The term tumor lysis syndrome (TLS) is used to describe the clinical consequences of serum accumulation of excessive cell lysis products. When cells die, the internal components are released into the serum circulation; these include potassium, phosphates, and nucleic acids. Normally the kidneys efficiently clear these byproducts so that no serum elevation occurs. However, when cell lysis exceeds the kidneys’ capacity or when the patient has pre-existing renal dysfunction, the cell byproducts cause clinical electrolyte disorders, with or without renal dysfunction (Davidson et al., 2004). According to Fernandez and colleagues (2006), clinical TLS is defined by specific laboratory evidence plus one or more of the following criteria: a serum creatinine greater than 1.5 times the upper limit of normal, cardiac dysrhythmia or sudden death, and seizure. These criteria are outlined in the Cairo-Bishop grading system (Table 48-1).
| Grade | Laboratory Evidence | Creatinine Level | Cardiac Dysrhythmias | Seizures |
|---|---|---|---|---|
| 0 | − | < 1.5× the upper limit of normal | None | None |
| 1 | + | 1.5 × the upper limit of normal | Intervention not indicated | None |
| 2 | + | > 1.5-3 × the upper limit of normal | Nonurgent medical intervention indicated. | One brief generalized seizure (or seizures) well controlled, or infrequent focal motor seizures that do not interfere with ADLs. |
| 3 | + | > 3-6 × the upper limit of normal | Symptomatic and incompletely controlled medically or controlled with a device. | Seizure in which consciousness is altered; poorly controlled seizure disorder; breakthrough generalized seizures despite medical intervention. |
| 4 | + | > 6 × the upper limit of normal | Life-threatening | Seizures of any kind that are prolonged, repetitive, or difficult to control. |
| 5 | + | Death | Death | Death |
Tumor lysis syndrome can be seen as early as 6 hours after initiation of antineoplastic therapy, although the peak incidence occurs within the first 24 to 48 hours (Secola, 2006; Davidson et al., 2004). In unusual cases, signs and symptoms may persist for 5 to 7days. The onset and duration of symptoms vary, depending on the tumor burden and its responsiveness to therapy. Rapidly responding tumors create a larger number of lysis products in a shorter time, which increases the risk of clinical effects.
EPIDEMIOLOGY AND ETIOLOGY
The patients most likely to develop TLS are those who have rapidly proliferating tumors, those who are highly sensitive and responsive to the antineoplastic therapy, and those with renal compromise. Hematologic malignancies are the most common reported risk factors, although TLS has been reported in a variety of other malignancies as well. The incidence perceptually decreased as a result of greater recognition of risk factors and implementation of preventive strategies. However, some believe that more effective antineoplastic therapies actually may have caused a higher incidence of TLS, although in a milder form (Reedy, 2006). Survival also has improved, increasing from 5% to 8% in the 1980s to the current 20% to 30%. This may be related to early pheresis management of high white blood cell counts in acute leukemia and newer renal replacement therapies (Cairo, 2006; Mato et al., 2006).
RISK PROFILE
• Individuals with rapidly proliferating white blood cells, such as the blasts (immature cells) associated with acute leukemia or Burkitt’s lymphoma, are at risk (Secola, 2006). In some of these individuals, high cell turnover exists even before treatment, which means that a degree of tumor lysis is seen at diagnosis. This usually worsens after definitive treatment causes more rapid lysis (Fernandez et al., 2006).
• Other tumors with high growth fractions (e.g., small cell lung cancer, testicular cancer, medulloblastoma, neuroblastoma) may also cause tumor lysis as a result of the rapid cell turnover rate (Rampello et al., 2006; Reedy, 2006).
• A large tumor mass, as indicated by high serum LDH levels, represents a greater risk of the development of TLS. LDH levels greater than 1500 mg/dL represent a greater risk for TLS (Cairo, 2006; Jeha, 2006; Rampello et al., 2006).
• Tumors that are highly responsive to antineoplastic therapy may also cause TLS. Tumor types that have been reported in the literature to induce TLS include breast carcinoma, Hodgkin’s and Non-Hodgkin’s lymphoma, medulloblastoma, melanoma, ovarian carcinoma, renal cell carcinoma, rhabdomyosarcoma, sarcoma, seminoma, small cell lung cancer, and thymoma (Cairo, 2006; Rampello et al., 2006; Yahata et al., 2006).
• Several monoclonal antibodies that target cell surface markers on tumor cells have been reported to cause acute tumor lysis. These include rituximab, campath, and bortezomib (Kenealy et al., 2006).
• Hypovolemia and dehydration lead to higher concentrations of cell lysis products, which can exacerbate the tendency for tumor lysis.
8. Steroid-sensitive tumors have been reported to produce tumor lysis even after hormonal replacement or premedication-dose dexamethasone (Chanimov et al., 2006).
PROGNOSIS
Although TLS has been associated with poor long-term outcomes, this often is due to its association with severe and advanced malignant disease rather than inadequate treatment of the complication. Only 30% of patients with TLS have severe symptoms such as dysrhythmias or renal failure, and only 5% to 8% of all patients with TLS die of the syndrome (Reedy, 2006). TLS complications reportedly are more severe in elder adults and children (Reedy, 2006).
The Cairo-Bishop grading system, mentioned previously, defines the severity of TLS based on the key clinical findings (see Table 48-1). Laboratory findings, renal dysfunction, cardiac dysrhythmias, and neuromuscular abnormalities are used to determine the severity score (Cairo & Bishop, 2004).
PROFESSIONAL ASSESSMENT CRITERIA (PAC)
A complete physical examination and evaluation of common diagnostic findings before antineoplastic therapy is started help differentiate the clinical findings associated with TLS. Initial and ongoing assessment of the following clinical criteria are important (Table 48-2).
1. Intake and output measurement. Large quantities of fluid are administered to enhance renal clearance of lysis products; intake greater than output is a high risk.
2. Urine output is an extremely important specific aspect of the intake and output totals. Elevated uric acid and calcium-phosphate precipitants may both cause oliguria, therefore a reduction in urine output could be a significant sign of renal impairment.
3. The pulse rate and regularity are important indicators of dysrhythmias, which can occur as a result of hypocalcemia and hypokalemia.
• If pulse irregularities occur, continuous cardiac monitoring is recommended to provide consistent feedback about the presence of dangerous rhythm disturbances, such as ventricular tachycardia or heart block, which can occur with TLS.
4. Blood pressure assessment helps verify complications such as hypervolemia (hypertension) or dysrhythmias (hypotension).
5. Edema may reflect fluid volume overload.
6. Respiratory assessment for rate and quality can reflect the workload of breathing. Oxygen saturation monitoring can reveal hypoxemia. Tachypnea and dyspnea provide information about compensation for pH imbalance or fluid overload.
7. When possible, the central venous pressure is monitored to assess for fluid volume overload and to adjust the rate of fluid administration. A normal central venous pressure is 0 to 6 mm Hg or 5 to 10 cm H2O. Patients with TLS usually require central venous pressures that are slightly higher to ensure continuous urine flow.
8. Muscle tone may vary, depending on the predominant electrolyte manifestation. Muscle cramping and tetany can occur as a result of hypocalcemia, and weakness or hypotonia is present with hyperkalemia.
9. The abdomen should be assessed for large tumor masses, hepatomegaly, or splenomegaly; these are indicative of a large tumor burden, which increases the risk for TLS (Secola, 2006).
10. Gastrointestinal distress, represented by anorexia, nausea, vomiting, and diarrhea, is a common symptom of TLS. These signs are due to the multitude of metabolic imbalances rather than one specific abnormality.
11. The serum chemistry should be monitored once to three times daily, depending on the degree of risk for TLS. Monitoring is more intense during the peak periods of occurrence (between 24 and 48 hours). Common electrolyte abnormalities include hyperkalemia, hyperphosphatemia, and hypocalcemia. More severe hyperphosphatemia is common in patients with therapy-induced TLS (Fernandez et al., 2006).
12. High phosphate levels are common with TLS. Close monitoring for a decline in phosphate levels, which indicates the end of TLS, allows for discontinuation of phosphate-binding therapies before a rebound hypophosphatemia is induced by overtreatment. The severity of hyperphosphatemia predicts the degree of hypocalcemia because of the inverse relationship of calcium and phosphate. High phosphate levels cause bone resorption or urinary excretion of calcium.
13. A method of calculating the risk of calcium-phosphate salt precipitants is calculation of the calcium-phosphorous solubility product. The formula is:
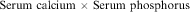
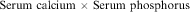
If the product is greater than 60, the risk of precipitation is high (Secola, 2006).
14. Uric acid is monitored daily. It reflects the end product of nucleic acid conversion to xanthine oxidase after release from destroyed tumor cells, and it increases the risk of renal failure. Uric acid levels that remain less than 9.8 mg/dL connote a better prognosis (Cairo, 2006). Higher uric acid levels occur in spontaneous TLS that develops before initiation of antineoplastic therapy (Fernandez et al., 2006). Specific therapies directed at preventing hyperuricemia or reducing uric acid levels are administered and should be discontinued when the TLS has resolved.
15. Serum blood urea nitrogen and creatinine levels are monitored at least daily during the high-risk period for tumor lysis. Renal failure is an infrequent but possible complication, and these laboratory values are the most accurate indicators of early renal insufficiency.
16. Arterial blood gases may be evaluated to assess pH balance and the degree of compensation by the respiratory system. Severe metabolic acidosis may occur with high uric acid levels, renal insufficiency, and fluid imbalances. If metabolic acidosis is severe, the respiratory workload to attempt to clear the acid may produce hypoxemia. To avoid frequent arterial blood punctures, the venous pH may be used for intermittent monitoring to validate the stability of the pH level.
17. LDH levels are indicative of the amount of tumor, and these levels are monitored daily during the period of high risk for TLS to assess tumor reduction (Secola, 2006).
18. In the past, the urine pH level has been used to titrate bicarbonate infusions. However, with decreased use of preventive bicarbonate infusions, this laboratory monitoring practice is not often performed.
19. If renal insufficiency occurs, a renal ultrasound examination is performed to rule out an obstructive process. Although unusual, uric acid crystallization and calcium-phosphate precipitation may cause kidney stones. The treatment for obstruction caused by stones is significantly different from that used for acute renal failure caused by tubular necrosis.
| Abnormal Laboratory Finding | Clinical Manifestations | Management |
|---|---|---|
| Potassium >5.5 mEq/L or >25% increase from baseline | • Increased bowel sounds • Abdominal cramping and diarrhea • Nausea • Muscle weakness, flaccidity • Fatigue • Paresthesias • Progressive ECG changes: First stage—peaked T waves, shortened PR interval, ST depression Second phase—widened QRS, prolonged PR interval, decreased amplitude of P wave; Third phase—flattened QRS into ventricular fibrillationz • Palpitations • Tachycardia (atrial, supraventricular, or ventricular) • Increased premature beats (atrial, junctional, or ventricular) | • Kayexylate given orally or by enema to bind with K+ ions and remove them via loose stool. • Sodium bicarbonate (1 mEq/kg) to cause potassium ions to move into the cells. • Calcium gluconate 1 amp (4.5 mEq) given intravenously (this is not the preferred choice if symptoms are mild or the only ECG change is peaked T waves). • Dextrose 50% and regular insulin 10 units given intravenously. • Force sodium-based fluids to enhance electrolyte excretion. • Loop diuretics (e.g. furosemide). • Dialysis. |
| Phosphate >8 mg/dL or 25% increase from baseline | • Renal dysfunction • Muscle weakness • Joint pain • Limited joint movement • Pruritus • Red eye or conjunctivitis • Mental status changes ranging from mild confusion to obtundation or seizures • Cataracts • Paresthesias • Prolonged QT segment on ECG • Leukopenia • Thrombocytopenia • Hypocalcemia | • Force sodium-based fluids to enhance electrolyte excretion. • Phosphate binding agents (e.g., aluminum hydroxide). • Dialysis. • Bone marrow growth factors. |
| Uric acid >8 mg/dL or increase from baseline | • Renal dysfunction, as evidenced by elevated creatinine • Oliguria, anuria • Hematuria • Enlarged, tender kidney with possible tubular obstruction | • Allopurinol • Rasburicase (Elitek) • Force fluids • Diuretics • Dialysis |
| Calcium <8 mg/dL or 25% decrease from baseline | • Muscle contraction/tetany • Painful twitching/fasciculation of small muscles • Increased deep tendon reflexes • Abdominal cramping, diarrhea, increased bowel sounds • Irritable heart rhythms (tachycardia, atrial fibrillation/flutter, premature beats) • Nausea | • Calcium replacement only after phosphorus has been reduced, • Phosphorus reduction. |
| Arterial pH <7.25 | • Bradycardia, junctional rhythm, heart block • Hypotension • Cyanosis • Smooth muscle contraction (abdominal, uterine cramping)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree

Get Clinical Tree app for offline access

|