CHAPTER 52 Platelet aggregation is a complex process that ends with formation of fibrinogen bridges between glycoprotein IIb/IIIa (GP IIb/IIIa) receptors on adjacent platelets (Fig. 52–1). In order for these bridges to form, GP IIb/IIIa receptors must first undergo activation—that is, they must undergo a configurational change that allows them to bind with fibrinogen. As indicated in Figure 52–1A, activation of GP IIb/IIIa can be stimulated by multiple factors, including thromboxane A2 (TXA2), thrombin, collagen, platelet activation factor, and ADP. Under the influence of these factors, GP IIb/IIIa changes its shape, binds with fibrinogen, and thereby causes aggregation (see Fig. 52–1B). The aggregated platelets constitute a plug that stops bleeding. This plug is unstable, however, and must be reinforced with fibrin if protection is to last. Coagulation is defined as production of fibrin, a thread-like protein that reinforces the platelet plug. Fibrin is produced by two convergent pathways (Fig. 52–2), referred to as the contact activation pathway (also known as the intrinsic pathway) and the tissue factor pathway (also known as the extrinsic pathway). As shown in Figure 52–2, the two pathways converge at factor Xa, after which they employ the same final series of reactions. In both pathways, each reaction in the sequence amplifies the reaction that follows. Hence, once this sequence is initiated, it becomes self-sustaining and self-reinforcing. The tissue factor pathway is turned on by trauma to the vascular wall, which triggers release of tissue factor,* also known as tissue thromboplastin. Tissue factor then combines with and thereby activates factor VII, which in turn activates factor X, which then catalyzes the conversion of prothrombin (factor II) into thrombin (factor IIa). As shown in Figure 52–2, thrombin then does three things. First, it catalyzes the conversion of fibrinogen into fibrin. Second, it catalyzes the conversion of factor V into its active form (Va), a compound that greatly increases the activity of factor Xa, even though it has no direct catalytic activity of its own. Third, thrombin catalyzes the conversion of factor VIII into its active form (VIIIa), a compound that greatly increases the activity of factor IXa in the contact activation pathway. The contact activation pathway is turned on when blood makes contact with collagen that has been exposed as a result of trauma to a blood vessel wall. Collagen contact stimulates conversion of factor XII into its active form, XIIa (see Figure 52–2). Factor XIIa then activates factor XI, which activates factor IX, which activates factor X. After this, the contact activation pathway is the same as the tissue factor pathway. As noted, factor VIIIa, which is produced under the influence of thrombin, greatly increases the activity of factor IXa, even though it has no direct catalytic activity of its own. Important to our understanding of anticoagulant drugs is the fact that four coagulation factors—factors VII, IX, X, and II (prothrombin)—require vitamin K for their synthesis. These factors appear in green boxes in Figure 52–2. The significance of the vitamin K–dependent factors will become apparent when we discuss warfarin, an oral anticoagulant. To protect against widespread coagulation, the body must inactivate any clotting factors that stray from the site of vessel injury. Inactivation is accomplished with antithrombin, a protein that forms a complex with clotting factors, and thereby inhibits their activity. The clotting factors that can be neutralized by antithrombin appear in yellow in Figure 52–2. As we shall see, antithrombin is intimately involved in the action of heparin, an injectable anticoagulant drug. The drugs considered in this chapter fall into three major groups: (1) anticoagulants, (2) antiplatelet drugs, and (3) thrombolytic drugs, also known as fibrinolytic drugs. Anticoagulants (eg, heparin, warfarin, dabigatran) disrupt the coagulation cascade, and thereby suppress production of fibrin. Antiplatelet drugs (eg, aspirin, clopidogrel) inhibit platelet aggregation. Thrombolytic drugs (eg, alteplase) promote lysis of fibrin, and thereby cause dissolution of thrombi. Drugs that belong to these groups are listed in Table 52–1. TABLE 52–1 Overview of Drugs for Thromboembolic Disorders Traditionally, anticoagulants have been grouped into two major classes: oral anticoagulants and parenteral anticoagulants. This scheme was reasonable because, until recently, all oral anticoagulants belonged to just one pharmacologic class: the vitamin K antagonists, of which warfarin is the principal member. Today, however, anticoagulants in two other pharmacologic classes—direct factor Xa inhibitors and direct thrombin inhibitors—can also be administered by mouth (see Table 52–1). Hence, grouping the anticoagulants by route of administration makes less sense than in the past. Accordingly, in this chapter, these drugs are grouped only by pharmacologic class, and not by whether they are given orally or by injection. Our discussion focuses on three preparations: unfractionated heparin, the low-molecular-weight (LMW) heparins, and fondaparinux. Although all three activate antithrombin, they do not have equal effects on thrombin and factor Xa. Specifically, heparin reduces the activity of thrombin and factor Xa more or less equally; the LMW heparins reduce the activity of factor Xa more than they reduce the activity of thrombin; and fondaparinux causes selective inhibition of factor Xa, having no effect on thrombin. Properties of the three preparations are summarized in Table 52–2. TABLE 52–2 Comparison of Drugs That Activate Antithrombin aPTT = activated partial thromboplastin time, LMW = low molecular weight. Heparin suppresses coagulation by helping antithrombin inactivate clotting factors, primarily thrombin and factor Xa. As shown in Figure 52–3, binding of heparin to antithrombin produces a conformational change in antithrombin that greatly enhances its ability to inactivate both thrombin and factor Xa. However, the process of inactivating these two clotting factors is not identical. In order to inactivate thrombin, heparin must simultaneously bind with both thrombin and antithrombin, thereby forming a ternary complex (see Fig. 52–3). In contrast, in order to inactivate factor Xa, heparin binds only with antithrombin; heparin itself does not bind with factor Xa. The risk of hemorrhage can be decreased in several ways. First, dosage should be carefully controlled so that the activated partial thromboplastin time (see below) does not exceed 2 times the control value. In addition, candidates for heparin therapy should be screened for risk factors (see Warnings and Contraindications). Finally, antiplatelet drugs (eg, aspirin, clopidogrel) should be avoided. • Use of an indwelling epidural catheter • Use of other anticoagulants (eg, warfarin, dabigatran) • Use of antiplatelet drugs (eg, aspirin, clopidogrel) • History of traumatic or repeated epidural or spinal puncture • History of spinal deformity, spinal injury, or spinal surgery Low-molecular-weight (LMW) heparins are simply heparin preparations composed of molecules that are shorter than those found in unfractionated heparin. LMW heparins are as effective as unfractionated heparin and are easier to use. Why? Because LMW heparins can be given using a fixed dosage and don’t require aPTT monitoring. As a result, LMW heparins can be used at home, whereas unfractionated heparin must be given in a hospital. Because of these advantages, LMW heparins are now considered first-line therapy for prevention and treatment of DVT. In the United States, three LMW heparins are available: enoxaparin [Lovenox], dalteparin [Fragmin], and tinzaparin [Innohep]. Differences between LMW heparins and unfractionated heparin are summarized in Table 52–2. Anticoagulant activity of LMW heparin is mediated by the same active pentasaccharide sequence that mediates anticoagulant action of unfractionated heparin. However, because LMW heparin molecules are short, they do not have quite the same effect as unfractionated heparin. Specifically, whereas unfractionated heparin is equally good at inactivating factor Xa and thrombin, LMW heparins preferentially inactivate factor Xa, being much less able to inactivate thrombin. Why the difference? In order to inactivate thrombin, a heparin chain must not only contain the pentasaccharide sequence that activates antithrombin, it must also be long enough to provide a binding site for thrombin. This binding site is necessary because inactivation of thrombin requires simultaneous binding of thrombin with heparin and antithrombin (see Fig. 52–3). In contrast to unfractionated heparin chains, most (but not all) LMW heparin chains are too short to allow thrombin binding, and hence LMW heparins are less able to inactivate thrombin. Use in atrial fibrillation requires comment. As discussed in Chapter 49 (Antidysrhythmic Drugs), atrial fibrillation carries a high risk of stroke secondary to clot formation in the atrium. (If the clot becomes dislodged, it can travel to the brain and block an artery, thereby causing ischemic stroke.) So, when people have atrial fibrillation, anticoagulant therapy is given long term to prevent clot formation. Until recently, warfarin was the only oral anticoagulant available, and hence has been the reference standard for stroke prevention. However, two new oral anticoagulants—dabigatran [Pradaxa, Pradax The objective of treatment is to raise the INR to an appropriate value. Recommended INR ranges are summarized in Table 52–3. As indicated, an INR of 2 to 3 is appropriate for most patients—although for some patients the target INR is 3 to 4.5. If the INR is below the recommended range, warfarin dosage should be increased. Conversely, if the INR is above the recommended range, dosage should be reduced. Unfortunately, since warfarin has a delayed onset and prolonged duration of action, the INR cannot be altered quickly: Once the dosage has been changed, it may take a week or more to reach the desired INR. TABLE 52–3 Monitoring Warfarin Therapy: Recommended Ranges of Prothrombin Time–Derived Values *Observed PT ratio = ratio of patient’s PT to a control PT value. In this table, the reagent used to determine the control PT value is one of the preparations of rabbit brain thromboplastin employed in the United States. Had a different preparation of thromboplastin been used, the observed PT ratio could be very different. †INR = international normalized ratio. This value is calculated from the observed PT ratio. The INR is equivalent to the PT ratio that would have been obtained if the patient’s PT has been compared to a PT value obtained using the International Reference Preparation, a standardized human brain thromboplastin prepared by the World Health Organization. In contrast to PT ratios, INR values are comparable from one laboratory to the next throughout the United States and the rest of the world. ‡For prevention of ischemic stroke and systemic embolism. Several measures can reduce the risk of bleeding. Candidates for treatment must be carefully screened for risk factors (see Warnings and Contraindications below). Prothrombin time must be measured frequently. A variety of drugs can potentiate warfarin’s effects (see below), and hence must be used with care. Patients should be given detailed verbal and written instructions regarding signs of bleeding, dosage size and timing, and scheduling of PT tests. When a patient is incapable of accurate self-medication, a responsible individual must supervise treatment. Patients should be advised to make a record of each dose, rather than relying on memory. A soft toothbrush can reduce gingival bleeding. An electric razor can reduce cuts from shaving.
Anticoagulant, antiplatelet, and thrombolytic drugs
Coagulation: physiology and pathophysiology
Hemostasis
Stage one: formation of a platelet plug.
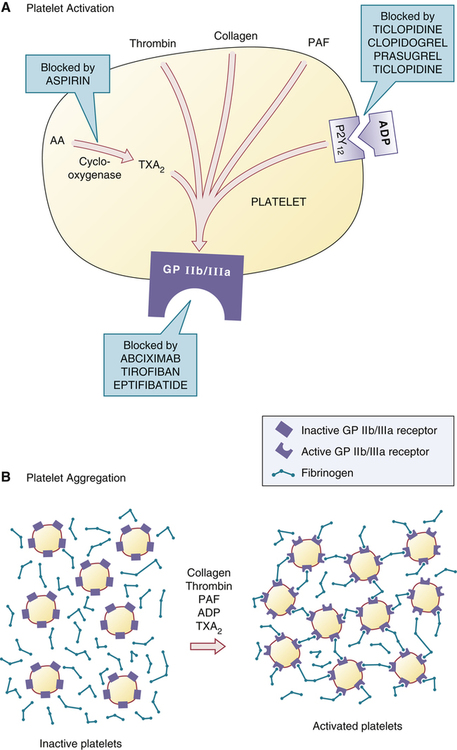
 Mechanism of platelet aggregation and actions of antiplatelet drugs.
Mechanism of platelet aggregation and actions of antiplatelet drugs.
A, Multiple factors—TXA2, thrombin, collagen, PAF, and ADP—promote activation of the GP IIb/IIIa receptor. Each platelet has 50,000 to 80,000 GP IIb/IIIa receptors, although only one is shown. B, Activation of the GP IIb/IIIa receptor permits binding of fibrinogen, which then causes aggregation by forming cross-links between platelets. After aggregation occurs, the platelet plug is reinforced with fibrin (not shown). (AA = arachidonic acid, ADP = adenosine diphosphate, GP IIb/IIIa = glycoprotein IIb/IIIa receptor, PAF = platelet activation factor, P2Y12 = P2Y12 ADP receptor, TXA2 = thromboxane A2.)
Stage two: coagulation.
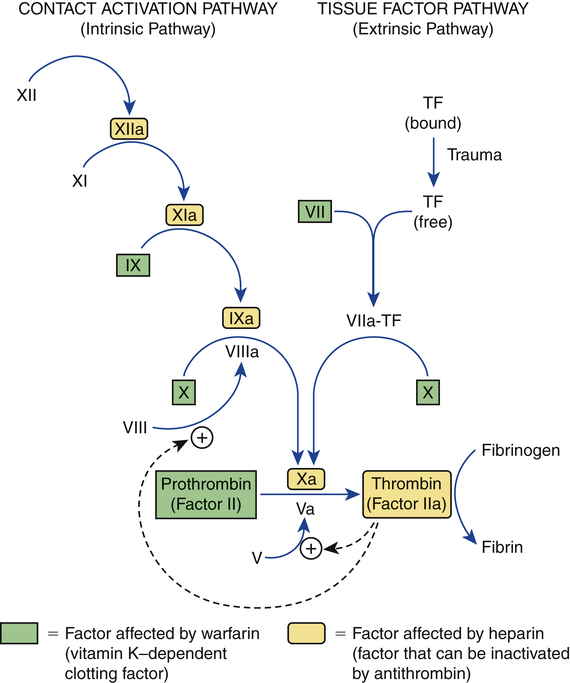
 Outline of coagulation pathways showing factors affected by warfarin and heparin.
Outline of coagulation pathways showing factors affected by warfarin and heparin.
TF = tissue factor. Common names for factors shown in roman numerals: V = proaccelerin, VII = proconvertin, VIII = antihemophilic factor, IX = Christmas factor, X = Stuart factor, XI = plasma thromboplastin antecedent, and XII = Hageman factor. The letter “a” after a factor’s name (eg, factor VIIIa) indicates the active form of the factor.
Keeping hemostasis under control.
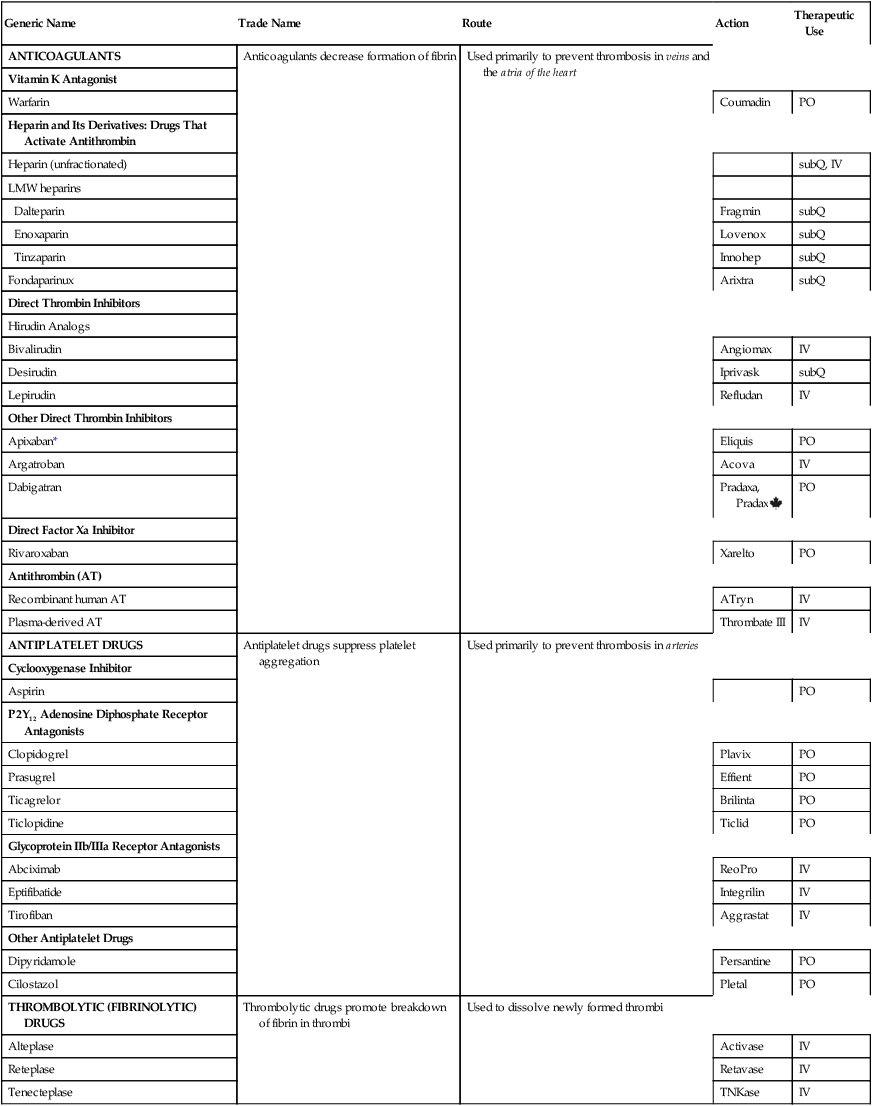
Overview of drugs for thromboembolic disorders

Generic Name
Trade Name
Route
Action
Therapeutic Use
ANTICOAGULANTS
Anticoagulants decrease formation of fibrin
Used primarily to prevent thrombosis in veins and the atria of the heart
Vitamin K Antagonist
Warfarin
Coumadin
PO
Heparin and Its Derivatives: Drugs That Activate Antithrombin
Heparin (unfractionated)
subQ, IV
LMW heparins
Dalteparin
Fragmin
subQ
Enoxaparin
Lovenox
subQ
Tinzaparin
Innohep
subQ
Fondaparinux
Arixtra
subQ
Direct Thrombin Inhibitors
Hirudin Analogs
Bivalirudin
Angiomax
IV
Desirudin
Iprivask
subQ
Lepirudin
Refludan
IV
Other Direct Thrombin Inhibitors
Apixaban*
Eliquis
PO
Argatroban
Acova
IV
Dabigatran
Pradaxa, Pradax ![]()
PO
Direct Factor Xa Inhibitor
Rivaroxaban
Xarelto
PO
Antithrombin (AT)
Recombinant human AT
ATryn
IV
Plasma-derived AT
Thrombate III
IV
ANTIPLATELET DRUGS
Antiplatelet drugs suppress platelet aggregation
Used primarily to prevent thrombosis in arteries
Cyclooxygenase Inhibitor
Aspirin
PO
P2Y12 Adenosine Diphosphate Receptor Antagonists
Clopidogrel
Plavix
PO
Prasugrel
Effient
PO
Ticagrelor
Brilinta
PO
Ticlopidine
Ticlid
PO
Glycoprotein IIb/IIIa Receptor Antagonists
Abciximab
ReoPro
IV
Eptifibatide
Integrilin
IV
Tirofiban
Aggrastat
IV
Other Antiplatelet Drugs
Dipyridamole
Persantine
PO
Cilostazol
Pletal
PO
THROMBOLYTIC (FIBRINOLYTIC) DRUGS
Thrombolytic drugs promote breakdown of fibrin in thrombi
Used to dissolve newly formed thrombi
Alteplase
Activase
IV
Reteplase
Retavase
IV
Tenecteplase
TNKase
IV
Anticoagulants
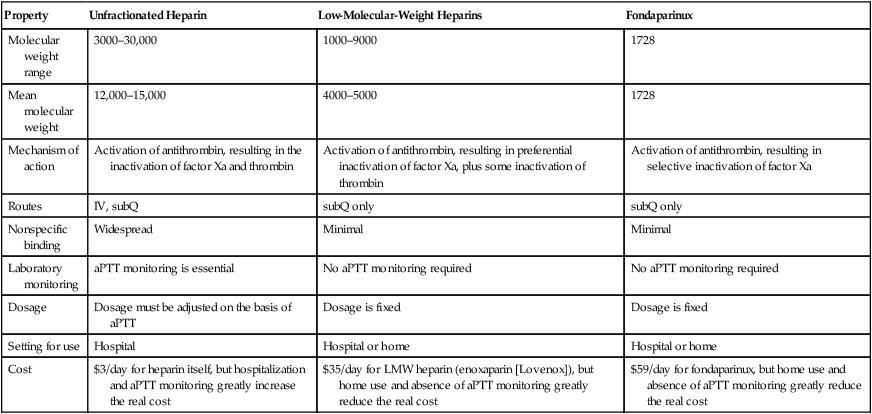
Heparin and its derivatives: drugs that activate antithrombin

Property
Unfractionated Heparin
Low-Molecular-Weight Heparins
Fondaparinux
Molecular weight range
3000–30,000
1000–9000
1728
Mean molecular weight
12,000–15,000
4000–5000
1728
Mechanism of action
Activation of antithrombin, resulting in the inactivation of factor Xa and thrombin
Activation of antithrombin, resulting in preferential inactivation of factor Xa, plus some inactivation of thrombin
Activation of antithrombin, resulting in selective inactivation of factor Xa
Routes
IV, subQ
subQ only
subQ only
Nonspecific binding
Widespread
Minimal
Minimal
Laboratory monitoring
aPTT monitoring is essential
No aPTT monitoring required
No aPTT monitoring required
Dosage
Dosage must be adjusted on the basis of aPTT
Dosage is fixed
Dosage is fixed
Setting for use
Hospital
Hospital or home
Hospital or home
Cost
$3/day for heparin itself, but hospitalization and aPTT monitoring greatly increase the real cost
$35/day for LMW heparin (enoxaparin [Lovenox]), but home use and absence of aPTT monitoring greatly reduce the real cost
$59/day for fondaparinux, but home use and absence of aPTT monitoring greatly reduce the real cost
Heparin (unfractionated)
Mechanism of anticoagulant action
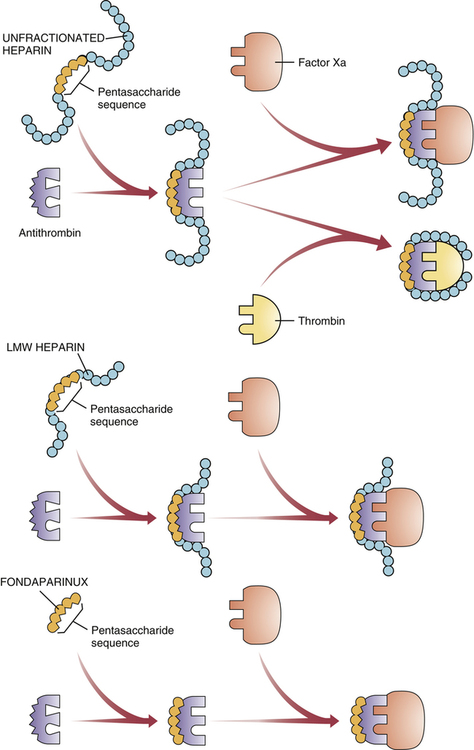
 Mechanism of action of heparin, LMW heparins, and fondaparinux.
Mechanism of action of heparin, LMW heparins, and fondaparinux.
All three drugs share a pentasaccharide sequence that allows them to bind with—and thereby activate—antithrombin, a protein that inactivates two major clotting factors: thrombin and factor Xa. All three drugs enable antithrombin to inactivate factor Xa, but only heparin also facilitates inactivation of thrombin.
Upper Panel: Unfractionated heparin binds with antithrombin, thereby causing a conformational change in antithrombin that greatly increases its ability to interact with factor Xa and thrombin. As shown, when the heparin-antithrombin complex binds with thrombin, heparin changes its conformation such that both heparin and antithrombin come in contact with thrombin. Formation of this ternary complex is necessary for thrombin inactivation. Inactivation of factor Xa is different: It only requires contact between activated antithrombin and factor Xa; contact between heparin and factor Xa is unnecessary.
Middle Panel: Low-molecular-weight (LMW) heparins have the same pentasaccharide sequence as unfractionated heparin, and hence can bind with and thereby activate antithrombin. However, in contrast to unfractionated heparin, which promotes inactivation of both thrombin and factor Xa, most molecules of LMW heparin can only inactivate factor Xa; they are unable to inactivate thrombin. Why? Because most molecules of LMW heparin are too small to form a ternary complex with thrombin and antithrombin.
Lower Panel: Fondaparinux is a synthetic pentasaccharide identical in structure to the antithrombin binding sequence found in unfractionated heparin and LMW heparins. Being even smaller than LMW heparins, fondaparinux is too small to form a ternary complex with thrombin, and hence can only inactivate factor Xa.
Adverse effects
Hemorrhage.
Spinal/epidural hematoma.
Low-molecular-weight heparins
Group properties
Mechanism of action.
Warfarin, a Vitamin K antagonist
Therapeutic uses
Atrial fibrillation.
![]() ] and rivaroxaban [Xarelto]—which are much easier to use than warfarin, are likely to displace warfarin as the treatment of choice for many patients.
] and rivaroxaban [Xarelto]—which are much easier to use than warfarin, are likely to displace warfarin as the treatment of choice for many patients.
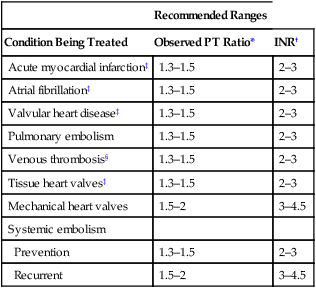
Monitoring treatment

Recommended Ranges
Condition Being Treated
Observed PT Ratio*
INR†
Acute myocardial infarction‡
1.3–1.5
2–3
Atrial fibrillation‡
1.3–1.5
2–3
Valvular heart disease‡
1.3–1.5
2–3
Pulmonary embolism
1.3–1.5
2–3
Venous thrombosis§
1.3–1.5
2–3
Tissue heart valves‡
1.3–1.5
2–3
Mechanical heart valves
1.5–2
3–4.5
Systemic embolism
Prevention
1.3–1.5
2–3
Recurrent
1.5–2
3–4.5
Adverse effects
Hemorrhage.
Get Clinical Tree app for offline access

Anticoagulant, antiplatelet, and thrombolytic drugs
Get Clinical Tree app for offline access


