However, in recent years, the intensive care nurse has taken over from the renal nurse in caring for those requiring continuous renal replacement therapy (CRRT). Specific nursing activities for CRRT are therefore beyond the scope of this book, but further reading can be found at the end of this chapter regarding the options available for renal replacement therapy (RRT).
Mortality
There are a number of patient groups for which AKI has a particularly high mortality rate. For example, AKI occurs in up to 65% of patients with septic shock and is independently associated with an increased risk of death in patients with sepsis (Bagshaw et al. 2009). The need for rapid identification of the cause of AKI and those patients at highest risk is essential so that the correct course of treatment can be adopted (DuBose et al. 1997). Therefore it is vital for the nurse to play a major role in assisting physicians in the treatment options available for this fragile group of patients. The development of new biochemical markers may enable a diagnosis to be made before changes are seen in either serum creatinine or the urine output.
It is important for nurses and carers to be able to understand that AKI is a serious condition that should never be underestimated. Constant improvements in dialysis technology, combined with a growing chronic kidney disease population and limited funds, have put clinicians under pressure to try and predict the outcomes of treatment. Acute kidney injury is costly to the NHS. Marion Kerr, an economist for NHS Kidney Care, estimated the cost of AKI care to be between £434 million and £620 million per annum. This is greater than the costs of lung and skin cancer combined (Laing 2012), although Kolhe et al. (2008) have suggested that a perfect mortality prediction model is still missing.
The National Confidential Enquiry into Patient Outcome and Death (NCEPOD) is a government-funded agency whose remit is to maintain and where necessary improve standards of medical and surgical care through confidential surveys, which aim to maintain and improve the quality of patient care. The NCEPOD undertook a study (National Confidential Enquiry into Patient Outcome and Death 2009) that examined the management of AKI. Their findings demonstrated that only 50% of patients dying with AKI received good care. It was identified that the recognition of acute illness, hypovolaemia and sepsis in patients with AKI was generally poor. Following on from the publication of this NCEPOD enquiry many initiatives have been introduced aiming to improve care and reduce the mortality. Examples include the London Acute Kidney Network (LAKIN) care bundles available at www.londonaki.net/ (accessed 20 May 2013) and the Yorkshire AKI Networks Acute Kidney Injury Patient Pathway (AKIPP) available at www.aki.org.uk/(accessed 20 May 2013).
Classification
AKI may be divided into three major categories, in which each category has a physiological location of the insult (Table 5.1):
Table 5.1 Acute kidney injury: major causes and aetiology.
| Stage | Major causes | Aetiology |
| Prerenal | Cardiovascular | Congestive cardiac failure |
| Myocardial infarction | ||
| Cardiogenic shock | ||
| Cardiac tamponade | ||
| Pulmonary embolism | ||
| Vasodilation | Sepsis | |
| Anaphylaxis | ||
| Hypovolaemia | Haemorrhage, including blood loss due to surgery | |
| Burns | ||
| Gastrointestinal loss | ||
| Renal loss | ||
| Renal (intrinsic) | Glomerulonephritis | Poststreptococcal infection |
| Systemic lupus erythematosus | ||
| Haemolytic-uraemic syndrome | ||
| Wegener’s granulomatosis | ||
| Goodpasture’s syndrome | ||
| Vascular | Vasculitis | |
| Hypertension | ||
| Eclampsia of pregnancy | ||
| Renal artery stenosis | ||
| Renal vein thrombosis | ||
| Intratubular | ||
| Pigment | Myoglobin (see rhabdomyolysis) | |
| Proteins | Myeloma | |
| Crystals | Nephrotoxins | |
| Postrenal | Obstruction of lower urinary tract | Prostatic hypertrophy |
| Obstruction of upper urinary tract | Ureteric obstruction (clots, extrinsic compression, calculi) |
- prerenal – relates to the ineffective perfusion of the kidneys, which are structurally normal
- renal (intrinsic) – damage to the renal parenchyma, sometimes secondary to prerenal problems
- postrenal – disordered urinary drainage of both kidneys or of a single functioning kidney.
An acute impairment may also present in the patient with existing chronic kidney disease, which may lead to further structural damage; this presentation is often referred to as acute-on-chronic renal failure and evidence would indicate that this is an increasing group (Hsu et al. 2008).
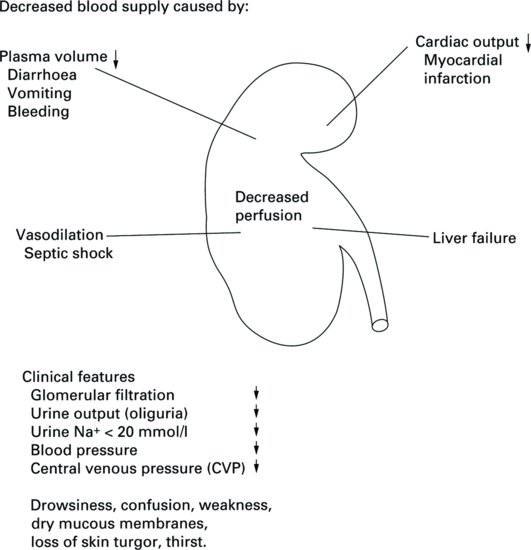
Prerenal renal failure
Prerenal causes of AKI are directly related to hypoperfusion states or a decline in the blood supply to the kidneys. The structure of the kidneys is normal. However, when the renal blood supply is restricted, glomerular filtration is reduced, causing decreased perfusion of the kidneys. The net effect is a decreased blood flow to the glomeruli, which therefore leads to ineffective filtration because of inadequate blood flow. Without an effective renal plasma flow rate the glomeruli are unable to filter waste from the blood but the structure of the renal tubules remains intact (Figure 5.2).
In this prerenal state, urine osmolarity is high and sodium low, which is consistent with renal hypoperfusion and well-preserved renal function. If, at this stage, renal blood flow can be restored, then normal renal function will return. However, if the prerenal state is prolonged, then this may lead to ischaemic damage due to poor perfusion, which in turn may lead to acute tubular necrosis (Devarajan 2006).
It is estimated that between 20 and 30% of cases of AKI are both predictable and avoidable (O’Donoghue and Matthews 2011). Early recognition, diagnosis and treatment are vital in prerenal failure, in order to prevent the condition progressing to renal failure with a degree of parenchymal damage. Nurses can play a large role in the recognition and management of this group of patients.
Renal (intrinsic) failure
This cause is sometimes referred to as intrinsic or intrarenal failure and is associated with structural damage to the glomeruli and renal tubules. The difference between pre- and postrenal failure and intrinsic failure is that in intrinsic failure the correction of the aetiology will not guarantee the complete recovery of renal function because of damage to the nephron itself. Here the episode of AKI may have a lengthy duration and can often lead to CKD.
The clinical course of intrinsic renal failure is often complex and, depending upon underlying disorders, the recovery may be prolonged for up to six weeks. As illustrated in Table 5.1, there are a wide variety of causes for intrinsic renal failure, which may involve multisystem disease or originate from a primary renal disorder, but often involve complicating severe illness that causes vasomotor nephropathy. Some specific causes are now discussed.
Acute interstitial nephritis
This condition often follows exposure to drugs in the form of antibiotics, analgesics and nonsteroidal antiinflammatory agents. Infections can cause a very similar clinical and pathological picture and these include Salmonella, Streptococcus, Meningococcus, leptospirosis and many viral disorders.
Other categories of interstitial nephritis are caused by systemic disease, such as systemic lupus erythematosus or sarcoidosis.
Clinical features
Fever, rash, arthralgia, back pain, and eosinophilia are clinical features of acute interstitial nephritis. Acute kidney injury may not develop for some weeks but in some cases renal dysfunction may occur within a few hours after exposure to a causative drug.
Rhabdomyolysis
Rhabdomyolysis is a result of the release of muscle contents, including myoglobin, into the plasma. It is often caused by trauma, for example a crush injury or pressure-induced muscle necrosis. This causes damage to muscle, which allows the pigment myoglobin to be released into the plasma. Myoglobin is an iron- and oxygen-binding protein and is only found in the bloodstream after muscle injury. At high plasma levels it becomes nephrotoxic. If treated early it can be successfully cured with fluid resuscitation (Russell 2005).
Clinical features
The urine is often brown or coffee coloured due to the presence of myoglobin. Patients often present with acute illness, with fever, weakness, pain, nausea and vomiting.
Renal failure and liver disease
AKI is often associated with acute liver injury that may result from:
- paracetamol overdose;
- circulatory shock;
- severe leptospirosis sepsis.
It may also be seen in surgery on the biliary tract.
For the patient with advanced liver disease, the onset of kidney injury is often referred to as hepato-renal syndrome. Septicaemia, fluid and electrolyte imbalance or hypovolaemia from gastrointestinal haemorrhage are common causes of the syndrome. These patients often require intensive care and the appropriate choice of therapy is of utmost importance (Rialp et al. 1996).
Cortical necrosis
Cortical necrosis may follow any course of intensive or prolonged ischaemia. The condition is also associated with sepsis, shock, transfusion reactions and burns.
Renal biopsy reveals pathology of patchy necrosis of the glomeruli, tubules and small vessels of the renal cortex. The renal medulla remains intact but the renal cortex becomes infarcted and calcifies, and this may be seen on plain abdominal X-ray.
The return of renal function is often slow but, if cortical necrosis is extensive, recovery is unlikely and the patient may become dialysis dependent.
Acute tubular necrosis
Acute kidney injury due to ischaemic changes or toxic renal injury presents a clinical syndrome that is often referred to as acute tubular necrosis (ATN). It is a very common cause of AKI and has a high mortality rate of around 50%. It causes damage to the tubular portion of the nephron. Unfortunately, despite 35 years of haemodialysis, little progress has been made in altering the outcome for ATN (Ricci et al. 2011).
Although the aetiology of ATN can vary, the common factor is that there is a reduction of oxygen and nutrients to the active tubular cells, which results in a lack of cell function and patchy necrosis. The tubular cells will regenerate at the basement membrane level. The aim is to keep the patient alive and well during this regeneration phase. An almost full recovery can be made provided the appropriate and timely treatment is undertaken (Pusnani and Hazra 1997).
Most intrinsic renal failure is caused by ischaemia through exposure to toxic agents, such as drugs or bacterial endotoxins.
Nephrotoxicity
In any patient with AKI, particularly ATN, a potential causative effect could be a therapeutic agent. Therapeutic agents can affect the kidney in any of the three categories listed, as illustrated in Table 5.2 (Perezella 2009). Contrast-induced acute kidney injury (CI-AKI) occurs within 72 hours of the patient receiving the contrast media. Acute kidney injury results from a combination of afferent arteriolar vasoconstriction and direct toxicity of the contrast media to the tubule epithelial cells. Patients with risk factors including the elderly and those with diabetes, have a higher incidence. It is recommended this group of patients receives appropriate fluid expansion before and after procedure (Lewington and Kanagasundaram 2011).
Table 5.2 Effects of therapeutic agents on the kidney.
| Clinical syndrome | Causative agents |
| Prerenal | Ciclosporin, radiocontrast, amphotericin B, ACE inhibitors, NSAIDs |
| Intrinsic: acute tubular necrosis | Aminoglycosides, amphotericin B, cephalosporins |
| Acute interstitial nephritis | Penicillins, cephalosporins, sulphonamides, rifampicin, NSAIDs, interferon, interleukin-2 |
| Postrenal | Aciclovir, analgesic abuse |
| Other heavy metals, including gold, lithium, mercury, silver | |
| ACE, angiotensin-converting enzyme; NSAIDs, non steroidal antiinflammatory drugs. | |
Ischaemic acute tubular necrosis is often associated with inadequate perfusion to the kidney, in that the efferent and afferent arterioles are unable to maintain their autoregulatory function and this leads to a fall in the glomerular filtration rate. This interruption in blood flow to the kidney may be due to surgical intervention, for example aortic repair, and is quite likely to cause ischaemia (Weldon and Monk 2000).
Postrenal failure
Postrenal conditions obstruct the flow of urine, so the obstruction has to be bilateral in order to cause failure. The rapidity of recovery will depend on the duration and completeness of the obstruction.
The urinary tract may be obstructed by three mechanisms:
- obstruction from within (e.g. ureteric stones);
- disease of the wall;
- obstruction from outside (e.g. prostatic hypertrophy).
As with all types of kidney injury, it is important to find the cause and start treatment as soon as possible since, in theory, all postrenal failure is reversible (Kellum 2008).
AKI classification systems
The Acute Dialysis Quality Initiative (ADQI), in 2004, proposed the introduction of a diagnostic criterion for AKI using serum creatinine levels and urine output (UO) volumes over standardised periods of time. The system was called RIFLE (Table 5.3) (Ricci 2007). The system provided a uniform standard for diagnosis and classification of AKI in order to optimise the treatment of patients with kidney disease. Many of the ADQI group collaborated at a later date to become the Acute Kidney Injury Network (AKIN), which modified the RIFLE with regards the creatinine measure, due to the clinical significance of relatively small rises in serum creatinine. As a result, the AKIN group modified RIFLE to the new AKI staging system, AKIN (Table 5.4), which reflects these smaller changes, with the AKIN corresponding to the earlier RIFLE stages. Stage 1 relates to risk, while AKIN stage 2 relates to injury and AKIN stage 3 corresponds to failure. The ‘loss’ and ‘end-stage kidney disease’ categories were dropped from the staging system but remain outcomes. The AKIN classification also introduced a baseline measure and dropped the use of GFR as this is not an accurate measure of kidney function in AKI.
Table 5.3 RIFLE Classification of AKI.
| Category | GFR criteria | Urine output (UO) |
| Risk | Increase in creatinine ×1.5 or GFR ≥ 25% | UO < 0.5 ml/kg/h × 6 h |
| Injury | Increase in creatinine ×2 or eGFR ≥ 50% | UO < 0.5 ml/kg/h × 12 h |
| Failure | Increase in creatinine ×3 or GFR ≥ 75% | UO < 0.3 ml/kg/h × 24 h or anuria × 12 h |
| Loss | Complete loss of kidney function ≥ 4 weeks | |
| ESKD | End stage kidney disease (≥ 3 months) |
Table 5.4 AKIN Classification of AKI.
| Category | Serum creatinine | Urine output |
| Stage 1 | Serum creatinine ≥ 150–200% from baseline | <0.5 ml/kg/h for > 6 h |
| Stage 2 | Serum creatinine ≥ 200–300% from baseline | <0.5 ml/kg/h for > 12 h |
| Stage 3 | Serum creatinine ≥ 300% from baseline OR serum creatinine ≥ 54 μmol/l with an acute rise of at least 44 μmol/l or commencement RRT | < 0.3 ml/kg/h for > 24 h or anuria for >12 h |
Lewington and Kanagasundaram (2011) recommend the use of the newest AKI staging system produced by International Kidney Disease: Improving Global Outcomes (KDIGO) staging classification (Table 5.5). All three systems provided guidance and the use of one of these classification systems should promote earlier detection of AKI, leading to appropriate and timely treatment of acute kidney injury.
Table 5.5 KDIGO classification of AKI.
| Category | Serum creatinine (SCr) | Urine output |
| Stage 1 | ≥ 1.5 to 1.9 × baseline or increase in SCr ≥0.3 mg/dl (≥26.5 μmol/l) | <0.5 ml/kg/h for 6-12 h |
| Stage 2 | ≥ 2–2.9 × baseline | <0.5 ml/kg/h >12 h |
| Stage 3 | 3 × baseline or increase in SCr ≥ 4 mg/dl (≥ 353.6 μmol/l) or initiation of RRT | < 0.3 ml/kg/h for ≥ 24 h or anuria for ≥ 12 h |
Management of Acute Kidney Injury
Since normal kidney function is essential to homeostasis of the body, particularly with regard to volume, electrolyte balance, acid-base balance and excretion of nitrogenous waste products, loss of these functions can lead to hyperkalaemia, volume overload, acidosis and uraemia.
Prevention is better than a cure, so early detection and treatment of AKI will prevent rapid deterioration. Some people are at a greater risk of AKI – these include patients who have chronic kidney disease, cardiac failure and liver disease, diabetes and those who are over 60 years of age. The clinical management goals for patients with AKI can be divided into three main categories:
- restoration of renal perfusion;
- minimising toxic effects;
- correction of metabolic derangements.
Hyperkalaemia
Hyperkalaemia is often a fatal complication in AKI. The failing kidney is unable to excrete potassium effectively when the patient is oliguric (<400 ml urine day) or, worse, anuric (no urine). It is further complicated by the very complex treatment of an individual who is often septic, hypoxic and requiring blood transfusions and potassium-containing drugs.
Renal replacement therapy is the most efficient treatment for hyperkalaemia but this may take time if vascular access is required. Other alternatives are available:
- The administration of intravenous insulin and dextrose or nebulised salbutamol will help move potassium ions back into the intracellular compartment and away from the extracellular compartment. It is important to monitor the patient’s blood sugar as hypo-hyperglycaemia can occur. Also it is important to measure the heart rate, which can increase in response to salbutamol.
- Calcium carbonate is recommended in most cases to reduce the cardiotoxicity and decrease the cardiac membrane excitability
- Oral or rectal potassium exchange agents in the form of calcium resonium (which is a slower acting treatment, often used as maintenance treatment for hyperkalaemia).
Volume overload
Successful volume homeostasis permits maintenance of a constant internal circulatory and extracellular volume despite consumption of varying quantities of water and salt intake and variable invisible losses of water.
The presence of oedema may be seen in the feet, legs and sacral area. This is often pitting in nature. The skin is particularly at risk at this stage and extra care must be taken. Shortness of breath and especially orthopnoea are indicative of pulmonary oedema.
Each patient in AKI should have an individual prescription for fluid and sodium intake. As a generalisation, the fluid intake volume should equal the daily urine output plus 300–500 ml. Patients with a large insensible loss, such as happens with burns, obviously need a larger fluid intake and special care should be taken. It is important that the patient and family are involved in accurate fluid balance.
Metabolic acidosis
The presence of AKI must not lead the nurse to think that it is the only cause of acidosis until other causes have been eliminated, for example ketoacidosis, lactic acidosis.
Acidosis in kidney injury occurs when the renal tubules fail to regenerate bicarbonate and secrete hydrogen ions into the urine, which in turn causes an acid-base imbalance.
As most acid comes from the breakdown of dietary protein, it is possible to reduce the level of acidosis by limiting the level of intake of protein. Another alternative is to infuse sodium bicarbonate but one has to be aware of fluid overload and hypernatraemia. The most efficient way of treating acute acidosis via RRT.
Uraemia
The accumulation of nitrogenous waste products will produce acute uraemia and symptoms of uraemia often include nausea, vomiting, hiccups, increasing bleeding, infection risks, neurological problems, irritability, confusion and twitching. As previously mentioned, it is necessary to begin appropriate dialysis.
Nutrition
Patients with acute kidney injury are often very ill and their metabolisms are often under great stress, resulting in the need for extra calories and extra protein (Kariyawasam 2012). Protein calorie malnutrition is believed to one of the leading factors in the high mortality rate seen in AKI (Murphy and Byrne 2010). Acute kidney injury causes major stress-induced hormonal and metabolic derangements within the body which result in negative nitrogen balance and depletion of body energy reserves (Casaer et al. 2008). Nitrogen balance is exceptionally negative in AKI and is often linked to sepsis, surgery and multiorgan dysfunction syndrome and renal factors such as uraemia, acidosis, inadequate protein intake and parathyroid hormone (Murphy and Byrne 2010). Nutritional intake is therefore of particular importance.
Patients require an individualised nutritional assessment and feeding plan, which will require specialist input from a dietitian, taking into consideration their complex metabolic state, fluid balance and current RRT. In the acutely ill patient, enteral nutrition, if tolerated, is considered to be the best treatment option by most experts (Cano et al. 2006) as it is cheaper, safer and more physiologically normal. The development of concentrated low-electrolyte feeds has proven invaluable in allowing delivery of optimal protein and calories with the minimum of fluid and electrolytes. However, these special feeds are not normally needed if the patient is receiving continuous renal replacement therapy.
The aims of nutritional support are to:
- prevent protein energy wasting;
- preserve lean body mass/prevent or minimise malnutrition;
- avoid further metabolic arrangements;
- stimulate immunocompetence;
- repair tissue damage;
- preserve organ function;
- maintain biochemistry/fluid balance;
- enhance recovery.
Kidney Disease Improving Global Outcomes (KDIGO) (2012) suggest the goal of any feeding regime should be to provide an energy intake of 20–30 kcal/kg/d. They advise against protein reduction with the aim of preventing or delaying initiation of RRT, recommending the administration of 0.8–1.0 g/kg/d of protein in people who are noncatabolic with AKI, who do not require dialysis. They recommend 1.0–1.5 g/kg/d for people with AKI on RRT, and up to a maximum of 1.7 g/kg/d in patients on continuous renal replacement therapy (CRRT). In patients who are hypercatabolic they recommend a maximum of 1.7 g/kg/day of amino acids if on CRRT (Lewington and Kanagasundaram 2011). Hypokalaemia and hypophosphataemia might be observed once a patient has commenced CRRT and supplementation of these electrolytes needs to be undertaken as required.
Patients with AKI are frequently anuric and hence monitoring of fluid balance and reduction of fluid intake is often necessary. In patients not requiring RRT it is likely that they will require dietary reductions in potassium, magnesium, and phosphate. The development of continuous renal replacement therapy has allowed fluid and electrolytes to be more easily managed without the need for the reduced fluid allowances that previously meant having to limit nutritional support. See Chapter 13.
Infection
Infection is known to contribute to the high mortality of patients with AKI (Hall and Esser 2008). Many patients with AKI are immuno-compromised due to uraemia (Perkins and Kisel 2005). They are therefore at an increased risk of developing infections such as pneumonia, urinary tract infections and sepsis, due to the large numbers of invasive devices they may need in situ. To reduce some of these risks it is a priority to remove all unnecessary lines and universal precautions and guidelines for maintaining asepsis should be adhered to at all times.
The Clinical Course of Acute Kidney Injury
The clinical course of AKI can be divided into four stages or phases:
- initiating stage;
- oliguric stage;
- diuretic stage;
- recovery stage.
Types of urine output can be found in Table 5.6.
Table 5.6 Types of urine output
| Anuria | No urine output |
| Oliguria | < 400 ml/day |
| Nonoliguria | > 400 ml/day |
| Polyuria | Normal or high urine output |
Initiating stage
This occurs when the kidneys are injured and when diagnosis is made and treatment established. It can last anything from hours to days.
Oliguric stage
This can last from 5 days to over 15 days. When AKI persists for weeks, endocrine problems, such as reduced erythropoietin production, are noticed. Functional renal changes occur, such as decreased tubular transport, reduced urine formation and lowered glomerular filtration. Renal healing will begin to occur, with the basement membrane being replaced with fibrous scar tissue and the nephron clogged with inflammatory products. The patient is particularly susceptible to bleeding and infection during this stage.
Diuretic stage
With continued healing the kidney begins to regain most of its lost function, but this depends on the severity of the initial injury. The signs and symptoms of the original condition begin to disappear. Urine output can begin to increase back to normal levels of up to 3 L day.
Recovery stage
The recovery stage can last from several months to over a year. The basement membrane is restored to its previous structure; scar tissue will remain but is not clinically significant. The kidneys respond in a regulatory excretory function to the body’s needs.
Futher reading can be found in Lameire et al. (2008).
Renal Replacement Therapy (RRT ) in Acute Kidney Injury
The purpose of RRT is to prevent morbidity and to support the kidney during its recovery phase. The amount, type and frequency of RRT are dictated by the severity of the patient’s condition (Dauguirdas 2000).
Indications for RRT are:
- uraemic symptoms, such as pericarditis;
- volume overload;
- hyperkalaemia;
- metabolic acidosis;
- ‘space-making’ – for example, nutrition, transfusions;
- AKIN / KDIGO stage 3 or RIFLE failure.
There are a variety of treatment options available for AKI and the choice will depend on physician preference, nurse expertise and availability of the appropriate equipment. The options fall broadly into two categories: intermittent treatments (acute haemodialysis and haemodiafiltration) and continuous renal replacement therapies, of which there are three basic types: continuous haemofiltration, and continuous haemodialysis and continuous haemodiafiltration. Treatments performed continuously over long periods of time allow optimal values to be obtained for urea and fluid exchange control, and electrolyte and acid-base balance.
Acute intermittent haemodialysis
In acute haemodialysis, certain factors need to be considered and these include time on dialysis (possibly only 2 h for the first treatment), frequency (daily dialysis may be required), potassium concentration of dialysate (3 mmol/l dialysate may be necessary), a biocompatible dialyser and good fluid balance control. For those patients who are too haemodynamically unstable for conventional haemodialysis, other forms of renal replacement therapy (RRT) may be appropriate.
Intermittent haemodiafiltration
Intermittent haemodialfiltration can be a therapy option for patients with AKI or established renal failure. It combines the advantages of diffusion (dialysis) with convection (haemofiltration), it is suitable for use in patients with cardiovascular instability, especially those with fluid overload. It has superior small solute clearence over haemofiltration. Blood flow rates are higher than those of continuous HDF and a requires an HDF membrane that is more permeable than a normal haemofilter. However due to the efficiency of small molecule clearence, this would not be a treatment of choice for the patient with severe uraemia at risk of disequilibrium.
Continuous renal replacement therapy
The use of CRRT has developed enormously during recent years and involves either dialysis (solute removal using diffusion) or filtration (solute and water removal using convection) or a combination of treatments. The advantage of continuous therapy is the slower rate of fluid or solute removal, thus making it better tolerated by critically ill patients.
The nurses in the intensive care unit have developed their skills, taking on this responsibility to ensure holistic care. It is recognised that intermittent haemodialysis may be contraindicated in patients with AKI who are critically ill. Complications such as cardiovascular instability, sepsis and multiorgan failure make conventional intermittent treatments difficult.
Continuous haemofiltration (HF)
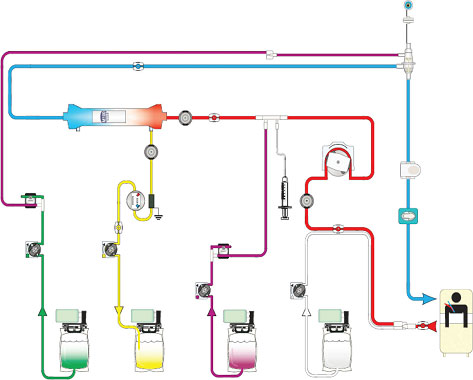
Continuous haemofiltration provides solute removal by convection. It offers high-volume ultrafiltration using replacement fluid, which can be administered in a variety of concentrations according to the patient’s biochemistry. The pump guarantees adequate blood flow to maintain required ultrafiltration rates. This method can be performed using several litres of replacement fluid each hour. This is dependent on the patient prescription, which is usually determined by their weight. The addition of a blood pump to the circuit removes the necessity for arterial access and provides a more reliable and controllable method of delivering treatment (Figure 5.3).
Figure 5.3 Continuous venovenous haemofiltration.
Source:With kind permission from Gambro Lundia AB.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree