Hyperuricemia, an excessive amount of uric acid in the blood, is a potentially life-threatening metabolic complication that results from overproduction or inefficient elimination of uric acid or from a combination of these processes. Nonmalignant exogenous causes of an increased serum uric acid level may be related to a dietary intake of foods high in nucleic acid (e.g., liver, kidney, anchovies, and “sweetbreads” consisting of thymus and pancreas tissue) (Wartmann, 2004). Overproduction of uric acid may arise from endogenous sources of purine production, such as excessive degradation of skeletal muscle ATP after strenuous physical exercise or status epilepticus. Accelerated ATP breakdown may also explain hyperuricemia related to myocardial infarction, smoke inhalation, and acute respiratory failure. Inefficient elimination of uric acid may result from decreased glomerular filtration, decreased tubular secretion, or enhanced tubular reabsorption. More than 90% of individuals with sustained hyperuricemia have a defect in renal handling of uric acid. Prolonged hyperuricemia predisposes these individuals to gouty arthritis, urolithiasis, and renal dysfunction (Wartmann, 2004; Cairo & Bishop, 2004).
Hyperuricemia may occur in patients with rapidly proliferating malignancies, such as Burkitt’s lymphoma or T cell acute lymphoblastic leukemia (ALL), and in patients with a malignancy involving a high tumor burden (Ribeiro & Pui, 2003; Schiffer, 2001). Hyperuricemia may be present when the cancer is diagnosed, or it may occur spontaneously before initiation of therapy, but it usually is present within 12 to 72 hours after initiation of therapy. Historically, it was associated with a high morbidity rate and delays in the delivery of chemotherapy (Rheingold & Lange, 2005; Pui et al., 1997). Early treatment and preventive measures now can prevent this potentially fatal complication.
Hyperuricemia occurs as a result of the release of purine nucleic acids, which are metabolized into hypoxanthine by the liver. The release of these acids may occur spontaneously as the result of ongoing cell death in a rapidly growing tumor, or as the result of cell death induced by chemotherapy. Hypoxanthine is converted into uric acid, a reaction that is catalyzed by xanthine oxidase. Uric acid crystals, in the presence of an acidic pH, precipitate in the renal tubules and obstruct urine flow in the tubules, causing acute obstructive uropathy, which may lead to renal failure (Del Toro et al., 2005; Fojo, 2005; Haut, 2005). Hyperuricemia may occur as an isolated metabolic complication or as one feature of a collection of metabolic derangements recognized as tumor lysis syndrome (see Chapter 48) (Shin et al., 2006).
EPIDEMIOLOGY AND ETIOLOGY
Hyperuricemia is present in approximately 5% of the general population and in up to 25% of hospitalized patients. Many of these individuals are completely asymptomatic with no clinical risk. However, if hyperuricemia is identified, the underlying cause should be determined and corrected (Wartmann, 2004).
The presence of hyperuricemia in the patient with cancer varies, depending on the type of malignancy. It is the direct result of the release of nucleic acids from destroyed malignant cells into the circulating blood system. Hyperuricemia has been reported in up to 50% of patients with acute myeloid leukemia (AML), and it may be associated with tumor lysis, although the full syndrome of tumor lysis is more frequently identified in acute lymphoblastic leukemia (ALL) (Greer et al., 2004). Hyperuricemia is a rare complication of cancer therapy for solid tumors, although reported cases likely underestimate the true incidence (Baeksgaard & Sorensen, 2003). A retrospective chart review of European cancer patients from 17 countries revealed that 13.6% of the reviewed cases had hyperuricemia alone, and an additional 5.3% had full tumor lysis syndrome (Annemans et al., 2003b).
If hyperuricemia is not prevented or goes untreated, the incidence of acute renal failure associated with the precipitation of uric acid crystals in the renal tubules may be as high as 30% in patients with Burkett’s lymphoma, and renal sequelae may be irreversible (Cohen et al., 1980).
RISK PROFILE
• Patients whose neoplastic cells have a very active purine metabolism, have high nucleic acid and phosphorus content, and are highly sensitive to chemotherapy (Ribeiro & Pui, 2003; Brant, 2002).
• Features at presentation include leukocytosis, massive hepatomegaly and splenomegaly, large abdominal mass, extrinsic obstructive uropathy, and decreased urinary flow (Ribeiro & Pui, 2003).
• Pre-existing renal compromise resulting in impaired ability to clear tumor byproducts (Brant, 2002).
• Dehydration
• Infection
• Medications that may increase uric acid, such as thiazide diuretics, probenecid, salicylates, corticosteroids, furosemide, gentamycin, methicillin, niacin, rifampin, propranolol, and phenothiazines (Brant, 2002), or medications that may impair renal function, such as nonsteroidal antiinflammatory agents (NSAIDs) (Cantril & Haylock, 2004).
• Male gender (Tsimberidou & Keating, 2005)
• Age under 25 years (Tsimberidou & Keating, 2005)
PROGNOSIS
Prevention and management of hyperuricemia usually are successful in lowering patient morbidity (Brant, 2002). The formation of uric acid crystals in the renal tubules and subsequent renal dysfunction can occur with uric acid levels near 8.3 mg/dL. If significant renal dysfunction develops before or during other electrolyte abnormalities accompanying tumor lysis syndrome, preventing those abnormalities from becoming life-threatening is much more difficult (Hutcherson et al., 2006). Approximately 15% of patients with acute lymphoid malignancies, mainly those with a high tumor burden at diagnosis, develop renal failure requiring dialysis, which may compromise the optimal delivery of chemotherapy (Ribeiro & Pui, 2003). Approximately 19% of patients with leukemia or lymphoma receiving induction therapy and 12.9% of patients receiving salvage therapies developed hyperuricemia (Annemans et al., 2003a).
PROFESSIONAL ASSESSMENT CRITERIA (PAC)
1. History of present illness: Time of onset of symptoms in relation to malignancy, abdominal pain or fullness, back pain, vomiting, diarrhea, lethargy, dehydration, and anorexia.
2. Past medical history and lifestyle: Dietary intake focusing on foods high in nucleic acids, medications taken for chronic conditions that may impair renal function (Rheingold & Lange, 2006; Wartmann, 2005; Cantril & Haylock, 2004). Assess patient for history of asthma, allergies, anaphylactic reaction or history of hypersensitivity reaction with urate oxidase, and history of glucose-6-phosphate dehydrogenase (G6PD) deficiency.
3. Laboratory studies: Complete blood count (CBC), sodium, potassium, chloride, calcium, phosphorus, carbon dioxide, blood urea nitrogen (BUN), creatinine, uric acid, and urinalysis.
4. Physical examination: If the exam yields an abdominal or pelvic mass, perform ultrasound and/or CT of the abdomen/pelvis to rule out obstructive renal failure. Renal failure from obstructive nephropathy can have the same signs and symptoms as that caused by hyperuricemia (Rheingold & Lange, 2006). If the examination yields signs and symptoms of infection, complete the appropriate diagnostic evaluation (cultures and/or diagnostic imaging as indicated).
5. Monitor the serum uric acid, serum creatinine, and BUN every 4 hours in the initial stage of therapy (Cope, 2004; Murphy-Ende & Chernecky, 2002).
6. Observe for signs of renal failure (e.g., oliguria, nausea, vomiting, lethargy, edema, heart failure, or seizure).
7. Observe for rapid weight gain (more than 2 pounds in 24 hours).
8. If using allopurinol, assess uric acid levels, because the maintenance dose is based on these levels. Observe precautions in patients with impaired renal or hepatic function, heart failure, diabetes, or hypertension.
9. If using rasburicase (urate oxidase enzyme): Assess serum G6PD before starting. Patients at high risk for G6PD deficiency include those of African, Mediterranean, or Asian ancestry (Wang et al., 2006; Cheson & Dutcher, 2005). Assess for hypersensitivity to rasburicase. Onset usually is abrupt and is characterized by bronchospasm, dyspnea, hypoxemia, hypotension, and cutaneous rash as urticaria (Lascombes et al., 1998; Pui et al., 1997). Assess for hemolytic anemia and methemoglobinemia. One of the byproducts of the breakdown of uric acid to allantoin is hydrogen peroxide, which can induce hemolytic anemia or methemoglobinemia in patients with G6PD deficiency. G6PD results in intravascular hemolysis after erythrocytes are subjected to oxidative stress (Pui et al., 2001). Three main symptoms of methemoglobinemia are illness out of proportion to the history of illness, cyanosis that does not resolve with administration of oxygen, and blood that appears darker than usual (Nelson & Hostetler, 2003).
NURSING CARE AND TREATMENT
1. The primary aims of treatment are to prevent hyperuricemia and ensure a high urine flow, because this reduces the likelihood of uric acid precipitation in the renal tissue (Baeksgaard & Sorensen, 2003).
2. The standard prophylaxis for and treatment of malignancy-associated hyperuricemia in the United States has been administration of allopurinol with vigorous hydration, urinary alkalinization, and osmotic diuresis (Pui et al., 1997). Allopurinol inhibits the enzyme xanthine oxidase, preventing the conversion of hypoxanthine and xanthine to uric acid and thereby reducing the renal load of uric acid. (Fig. 28.1). It may take several days for the uric acid level to normalize.
• If allopurinol is given PO, it may be given with meals. Instruct the patient to drink 10 to 12 8-ounce glasses of water a day.
• If the dosage of allopurinol is greater than 300 mg/day, it should be given in divided doses. The oral dosage is 100 mg/m2/dose every 8 hours (10 mg/kg/day in 3 divided doses) to a maximum of 800 mg/day.
• For IV administration: Reconstitute a 500 mg dose to a concentration of 20 mg/mL. Infuse over 30 to 60 minutes. The IV dosage is 200-400 mg/m2/day in 1-3 divided doses to a maximum of 600 mg/day. The maintenance dose is based on the serum uric acid levels.
• Hydration maintains both blood volume and blood pressure, which ensures renal perfusion even with loss of autoregulation (Ronco et al., 2004).
• Vigorous diuresis may also prevent crystallization of xanthine and other purine metabolites, the excretion of which is increased with the use of allopurinol.
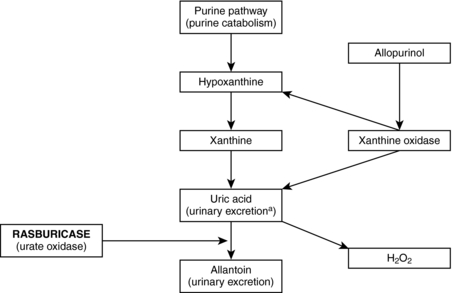 |
| Fig. 28.1Allopurinol blocks xanthine oxidase, inhibiting the formation of uric acid. Rasburicase (urate oxidase) metabolizes uric acid to allantoin, a protein five to 10 times more soluble than uric acid that is easily excreted. In the conversion of uric acid to allantoin, hydrogen peroxide is produced. This fact is important, because patients who are deficient in glucose-6-phosphate dehydrogenase should not receive rasburicase.(From Cheson, B. D., & Dutcher, B. S. [2005]. Managing malignancy-associated hyperuricemia with rasburicase. Journal of Supportive Oncology, 3:117-124.) |
3. Rasburicase is a recombinant urate oxidase enzyme that rapidly catalyzes the enzymatic oxidation of uric acid into allantoin and hydrogen peroxide (see Fig. 28.1) (Hutcherson et al., 2006). Rasburicase should never be administered as a bolus. It should be infused over 30 minutes, without a filter, in a dedicated intravenous line. If it is not possible to use a line different from that through which other medications are being given, flush the line with 15 mL of normal saline before and after the infusion of rasburicase. The recommended dosage is 0.15-0.2 mg/kg daily for 5 consecutive days (Cheson & Dutcher, 2005). Recent studies have shown that less aggressive treatment is successful (see Evidence-Based Practice Updates). Monitor carefully for signs and symptoms of anaphylaxis. Make sure that emergency drugs (epinephrine, diphenhydramine, and steroids), oxygen, and emergency equipment are available before rasburicase is administered (Brant, 2002).
4. Ensure the accuracy of uric acid determinations by collecting the blood samples in prechilled tubes and sending them to the laboratory in an ice bath. Rasburicase causes enzymatic degradation of uric acid in blood samples if they are left at room temperature, resulting in falsely low values (Fojo, 2005; Brant, 2002).
5. Weigh the patient daily, or every 12 hours if fluid retention becomes apparent.
6. Maintain strict intake and output measurement, with accurate documentation every 4 hours (Ribeiro & Pui, 2003).
7. Provide aggressive IV hydration before and during chemotherapy administration; frequent monitoring of electrolytes and uric acid; allopurinol and/or rasburicase or other agents for hyperuricemia; urinary alkalinization with the addition of sodium bicarbonate to IV fluids (Haut, 2005).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access
