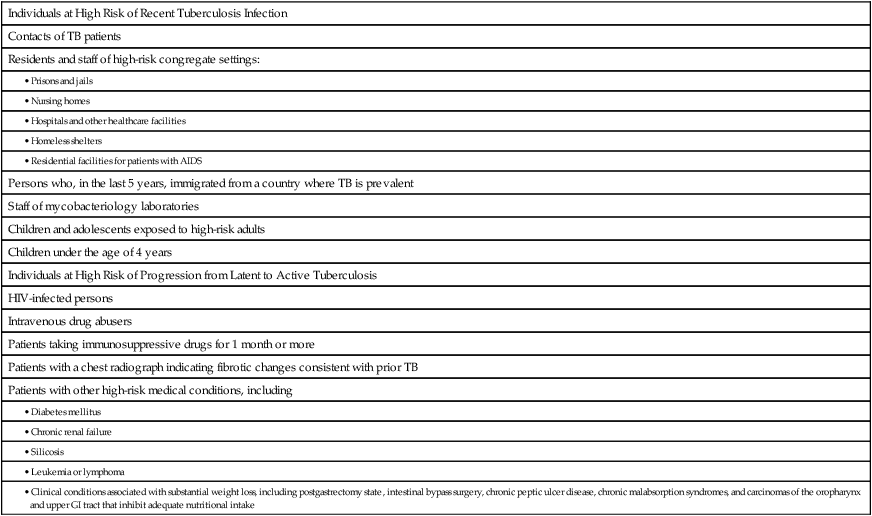
Who should be tested for latent tuberculosis?

Individuals at High Risk of Recent Tuberculosis Infection
Contacts of TB patients
Residents and staff of high-risk congregate settings:
Persons who, in the last 5 years, immigrated from a country where TB is prevalent
Staff of mycobacteriology laboratories
Children and adolescents exposed to high-risk adults
Children under the age of 4 years
Individuals at High Risk of Progression from Latent to Active Tuberculosis
HIV-infected persons
Intravenous drug abusers
Patients taking immunosuppressive drugs for 1 month or more
Patients with a chest radiograph indicating fibrotic changes consistent with prior TB
Patients with other high-risk medical conditions, including
How do we test for latent tuberculosis?
Tuberculin skin test.

Risk Category
Who Is In the Risk Category?
Test Result Considered Positive
High
HIV-positive people
Recent contacts of patients with TB
People with fibrotic changes on their chest radiograph consistent with prior TB
People taking immunosuppressive drugs for more than 1 month
5 mm of induration
Moderate
Recent immigrants from countries with a high prevalence of TB
Intravenous drug abusers
Residents and staff of high-risk congregate settings (eg, prisons, nursing homes, hospitals, homeless shelters)
Mycobacteriology laboratory personnel
Persons with high-risk medical conditions (eg, diabetes mellitus, chronic renal failure, silicosis, leukemia, lymphoma)
Children and adolescents exposed to high-risk adults
Children under 4 years old
10 mm of induration
Low
Persons with no risk factors for TB
15 mm of induration



