Chapter 23. Antibacterial drugs
Introduction302
Gram-positive and gram-negative bacteria302
Choosing an antibiotic303
The nitrofurans305
The quinolones305
Chloramphenicol313
Miscellaneous antibiotics314
Prevention of surgical sepsis321
Practical points in the administration of antibiotics322
Deaths involving Clostridium difficile and Staphylococcus aureus in England and Wales322
At the end of this chapter, the reader should be able to:
• explain how the bacterial cell wall is a target for antibiotics
• define the terms Gram-positive and Gram-negative bacteria
• discuss how an antibiotic is chosen
• distinguish the different classes of antibiotics and give important examples of each
• give an account of what TB is, how it is diagnosed and treated with drugs, and about immunization
• describe resistance to antibiotics, and how it happens with penicillins
• explain how antibiotics are used to treat common infections
• state the practicalities and precautions of antibiotic handling and administration
Introduction
Antibacterial drugs have revolutionized the treatment of infection, and many diseases such as bacterial meningitis, which previously were often fatal, are now usually curable, but it must be realized that the battle against pathogenic bacteria is by no means over. Many organisms have become resistant to antibacterial drugs and this requires a continuing search for new drugs and modification of those already in use. It also means that they should not be used unnecessarily.
It should be remembered that even with powerful antibacterial drugs the patient’s natural resistance plays an important part in combating infection. The nurse will note that patients with impaired immunity, due to prolonged illness, old age or perhaps the use of cytotoxic or immunosuppressive drugs, respond poorly to antibacterial drugs and the infection is much harder to eradicate.
How antibacterial substances work
Antibiotics exert their effects in two main ways:
• Bactericidal agents kill bacteria rapidly (e.g. aminoglycosides, polymyxin).
• Bacteriostatic agents prevent bacteria from replicating, but do not kill them (e.g. sulphonamides, tetracyclines, chloramphenicol).
The distinction between these two categories is not clear. Many antibiotics that operate principally as bacteriostatic agents can become bactericidal under favourable circumstances. Factors affecting the mode of action include the concentration of the drug and the number and type of organisms. When relatively modest numbers of bacteria are present and the drug is given in high doses to highly sensitive organisms, a normally bacteriostatic agent such as penicillin may become bactericidal.
Antibiotics and the bacterial cell wall
Each of the main groups of antibiotics has a different molecular structure and this is the factor that determines its mode of action. Many antibiotics exert their effects directly on the bacterial cell wall or must pass through it before disrupting bacterial metabolism at the intracellular level. The cell walls of all bacteria are composed of layers of protein molecules bound together by cross-linkages, resulting in a large, complex chemical aggregate, but their fine structure depends on whether they are Gram-positive or Gram-negative. The fine structure of the cell wall influences susceptibility to the different groups of antibiotics. For example, erythromycin is able to penetrate the cell walls of Gram-positive bacteria and is effective in the treatment of some staphylococcal and streptococcal infections, but has no effect on Gram-negative bacteria. Some of the diverse ways in which the different groups of antibiotics exert their effects are illustrated in Figure 23.1.
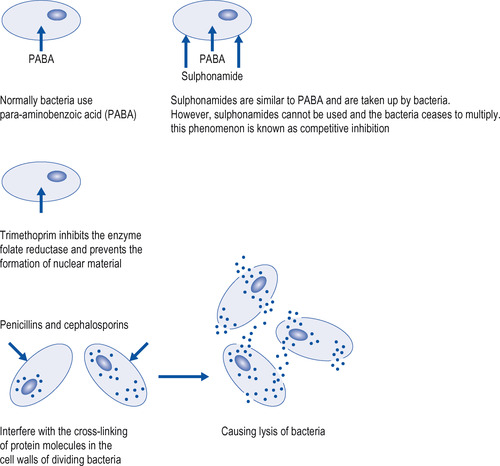 |
| Figure 23.1 Some of the modes of action of antibacterial drugs. |
Gram-positive and gram-negative bacteria
With few exceptions, bacteria may be classified as Gram-positive or Gram-negative, according to a staining technique used in laboratory identification. Generally, Gram-positive bacteria are able to withstand desiccation better than can Gram-negative bacteria, and many form spores that resist drying. Gram-negative species multiply rapidly in the presence of moisture even when provided with minimal nourishment. Several Gram-positive and Gram-negative bacteria have had a long association with hospital infection and, because they can be carried on the hands, may be spread by cross-infection in any health care setting (Table 23.1).
| Gram-positive | Gram-negative |
|---|---|
| Staphylococcus aureus | Haemophilus influenzae |
| Streptococcus pyogenes | Neisseria gonorrhoeae (Gonococcus) |
| Streptococcus viridans | Neisseria meningitides (Meningococcus) |
| Streptococcus pneumoniae (Pneumococcus) | E. coliProteusPseudomonasKlebsiella |
Anaerobes
This term refers to bacteria that can live and multiply in the absence of free oxygen. In the laboratory they require special conditions before they will grow in culture, but they are able to cause severe infections given the correct circumstances. Anaerobic bacteria naturally inhabiting the gut may cause severe sepsis following abdominal surgery. If Clostridium tetani gains access to a penetrating wound that is free of oxygen the organism multiplies and produces a toxin that causes tetanus.
Choosing an antibiotic
When faced with a patient with an infectious disease, the first consideration is whether an antibiotic is required. Many infections (e.g. a mild sore throat) get better quickly without specific treatment. If antibiotic treatment is needed, the following points should be considered:
• The infecting organism: this is often suspected from the nature of the disease, and initial treatment is usually based on this guess. If possible, the nature of the organism should be confirmed by culture of blood, sputum, urine, etc., although in practice this is not always possible.
• The correct antibiotic to eradicate the infection.
• The ability of the antibiotic to penetrate the site of infection (for example, in meningitis not all drugs enter the cerebrospinal fluid).
• The route of administration: some antibiotics are ineffective orally and injection may also be required for rapid action.
• A drug history is essential (as always) to ensure that the patient has not previously had an adverse reaction to the chosen antibiotic, or that the antibiotic will not compromise any other drug being used, e.g. rifampicin and the Pill (see below).
• Possible complicating factors such as pregnancy, and renal or hepatic failure.
• In these difficult times, the cost of the drug, although this decision should not jeopardize the patient’s safety or return to health.
Note that antibiotic is a term used to describe any substance produced by or derived from an organism which inhibits the growth of or destroys another organism, usually a bacterial or fungal infection.
Case History 23.1 gives an example of the procedures used.
Case History 23.1
Miss B, aged 27, developed a cough which produced phlegm and she bought a proprietary cough mixture, which did not stop the cough. She also became troubled by shortness of breath, especially when she lay on her back, and a severe headache; therefore, she decided to visit her GP. The GP, who listened to her chest and said there was nothing untoward except a slight sound on one side, diagnosed a bacterial infection. A sputum specimen was taken and sent away for analysis. There was indeed a bacterial infection and a course of clarithromycin was prescribed to treat the chest infection; Miss B was told to complete the full course of treatment. Salbutamol, an α 2 agonist, was prescribed for the shortness of breath, together with a spacer to help deliver the full dose of salbutamol. It was also recommended that Miss B take aspirin for the headache.
The sulphonamides
This is one of the oldest groups of antibacterial agents. They differ to some extent in the range of organisms they attack, but most of their pharmacological properties are similar. They have been largely replaced because of the development of bacterial resistance and adverse side-effects, and most have disappeared from use. They are, with few exceptions, well and rapidly absorbed from the intestinal tract. They circulate widely in the body fluids and cross the meningeal barrier to enter the cerebrospinal fluid (CSF).
After absorption, the liver begins to acetylate the sulphonamides. The acetylated drugs together with unaltered sulphonamide are excreted in the urine. The acetylated sulphonamides are very poorly soluble and therefore there is a danger that they will precipitate in the urine unless an adequate flow is maintained. Most of the sulphonamides are effective against a fairly wide range of bacteria. Unfortunately, certain of these bacteria have become resistant. Table 23.2 gives a number of available sulphonamides.
| Drug | Important features |
|---|---|
| Sulfadimidine | Well absorbed orally |
| Sulfasalazine | Used in ulcerative colitis. It is particularly useful for long-term maintenance treatment |
| Silver sulfadiazine | Applied locally as a cream to prevent infection in severe burns |
Therapeutic use of sulphonamides
Sulfadimidine and sulfasalazine
Sulfadimidine is still used occasionally in urinary infections provided that the infecting organism is susceptible. Sulfasalazine is given in the long-term treatment of ulcerative colitis and Crohn’s disease (see p. 151). Sulfasalazine can also be used in the treatment of rheumatoid arthritis.
Adverse effects
• Rashes of various types, sometimes with fever.
• Blood dyscrasias: polyarteritis nodosa is a rare, but dangerous, complication.
• The sperm count is reduced by sulfasalazine, but recovers on stopping the drug.
• Precipitation in the urinary tract causing obstruction; the patient should be given 2–3 litres of fluid daily to maintain a good urinary flow when receiving sulfadimidine.
Co-trimoxazole (trimethoprim+sulfamethoxazole)
Mechanism of action
Sulphonamides affect bacteria by interfering with their use of para-aminobenzoic acid (PABA), a precursor of folic acid, which is ultimately essential in cell division (see Fig. 23.1). Trimethoprim interferes with folic acid metabolism at the phase when folic acid is changed to folinic acid to build up the cell nucleus. This requires the action of an enzyme, and, by combining with that enzyme, trimethoprim stops the reaction and the cell dies. The combination of a sulphonamide with trimethoprim is particularly effective in preventing bacterial cell division and is also bactericidal. Co-trimoxazole is effective against Streptococcus pyogenes, Streptococcus pneumoniae, E. coli, Haemophilus (H.) influenzae, Salmonella and Pneumocystis carinii (in large doses).
Therapeutic use
The combined tablet of trimethoprim plus sulfamethoxazole, co-trimoxazole, has been widely and successfully used in exacerbations of chronic bronchitis and in urinary infections. It also has a place in the treatment of the more severe salmonella and other infections. Unfortunately, its adverse-effect profile has led to its use being largely confined to pneumocystis pneumonia. In large doses it is used to treat pneumocystis infection of the lung, a disease which occurs in patients whose immunity has been suppressed, often as a result of cancer chemotherapy or HIV.
Adverse effects
These are largely those of the sulphonamides, namely nausea and vomiting, and occasionally blood disorders. More serious is the occasional development of the Stevens–Johnson syndrome with a bullous rash, mouth ulceration and fever, which can be fatal.
Trimethoprim
Trimethoprim can also be used alone. At present it is largely used to treat urinary infection. It can also be used to treat bronchitis.
Adverse effects
These include nausea, rashes and, rarely, depression of the blood count.
Contraindication
It should not be used in the first 3 months of pregnancy.
The nitrofurans
This group of chemotherapeutic agents has been investigated sporadically for over 30 years. The only one currently used is nitrofurantoin.
Nitrofurantoin has a fairly wide antibacterial spectrum and is considerably concentrated in the urine. It is used in the treatment of urinary tract infections, or as a prophylactic. Nausea sometimes occurs but can be minimized by giving the drug after food. Other adverse effects include rashes and fever. It should not be used in patients with renal failure, as accumulation will occur.
The quinolones
The quinolones comprise:
• ciprofloxacin
• levofloxacin
• nalidixic acid
• norfloxacin
• ofloxacin.
This group of antibacterial drugs is increasingly important; several are available already and more will probably be introduced in the next few years. They interfere with an enzyme necessary for the cell division of bacteria.
Ciprofloxacin
Therapeutic use
Ciprofloxacin acts against a wide range of organisms, but is not very effective against some Gram-positive organisms, particularly pneumococci. It is given orally twice daily or by infusion, which is very expensive. At present, its use should be confined to patients for whom older antibacterial drugs are unsatisfactory, particularly for typhoid, urinary infection and gonorrhoea. It is the preferred drug in adults as a prophylactic for close contact with meningococcal meningitis. There has been a large increase in resistance to ciprofloxacin in recent years.
Adverse effects
These include gastrointestinal upsets and rashes. It should be avoided, if possible, in patients with epilepsy, as it has a potential to cause seizures, and in children it may cause damage to developing weight-bearing joints. It can also cause pain and inflammation of tendons, especially in older people.
Drug interactions
Ciprofloxacin raises the blood levels of theophylline. The action of warfarin is increased.
Other quinolones
Ofloxacin is similar to ciprofloxacin. Levofloxacin is more active against Streptococcus pneumoniae and can be used in community-acquired pneumonia but offers no real advantage over the usual antibiotics.
Norfloxacin and nalidixic acid are effective in uncomplicated urinary tract infections and are used when the infecting organism is resistant to the older antibacterial drugs. For an uncomplicated infection a 3-day course is adequate, but prolonged treatment is required for severe or recurrent infections. They should be avoided in children and pregnancy, in cases of porphyria and renal impairment.
β-Lactam antibiotics
The β-lactam antibiotics can be divided into:
• the penicillins
• the cephalosporins
• others.
The β-lactam antibiotics all contain the β-lactam ring, which is a chemical structure essential for their antibacterial activity. The family of β-lactams is summarized in Figure 23.2.
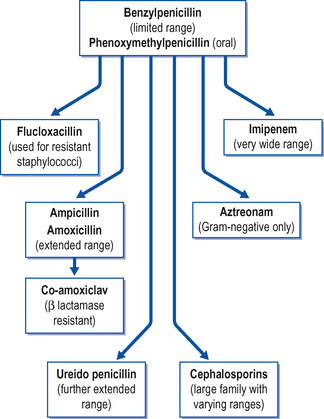 |
| Figure 23.2 The β-lactam family of antibiotics. |
The penicillins
The penicillins comprise:
• amoxicillin
• ampicillin
• azlocillin
• benzylpenicillin
• co-amoxiclav
• flucloxacillin
• phenoxymethylpenicillin
• piperacillin
• pivampicillin
• ticarcillin.
The penicillins were the first antibiotics to be isolated. Over the years their structure has been repeatedly modified to deal with the problem of resistance and to extend their antibacterial range. They are still probably the most widely used family of antibiotics.
Benzylpenicillin
Benzylpenicillin was the first penicillin to be used clinically. It is usually given by deep intramuscular injection, which is painful; if a large single dose is needed, it should be given intravenously. It enters the circulation rapidly and spreads through the body; it does not, however, cross into the CSF in any great quantity, although this may be increased if the meninges are inflamed.
Elimination of penicillins
All penicillins are excreted by the kidneys, partially through the glomeruli but the major part via the renal tubules. The excretion is rapid and blood levels fall nearly to zero 4 hours after injection. If benzylpenicillin is given orally, it is partially broken down by the gastric acid, and is not now given by this route.
Range of benzylpenicillin
Benzylpenicillin is effective against a fairly wide range of organisms. Table 23.3 lists the most common.
| *Note that a high proportion of staphylococci found in hospital are now resistant to benzylpenicillin. | |
| Organisms | Disease |
|---|---|
| Streptococcus pyogenes | Tonsillitis, scarlet fever, septicaemia |
| Streptococcus viridans | Subacute bacterial endocarditis |
| Staphylococcus aureus* | Carbuncles, osteomyelitis, septicaemia, boils |
| Streptococcus pneumoniae | Pneumonia |
| Neisseria gonorrhoeae | Gonorrhoea |
| Neisseria meningitidis | Meningococcal meningitis |
| Treponema pallidum | Syphilis |
| Clostridium perfringens | Gangrene |
| Clostridium tetani | Tetanus |
| Actinomyces | Actinomycosis |
Penicillins are bacteriostatic and in higher doses are bactericidal. When treating infection it is ideal to maintain the blood level of penicillin continually at bactericidal levels, and this requires injections every 4 hours. In milder infections, however, it is often adequate to give less frequent injections. Even after the blood levels of penicillin have dropped below bactericidal or bacteriostatic levels, the organism may take some time to recover and by that time the blood level of penicillin has risen again following a further injection.
Procaine benzylpenicillin
Numerous attempts have been made to prolong the action of benzylpenicillin after injection by slowing down its release from the injection site. A successful method is to combine benzylpenicillin with procaine. The combination is called procaine benzylpenicillin and will maintain a satisfactory blood level for at least 12 hours. This preparation is, however, rather slow to produce a satisfactory blood level, so that if a rapid effect is required, benzylpenicillin should be given as well.
The action of penicillin can be augmented and prolonged by slowing down its excretion. This can be done by giving probenecid, a drug that blocks the tubular secretion of penicillin, thus allowing the drug to accumulate in the body.
Penicillin resistance: penicillinase and the β-lactam ring
Certain organisms develop resistance to the action of penicillin. Organisms which were originally sensitive appear to adapt themselves to the penicillin by producing an enzyme called penicillinase, which inactivates penicillin by attacking part of the penicillin molecule known as the β– lactam ring. This structure is an essential part of penicillins and cephalosporins, and the family of enzymes involved is sometimes known as the β-lactamases. This is particularly so in the case of staphylococci, and strains of this organism which are resistant to penicillin and other antibiotics are a serious clinical problem. There is now a considerable degree of alarm because of the rapidly reducing number of effective antibiotics, and there is growing realization that we may be in danger of entering a period analogous to that before antibiotics were originally introduced.
Other penicillins
These comprise:
• phenoxymethylpenicillin
• flucloxacillin
• broad-spectrum penicillins
• extended-spectrum penicillins (ureido penicillins).
There are a number of penicillins that are similar to benzylpenicillin but are effective by mouth. Some of the examples given below are not destroyed by the acid in the stomach and are fairly well absorbed from the intestinal tract.
Phenoxymethylpenicillin
Adequate absorption with a satisfactory therapeutic response usually occurs with oral penicillin but it is important that it is taken 30 minutes before a meal. The patient must be carefully observed in case the drug is ineffective due to vomiting or inadequate absorption. Penicillin must then be given by injection.
Flucloxacillin
The elucidation of the structure of the penicillin nucleus has made it possible to produce penicillins which are not broken down by penicillinase and are therefore effective against organisms, particularly staphylococci, which have become resistant to benzylpenicillin. Flucloxacillin is a commonly used example. It is used almost exclusively for treating staphylococcal infections.
Strains of staphylococci have emerged which are resistant even to flucloxacillin. They are called meticillin-resistantStaphylococcus aureus(MRSA) and respond only to vancomycin or teicoplanin (see p. 314).
Broad-spectrum penicillins
These include ampicillin, amoxicillin and pivampicillin.
Ampicillin is effective against a number of bacteria, including salmonellae, E. coli, Shigella and H. influenzae, which are little affected by benzylpenicillin. It has proved particularly useful in chronic bronchitis, urinary infections and typhoid. It can be given orally or by injection.
Amoxicillin is very similar to ampicillin but is better absorbed, so a smaller dose is required. For this reason it is perhaps to be preferred to ampicillin.
Pivampicillin consists of ampicillin linked to another molecule that facilitates absorption. As it passes through the gut wall it is split and ampicillin is released. It therefore has no advantage over ampicillin except that it is better absorbed.
Co-amoxiclav: Some bacteria produce β-lactamases capable of breaking down both ampicillin and amoxicillin together with other antibiotics. Antibiotics can be combined with a substance called clavulanate, which prevents this breakdown and thus enables it to destroy β-lactamase-producing bacteria. The combined preparation of amoxicillin and clavulanate is called co-amoxiclav.
Extended-spectrum penicillins (ureido penicillins)
These include azlocillin, piperacillin and ticarcillin. This group of extended-spectrum penicillins (ureido penicillins) have much the same antibacterial spectrum as ampicillin but are also effective against Pseudomonas aeruginosa and Proteus morganii. They are, however, inactivated by some β-lactamases and are therefore not active against penicillin-resistant staphylococci and, in addition, are not very effective against other Gram-positive organisms (e.g. Streptococcus pneumoniae). They are not absorbed from the gut and must be given by injection. They are reserved for serious infections with pseudomonads or when the causative organism is not known. In this case, due to deficiencies in their antibacterial spectrum, they are usually combined with an aminoglycoside, but must not be mixed in the same infusion or syringe. They are very expensive.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access
