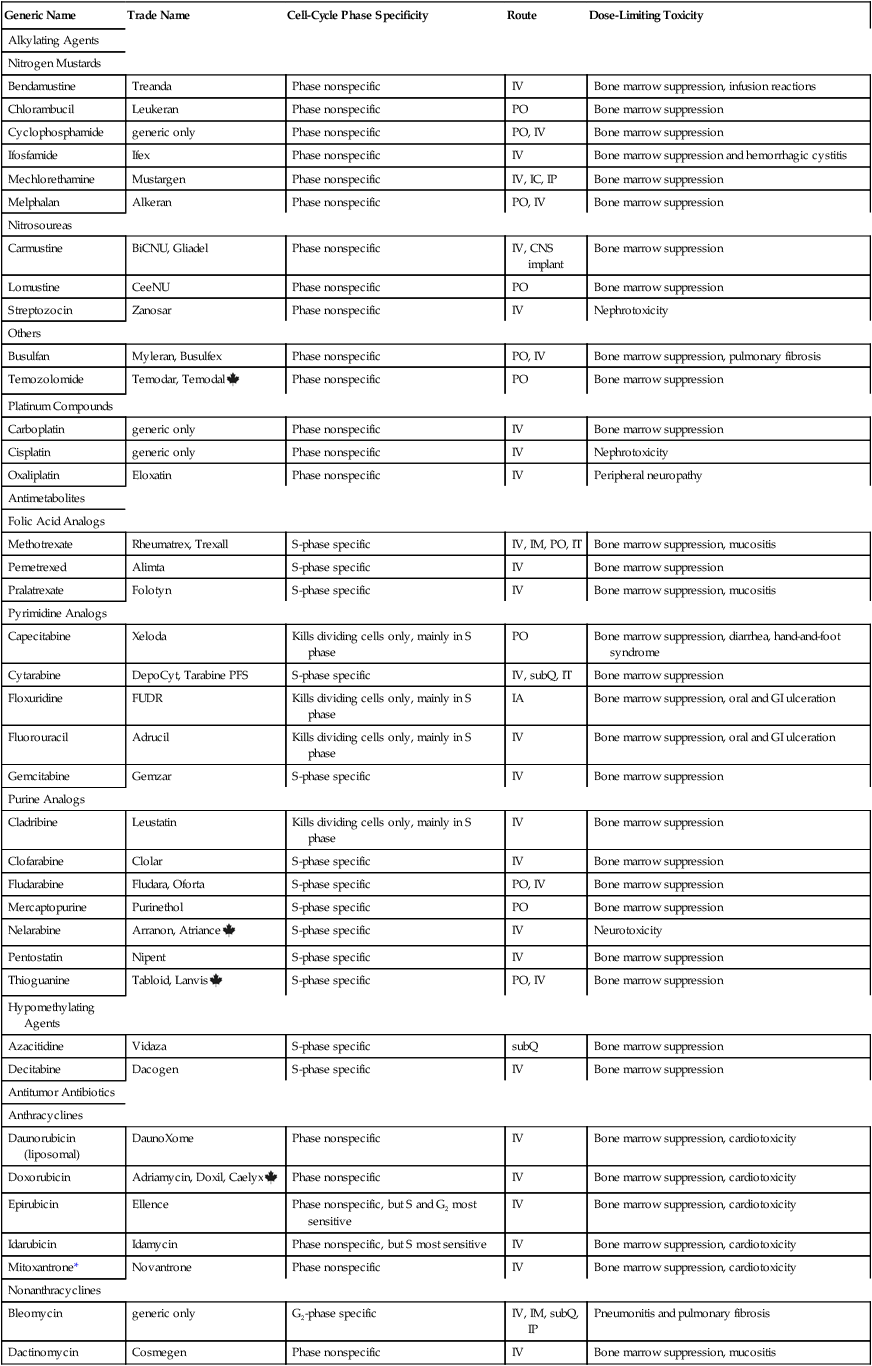
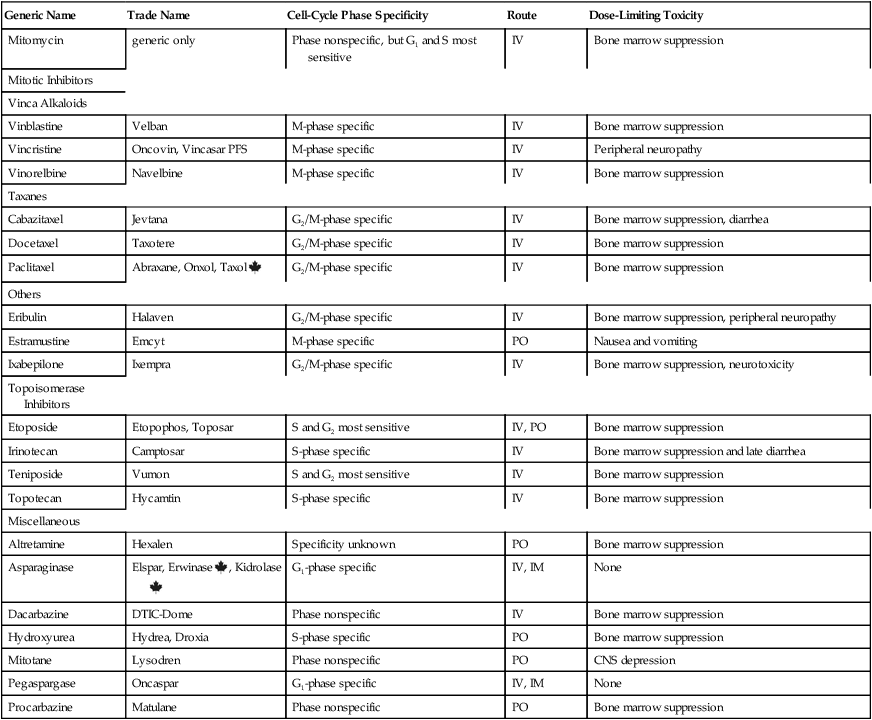
CHAPTER 102 The cytotoxic agents constitute the largest class of anticancer drugs. As their name implies, these agents act directly on cancer cells to cause their death. The cytotoxic drugs can be subdivided into eight major groups: (1) alkylating agents, (2) platinum compounds, (3) antimetabolites, (4) hypomethylating agents, (5) antitumor antibiotics, (6) mitotic inhibitors, (7) topoisomerase inhibitors, and (8) miscellaneous cytotoxic drugs. I don’t discuss each drug in detail. Rather, I focus on selected representative agents. Individual cytotoxic agents are listed in Table 102–1. TABLE 102–1 *Mitoxantrone is classified chemically as an anthracenedione, which is very similar to an anthracycline. Table 102–2 summarizes the principal mechanisms by which the cytotoxic anticancer drugs act. As the table shows, most cytotoxic agents disrupt processes related to synthesis of DNA or its precursors. In addition, some agents (eg, vinblastine, vincristine) act specifically to block mitosis, and one drug—asparaginase—disrupts synthesis of proteins. Note that, with the exception of asparaginase, all of the cytotoxic drugs disrupt processes carried out exclusively by cells that are undergoing replication. As a result, these drugs are most toxic to tissues that have a high growth fraction (ie, a high proportion of proliferating cells). TABLE 102–2 Actions of Representative Cytotoxic Anticancer Drugs As discussed in Chapter 101, the cell cycle is the sequence of events that a cell goes through from one mitotic division to the next. Some anticancer agents, known as cell-cycle phase–specific drugs, are effective only during a specific phase of the cell cycle. Other anticancer agents, known as cell-cycle phase–nonspecific drugs, can affect cells during any phase of the cell cycle. About half of the cytotoxic anticancer drugs are phase specific, and the other half are phase nonspecific. The phase specificity of individual cytotoxic agents is summarized in Table 102–1. As discussed in Chapter 101, many anticancer drugs are toxic to normal tissues—especially tissues that have a high percentage of proliferating cells (bone marrow, hair follicles, GI epithelium, germinal epithelium). The common major toxicities of the cytotoxic anticancer drugs, together with management procedures, are discussed at length in Chapter 101. Therefore, as we consider individual anticancer agents in this chapter, discussion of most toxicities is brief. As discussed in Chapter 101, extravasation of vesicants can cause severe local injury, sometimes requiring surgical débridement and skin grafting. Drugs with strong vesicant properties include carmustine, dacarbazine, dactinomycin, daunorubicin, doxorubicin, mechlorethamine, mitomycin, plicamycin, streptozocin, vinblastine, and vincristine. To minimize the risk of injury, IV administration should be performed only into a vein with good flow. Sites of previous irradiation should be avoided. If extravasation occurs, the infusion should be discontinued immediately. The family of alkylating agents consists of nitrogen mustards, nitrosoureas, and other compounds. Before considering the properties of individual alkylating agents, we discuss the characteristics of the group as a whole. The alkylating agents are listed in Table 102–1. The alkylating agents are highly reactive compounds that can transfer an alkyl group to various cell constituents. Cell kill results primarily from alkylation of DNA. As a rule, alkylating agents interact with DNA by forming a covalent bond with a specific nitrogen atom in guanine (Fig. 102–1). Some alkylating agents have two reactive sites, whereas others have only one. Alkylating agents with two reactive sites (bifunctional agents) are able to bind DNA in two places to form cross-links. These bridges may be formed within a single DNA strand or between parallel DNA strands. Figure 102–1 illustrates the production of interstrand cross-links by nitrogen mustard. Alkylating agents with only one reactive site (monofunctional agents) lack the ability to form cross-links, but can still bind to a single guanine in DNA.
Anticancer drugs I: cytotoxic agents
Introduction to the cytotoxic anticancer drugs

Generic Name
Trade Name
Cell-Cycle Phase Specificity
Route
Dose-Limiting Toxicity
Alkylating Agents
Nitrogen Mustards
Bendamustine
Treanda
Phase nonspecific
IV
Bone marrow suppression, infusion reactions
Chlorambucil
Leukeran
Phase nonspecific
PO
Bone marrow suppression
Cyclophosphamide
generic only
Phase nonspecific
PO, IV
Bone marrow suppression
Ifosfamide
Ifex
Phase nonspecific
IV
Bone marrow suppression and hemorrhagic cystitis
Mechlorethamine
Mustargen
Phase nonspecific
IV, IC, IP
Bone marrow suppression
Melphalan
Alkeran
Phase nonspecific
PO, IV
Bone marrow suppression
Nitrosoureas
Carmustine
BiCNU, Gliadel
Phase nonspecific
IV, CNS implant
Bone marrow suppression
Lomustine
CeeNU
Phase nonspecific
PO
Bone marrow suppression
Streptozocin
Zanosar
Phase nonspecific
IV
Nephrotoxicity
Others
Busulfan
Myleran, Busulfex
Phase nonspecific
PO, IV
Bone marrow suppression, pulmonary fibrosis
Temozolomide
Temodar, Temodal ![]()
Phase nonspecific
PO
Bone marrow suppression
Platinum Compounds
Carboplatin
generic only
Phase nonspecific
IV
Bone marrow suppression
Cisplatin
generic only
Phase nonspecific
IV
Nephrotoxicity
Oxaliplatin
Eloxatin
Phase nonspecific
IV
Peripheral neuropathy
Antimetabolites
Folic Acid Analogs
Methotrexate
Rheumatrex, Trexall
S-phase specific
IV, IM, PO, IT
Bone marrow suppression, mucositis
Pemetrexed
Alimta
S-phase specific
IV
Bone marrow suppression
Pralatrexate
Folotyn
S-phase specific
IV
Bone marrow suppression, mucositis
Pyrimidine Analogs
Capecitabine
Xeloda
Kills dividing cells only, mainly in S phase
PO
Bone marrow suppression, diarrhea, hand-and-foot syndrome
Cytarabine
DepoCyt, Tarabine PFS
S-phase specific
IV, subQ, IT
Bone marrow suppression
Floxuridine
FUDR
Kills dividing cells only, mainly in S phase
IA
Bone marrow suppression, oral and GI ulceration
Fluorouracil
Adrucil
Kills dividing cells only, mainly in S phase
IV
Bone marrow suppression, oral and GI ulceration
Gemcitabine
Gemzar
S-phase specific
IV
Bone marrow suppression
Purine Analogs
Cladribine
Leustatin
Kills dividing cells only, mainly in S phase
IV
Bone marrow suppression
Clofarabine
Clolar
S-phase specific
IV
Bone marrow suppression
Fludarabine
Fludara, Oforta
S-phase specific
PO, IV
Bone marrow suppression
Mercaptopurine
Purinethol
S-phase specific
PO
Bone marrow suppression
Nelarabine
Arranon, Atriance ![]()
S-phase specific
IV
Neurotoxicity
Pentostatin
Nipent
S-phase specific
IV
Bone marrow suppression
Thioguanine
Tabloid, Lanvis ![]()
S-phase specific
PO, IV
Bone marrow suppression
Hypomethylating Agents
Azacitidine
Vidaza
S-phase specific
subQ
Bone marrow suppression
Decitabine
Dacogen
S-phase specific
IV
Bone marrow suppression
Antitumor Antibiotics
Anthracyclines
Daunorubicin (liposomal)
DaunoXome
Phase nonspecific
IV
Bone marrow suppression, cardiotoxicity
Doxorubicin
Adriamycin, Doxil, Caelyx ![]()
Phase nonspecific
IV
Bone marrow suppression, cardiotoxicity
Epirubicin
Ellence
Phase nonspecific, but S and G2 most sensitive
IV
Bone marrow suppression, cardiotoxicity
Idarubicin
Idamycin
Phase nonspecific, but S most sensitive
IV
Bone marrow suppression, cardiotoxicity
Mitoxantrone*
Novantrone
Phase nonspecific
IV
Bone marrow suppression, cardiotoxicity
Nonanthracyclines
Bleomycin
generic only
G2-phase specific
IV, IM, subQ, IP
Pneumonitis and pulmonary fibrosis
Dactinomycin
Cosmegen
Phase nonspecific
IV
Bone marrow suppression, mucositis
Mitomycin
generic only
Phase nonspecific, but G1 and S most sensitive
IV
Bone marrow suppression
Mitotic Inhibitors
Vinca Alkaloids
Vinblastine
Velban
M-phase specific
IV
Bone marrow suppression
Vincristine
Oncovin, Vincasar PFS
M-phase specific
IV
Peripheral neuropathy
Vinorelbine
Navelbine
M-phase specific
IV
Bone marrow suppression
Taxanes
Cabazitaxel
Jevtana
G2/M-phase specific
IV
Bone marrow suppression, diarrhea
Docetaxel
Taxotere
G2/M-phase specific
IV
Bone marrow suppression
Paclitaxel
Abraxane, Onxol, Taxol ![]()
G2/M-phase specific
IV
Bone marrow suppression
Others
Eribulin
Halaven
G2/M-phase specific
IV
Bone marrow suppression, peripheral neuropathy
Estramustine
Emcyt
M-phase specific
PO
Nausea and vomiting
Ixabepilone
Ixempra
G2/M-phase specific
IV
Bone marrow suppression, neurotoxicity
Topoisomerase Inhibitors
Etoposide
Etopophos, Toposar
S and G2 most sensitive
IV, PO
Bone marrow suppression
Irinotecan
Camptosar
S-phase specific
IV
Bone marrow suppression and late diarrhea
Teniposide
Vumon
S and G2 most sensitive
IV
Bone marrow suppression
Topotecan
Hycamtin
S-phase specific
IV
Bone marrow suppression
Miscellaneous
Altretamine
Hexalen
Specificity unknown
PO
Bone marrow suppression
Asparaginase
Elspar, Erwinase ![]() , Kidrolase
, Kidrolase![]()
G1-phase specific
IV, IM
None
Dacarbazine
DTIC-Dome
Phase nonspecific
IV
Bone marrow suppression
Hydroxyurea
Hydrea, Droxia
S-phase specific
PO
Bone marrow suppression
Mitotane
Lysodren
Phase nonspecific
PO
CNS depression
Pegaspargase
Oncaspar
G1-phase specific
IV, IM
None
Procarbazine
Matulane
Phase nonspecific
PO
Bone marrow suppression
Mechanisms of cytotoxic action

Drug
Drug Action
Cellular Process Disrupted
Cyclophosphamide
Alkylates DNA, causing cross-links and strand breakage
DNA and RNA synthesis
Methotrexate
Inhibits 1-carbon transfer reactions
Synthesis of DNA precursors (purines, dTMP)
Hydroxyurea
Inhibits ribonucleotide reductase
Synthesis of DNA precursors (blocks conversion of ribonucleotides into deoxyribonucleotides)
Thioguanine, mercaptopurine
Inhibit purine ring synthesis and nucleotide interconversion
Synthesis of DNA precursors (purines, pyrimidines, ribonucleotides, and deoxyribonucleotides)
Fluorouracil
Inhibits thymidylate synthetase
Synthesis of dTMP, a DNA precursor
Cytarabine
Inhibits DNA polymerase
DNA synthesis
Bleomycin
Breaks DNA strands and prevents their repair
DNA synthesis
Doxorubicin
Intercalates between base pairs of DNA and inhibits topoisomerase II
DNA and RNA synthesis
Vinblastine, vincristine
Block microtubule assembly
Mitosis
Asparaginase
Deaminates asparagine, depriving cells of this amino acid
Protein synthesis
Topotecan
Inhibits topoisomerase I and thereby prevents resealing of DNA strand breaks
Impairs DNA replication
Cell-cycle phase specificity
Toxicity
Dosage, handling, and administration
Administering vesicants.
Alkylating agents
Shared properties
Mechanism of action.
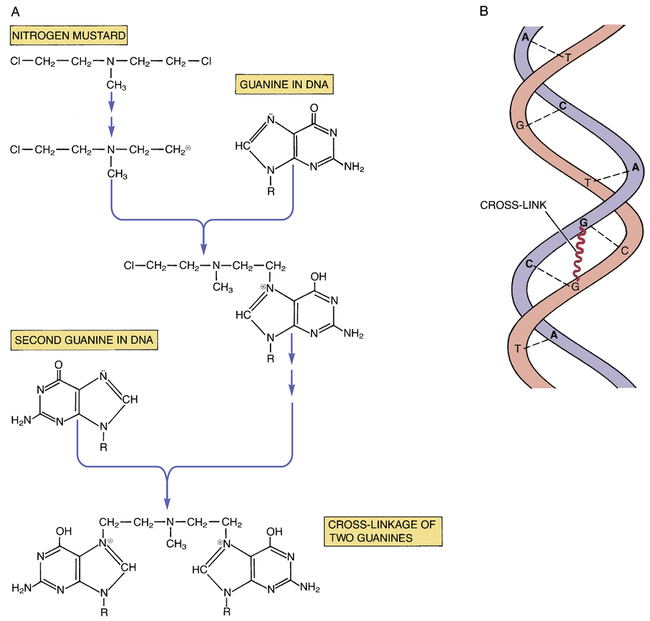
 Cross-linking of DNA by an alkylating agent.
Cross-linking of DNA by an alkylating agent.
A, Reactions leading to cross-linkage between guanine moieties in DNA. B, Schematic representation of interstrand cross-linking within the DNA double helix. (A = adenine, C = cytosine, G = guanine, T = thymine.)



