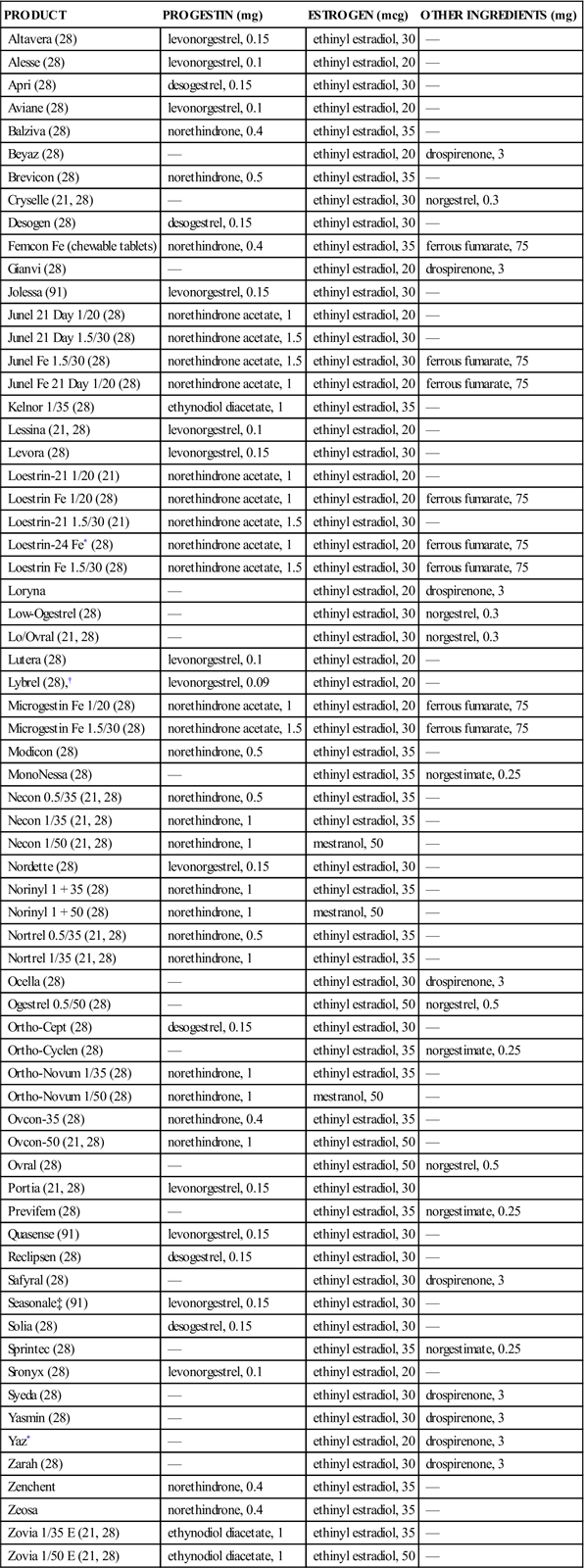
3 Differentiate between the actions and the benefits of the combination pill and the minipill. 4 Describe the major adverse effects and contraindications to the use of oral contraceptive agents. 6 Describe pharmacologic treatments of benign prostatic hyperplasia. 7 Describe the pharmacologic treatment of erectile dysfunction. leukorrhea ( sexually transmitted infections ( dysmenorrhea ( Secretions from the vagina usually represent a normal physiologic process, but if the discharge becomes excessive, it is known as leukorrhea, an abnormal, usually whitish, vaginal discharge that may occur at any age. It affects almost all females at some time in their lives. Leukorrhea is not a disease but a symptom of an underlying disorder. The most common cause is an infection of the lower reproductive tract, but other physiologic and noninfectious causes of vaginal discharge are well known (Box 41-1). The most common organisms causing the infectious type of leukorrhea are Candida albicans, Trichomonas vaginalis, and Gardnerella vaginalis (Box 41-2). Occasionally, C. albicans infections of the mouth, gastrointestinal tract, or vagina may develop as secondary infections during the use of broad-spectrum antibiotics, such as penicillins, tetracyclines, and cephalosporins. Pathogens that are commonly transmitted by sexual contact are called sexually transmitted infections (STIs). In some diseases, such as gonorrhea, syphilis, chlamydia, and genital herpes simplex virus, and in human papillomavirus (HPV) infection, sexual transmission is the primary mode of transmission. The medications used to treat these infections are found in Table 41-1. In other diseases, such as giardiasis, shigellosis, and the hepatitis viruses, other important nonsexual means of transmission also exist. Unfortunately, the true incidence of STIs is not known in the United States because of large numbers of unreported cases. Table 41-1 Causative Organisms and Products Used to Treat Genital Infections Data from Centers for Disease Control and Prevention (CDC), Workowski KA, Berman SM: Sexually transmitted diseases treatment guidelines, MMWR Morb Mortal Wkly Rep 55(RR-11):1-94, 2006; Centers for Disease Control and Prevention (CDC): Update to CDC’s sexually transmitted diseases treatment guidelines, 2010: fluoroquinolones no longer recommended for treatment of gonococcal infections, MMWR Morb Mortal Wkly Rep 56:332-336, 2007. See Table 41-1. The following assessment questions apply to all age groups. • Usual pattern of menses: Duration, number of pads used, last menstrual period • Pain, discomfort, spotting between periods, or extended time of menstrual flow • Number of pregnancies, live births, miscarriages, or abortions • Postmenopausal women: Has there been any vaginal bleeding? • History and frequency of Papanicolaou (Pap) smears • Reproductive problems (e.g., endometriosis, ovarian cysts, and uterine fibroids) • Presence of a urethral discharge or genital or perianal lesions. Is there any swelling of the penis? • Is there pain in the lower back, perineum, or pelvis? • History of prostatitis, benign prostatic hyperplasia, or prostatic cancer • History of erectile dysfunction and description of pattern of altered erectile functioning • History of arthralgia, fever, chills, malaise, pharyngitis, or oral lesions Ask the patient to describe the current problem or problems that initiated this visit. How long have the symptoms existed? Is there a recurrence of symptoms that were treated previously? STIs cause a high degree of anxiety. The intimate nature of the questioning required to obtain a sexual history may be embarrassing. Vaginal or urethral discharge may also be alarming to the patient seeking health care. When an STI diagnosis is suspected, explain the confidentiality policy of the facility before asking about sexual partners. (Many individuals do not return for followup appointments; there may be only one chance to obtain relevant information about contacts.) Ask about lifestyle orientation (e.g., heterosexual, bisexual, or homosexual and number of partners). Has there been known contact with people with STIs? Are precautions used during sexual contacts? Assess the level of anxiety present and adaptive responses and coping mechanisms used. • Record basic patient data (e.g., height, weight, vital signs). • Prepare the patient for, and assist with, a physical examination. • Assist with specimen collection (e.g., vaginal smears, cultures of discharge). • Inspect the penis and scrotum for swelling or abnormalities; observe for urethral discharge. • Provide psychological support and refer for available counseling, as appropriate. The rate of STIs is high in this age group, so it is important to do a thorough assessment of sexual activity and practices. For those who are sexually active, counseling regarding safe sex practices and voluntary testing and treatment should be offered. Medical care for STIs can be provided without parental consent or knowledge. Check individual state laws for those that allow testing and counseling for HIV. All adolescents should be taught thoroughly about alternatives for abstinence and about safe sexual practices. For Women. Teach the patient the proper way to apply medications topically or intravaginally using ointments, troches, or suppositories. It is imperative that proper cleansing of the genital area be done regularly using soap and water; rinse and dry well. Hands should be washed before and after the application or insertion of medications and before and after toileting. After every use, the vaginal applicator should be thoroughly washed with soap and water and then dried. After inserting a vaginal medication (cream or suppository), the woman should remain in a recumbent position for 30 minutes to allow time for drug absorption. A minipad can be worn to catch remaining drainage. (See Chapter 8, Figure 8-13 for proper administration of vaginal medication.) With oral contraceptive therapy, teach not only the medication schedule and dosage but also what to do if a dose is missed, frequency of follow-up care, and common and serious adverse effects. For Men and Women. Teach the medication regimen and who must take the medications—both partners in a sexual relationship. Enlist the patient’s aid in developing and maintaining a written record of monitoring parameters (e.g., blood pressure, pulse, weight, degree of relief from menstrual pain, menstrual cycle information for women on oral contraceptives). (See Self-Monitoring Drug Therapy Record on the Evolve Oral (hormonal) contraceptives (birth control pills) became available in 1960. They now represent one of the most common forms of artificial birth control in the United States. It is estimated that approximately one third of all women between 18 and 44 years of age use oral contraceptives. Estrogens and progestins, to some extent, induce contraception by inhibiting ovulation. The estrogens block pituitary release of follicle-stimulating hormone (FSH), preventing the ovaries from developing a follicle from which the ovum is released. Progestins inhibit pituitary release of luteinizing hormone (LH), the hormone responsible for releasing an ovum from a follicle. Other mechanisms play a contributory role in preventing conception. Estrogens and progestins alter cervical mucus by making it thick and viscous, inhibiting sperm migration. Hormones also change the endometrial wall, impairing implantation of the fertilized ovum. The progestin-only pills, or minipills, represent a common form of oral contraceptive therapy. Many adverse effects of combination-type contraceptives are caused by the estrogen component of the tablet. For those women particularly susceptible to adverse effects of estrogen therapy, the minipill provides an alternative. Women who might prefer the minipill are those with a history of migraine headaches, hypertension, mental depression, weight gain, and breast tenderness and those who want to breastfeed postpartum. The minipill is not without its disadvantages, however. Between 30% and 40% of women on the minipill continue to ovulate. Dysmenorrhea, manifested by irregular periods, infrequent periods, and spotting between periods, is common in women taking the minipill. Birth control is maintained by progestin activity on cervical mucus, uterine and fallopian transport, and implantation. There is a slightly higher incidence of both uterine and tubal pregnancies. There are two types of oral contraceptives: the combination pill, which contains both an estrogen and a progestin, and the minipill, which contains only a progestin. The combination pills are subdivided into fixed combination or monophasic (Table 41-2), biphasic (Table 41-3), triphasic and quadriphasic (Table 41-4) products. The monophasic combination pills contain a fixed ratio of estrogen and progestin given daily for 21 days, beginning on day 5 of the menstrual cycle. The biphasic product contains a fixed dose of estrogen and a progestin dose on days 1 to 10 that is lower than that on days 11 to 21 of the menstrual cycle. The triphasic combination pills provide three concentrations of estrogen and progestin, and the quadriphasic pills provide four concentrations of hormones. The purpose of the variable concentrations is to provide contraception with the lowest necessary dose of hormones. Most of the combination pills are also packaged in 28-tablet containers. The last 7 tablets are inert but are supplied so that there is no break in the routine of taking 1 tablet daily. The progestin-only products (Box 41-3, p. 651) are packaged in units of 28 tablets. All tablets contain active hormone; 1 tablet should be taken daily at approximately the same time each day. Monophasic Oral Contraceptives *Take one active tablet daily for 24 days, followed by four inert tablets. †Take one active tablet daily for 28 days, followed by a new cycle of active tablets. These products contain no inert tablets.
Drugs Used in Men’s and Women’s Health
Objectives
Key Terms
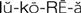 ) (p. 642)
) (p. 642)
 ) (p. 642)
) (p. 642)
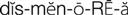 ) (p. 647)
) (p. 647)
Vaginitis
![]() http://evolve.elsevier.com/Clayton
http://evolve.elsevier.com/Clayton
CAUSATIVE ORGANISM
GENERIC NAME
BRAND NAME
DRUG MONOGRAPH, NURSING IMPLICATIONS
Vulvovaginitis
Candida albicans (fungus)
butoconazole vaginal cream
Gynazole l; Mycelex-3
(p. 760)
clotrimazole vaginal cream, vaginal tablets
Gyne-Lotrimin, Mycelex-7
(p. 760)
fluconazole oral tablets
Diflucan
(p. 760)
itraconazole oral capsules
Sporanox
(p. 766)
miconazole vaginal cream, suppositories
Monistat 3, 7
(p. 760)
terconazole vaginal cream, suppositories
Terazol 7, Terazol 3
(p. 760)
tioconazole vaginal ointment
Vagistat
(p. 760)
Trichomonas vaginalis (protozoa)
metronidazole oral tablets
Flagyl
(p. 757)
tinidazole oral tablets
Tindamax
(p. 758)
Bacterial vaginosis (formerly known as Gardnerella vaginalis [bacteria])
metronidazole oral tablets, vaginal gel
Flagyl; MetroGel-Vaginal
(p. 757)
tinidazole oral tablets
Tindamax
(p. 758)
clindamycin vaginal cream
Cleocin
(p. 754)
Gonorrhea
Neisseria gonorrhea (bacteria)
ceftriaxone
Rocephin
(p. 736)
cefotaxime
Claforan
(p. 736)
ceftizoxime
Cefizox
(p. 736)
azithromycin
Zithromax
(p. 740)
doxycycline
Vibramycin
(p. 750)
Syphilis
Treponema pallidum (spirochete)
penicillin G, benzathine
Bicillin L-A
(p. 743)
tetracycline
Tetracycline
(p. 750)
doxycycline
Vibramycin
(p. 750)
azithromycin (resistance reported)
Zithromax
(p. 740)
Genital Herpes
Herpes simplex genitalis (virus)
acyclovir oral capsules
Zovirax
(p. 770)
famciclovir oral tablets
Famvir
(p. 777)
valacyclovir oral tablets
Valtrex
(p. 786)
Chlamydiae
Chlamydia trachomatis (chlamydia)
azithromycin
Zithromax
(p. 740)
doxycycline
Vibramycin
(p. 750)
erythromycin
Erythromycin
(p. 740)
levofloxacin
Levaquin
(p. 746)
ofloxacin
Floxin
(p. 746)
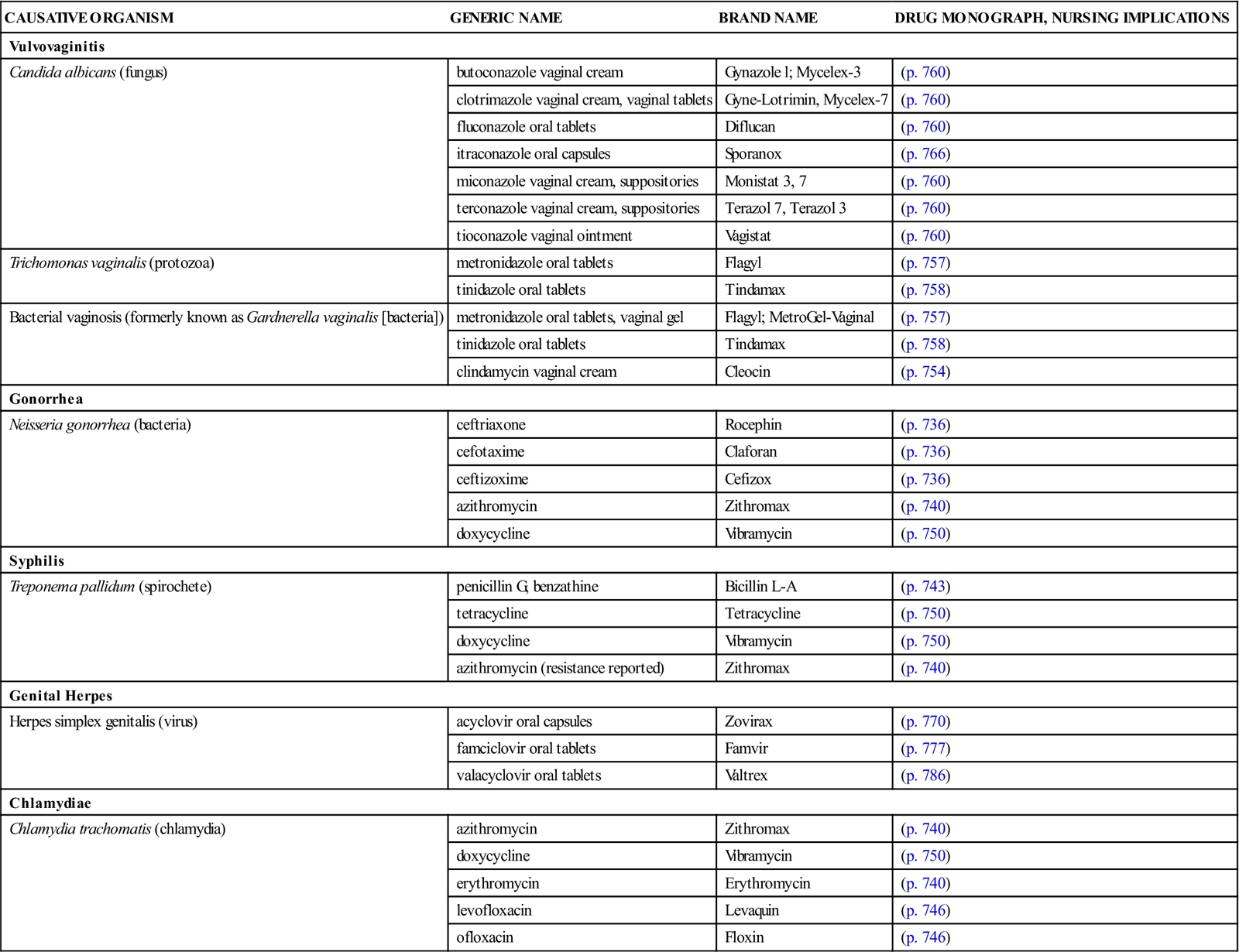
Drug Therapy for Leukorrhea and Genital Infections
![]() Nursing Implications for Men’s and Women’s Health
Nursing Implications for Men’s and Women’s Health
Assessment
Female Reproductive History.
Male Reproductive History.
History of Current Symptoms.
Medication History
Psychosocial.
Laboratory and Diagnostic Studies
Physical Examination
Implementation
![]() Patient Teaching and Health Promotion
Patient Teaching and Health Promotion
Instructions for Adolescents.
Instructions for Women
Instructions for Men
Instructions for Women and Men
Medications
Fostering Health Maintenance
Written Record.
![]() Web site at http://evolve.elsevier.com/Clayton.) For patients with STIs, a listing of the symptoms present and degree of relief obtained may be appropriate. Complete the Premedication Data column for use as a baseline to track response to drug therapy. Ensure that the patient understands how to use the form, and instruct the patient to bring the completed form to follow-up visits. During follow-up visits, focus on issues that will foster adherence with the therapeutic interventions prescribed.
Web site at http://evolve.elsevier.com/Clayton.) For patients with STIs, a listing of the symptoms present and degree of relief obtained may be appropriate. Complete the Premedication Data column for use as a baseline to track response to drug therapy. Ensure that the patient understands how to use the form, and instruct the patient to bring the completed form to follow-up visits. During follow-up visits, focus on issues that will foster adherence with the therapeutic interventions prescribed.
Drug Therapy for Contraception
Drug Class: Oral Contraceptives
Actions
Uses
![]() Table 41-2
Table 41-2
PRODUCT
PROGESTIN (mg)
ESTROGEN (mcg)
OTHER INGREDIENTS (mg)
Altavera (28)
levonorgestrel, 0.15
ethinyl estradiol, 30
—
Alesse (28)
levonorgestrel, 0.1
ethinyl estradiol, 20
—
Apri (28)
desogestrel, 0.15
ethinyl estradiol, 30
—
Aviane (28)
levonorgestrel, 0.1
ethinyl estradiol, 20
—
Balziva (28)
norethindrone, 0.4
ethinyl estradiol, 35
—
Beyaz (28)
—
ethinyl estradiol, 20
drospirenone, 3
Brevicon (28)
norethindrone, 0.5
ethinyl estradiol, 35
—
Cryselle (21, 28)
—
ethinyl estradiol, 30
norgestrel, 0.3
Desogen (28)
desogestrel, 0.15
ethinyl estradiol, 30
—
Femcon Fe (chewable tablets)
norethindrone, 0.4
ethinyl estradiol, 35
ferrous fumarate, 75
Gianvi (28)
—
ethinyl estradiol, 20
drospirenone, 3
Jolessa (91)
levonorgestrel, 0.15
ethinyl estradiol, 30
—
Junel 21 Day 1/20 (28)
norethindrone acetate, 1
ethinyl estradiol, 20
—
Junel 21 Day 1.5/30 (28)
norethindrone acetate, 1.5
ethinyl estradiol, 30
—
Junel Fe 1.5/30 (28)
norethindrone acetate, 1.5
ethinyl estradiol, 30
ferrous fumarate, 75
Junel Fe 21 Day 1/20 (28)
norethindrone acetate, 1
ethinyl estradiol, 20
ferrous fumarate, 75
Kelnor 1/35 (28)
ethynodiol diacetate, 1
ethinyl estradiol, 35
—
Lessina (21, 28)
levonorgestrel, 0.1
ethinyl estradiol, 20
—
Levora (28)
levonorgestrel, 0.15
ethinyl estradiol, 30
—
Loestrin-21 1/20 (21)
norethindrone acetate, 1
ethinyl estradiol, 20
—
Loestrin Fe 1/20 (28)
norethindrone acetate, 1
ethinyl estradiol, 20
ferrous fumarate, 75
Loestrin-21 1.5/30 (21)
norethindrone acetate, 1.5
ethinyl estradiol, 30
—
Loestrin-24 Fe* (28)
norethindrone acetate, 1
ethinyl estradiol, 20
ferrous fumarate, 75
Loestrin Fe 1.5/30 (28)
norethindrone acetate, 1.5
ethinyl estradiol, 30
ferrous fumarate, 75
Loryna
—
ethinyl estradiol, 20
drospirenone, 3
Low-Ogestrel (28)
—
ethinyl estradiol, 30
norgestrel, 0.3
Lo/Ovral (21, 28)
—
ethinyl estradiol, 30
norgestrel, 0.3
Lutera (28)
levonorgestrel, 0.1
ethinyl estradiol, 20
—
Lybrel (28),†
levonorgestrel, 0.09
ethinyl estradiol, 20
—
Microgestin Fe 1/20 (28)
norethindrone acetate, 1
ethinyl estradiol, 20
ferrous fumarate, 75
Microgestin Fe 1.5/30 (28)
norethindrone acetate, 1.5
ethinyl estradiol, 30
ferrous fumarate, 75
Modicon (28)
norethindrone, 0.5
ethinyl estradiol, 35
—
MonoNessa (28)
—
ethinyl estradiol, 35
norgestimate, 0.25
Necon 0.5/35 (21, 28)
norethindrone, 0.5
ethinyl estradiol, 35
—
Necon 1/35 (21, 28)
norethindrone, 1
ethinyl estradiol, 35
—
Necon 1/50 (21, 28)
norethindrone, 1
mestranol, 50
—
Nordette (28)
levonorgestrel, 0.15
ethinyl estradiol, 30
—
Norinyl 1 + 35 (28)
norethindrone, 1
ethinyl estradiol, 35
—
Norinyl 1 + 50 (28)
norethindrone, 1
mestranol, 50
—
Nortrel 0.5/35 (21, 28)
norethindrone, 0.5
ethinyl estradiol, 35
—
Nortrel 1/35 (21, 28)
norethindrone, 1
ethinyl estradiol, 35
—
Ocella (28)
—
ethinyl estradiol, 30
drospirenone, 3
Ogestrel 0.5/50 (28)
—
ethinyl estradiol, 50
norgestrel, 0.5
Ortho-Cept (28)
desogestrel, 0.15
ethinyl estradiol, 30
—
Ortho-Cyclen (28)
—
ethinyl estradiol, 35
norgestimate, 0.25
Ortho-Novum 1/35 (28)
norethindrone, 1
ethinyl estradiol, 35
—
Ortho-Novum 1/50 (28)
norethindrone, 1
mestranol, 50
—
Ovcon-35 (28)
norethindrone, 0.4
ethinyl estradiol, 35
—
Ovcon-50 (21, 28)
norethindrone, 1
ethinyl estradiol, 50
—
Ovral (28)
—
ethinyl estradiol, 50
norgestrel, 0.5
Portia (21, 28)
levonorgestrel, 0.15
ethinyl estradiol, 30
Previfem (28)
—
ethinyl estradiol, 35
norgestimate, 0.25
Quasense (91)
levonorgestrel, 0.15
ethinyl estradiol, 30
—
Reclipsen (28)
desogestrel, 0.15
ethinyl estradiol, 30
—
Safyral (28)
—
ethinyl estradiol, 30
drospirenone, 3
Seasonale‡ (91)
levonorgestrel, 0.15
ethinyl estradiol, 30
—
Solia (28)
desogestrel, 0.15
ethinyl estradiol, 30
—
Sprintec (28)
—
ethinyl estradiol, 35
norgestimate, 0.25
Sronyx (28)
levonorgestrel, 0.1
ethinyl estradiol, 20
—
Syeda (28)
—
ethinyl estradiol, 30
drospirenone, 3
Yasmin (28)
—
ethinyl estradiol, 30
drospirenone, 3
Yaz*
—
ethinyl estradiol, 20
drospirenone, 3
Zarah (28)
—
ethinyl estradiol, 30
drospirenone, 3
Zenchent
norethindrone, 0.4
ethinyl estradiol, 35
—
Zeosa
norethindrone, 0.4
ethinyl estradiol, 35
—
Zovia 1/35 E (21, 28)
ethynodiol diacetate, 1
ethinyl estradiol, 35
—
Zovia 1/50 E (21, 28)
ethynodiol diacetate, 1
ethinyl estradiol, 50
—
![]() Table 41-3
Table 41-3
PRODUCT
PROGESTIN (mg)
ESTROGEN (mcg)
OTHER INGREDIENTS (mg)
Amethia
—
Azurette
—
Camrese
—
Kariva* (28)
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

41. Drugs Used in Men’s and Women’s Health
Only gold members can continue reading. Log In or Register to continue
Get Clinical Tree app for offline access