Care of Patients with Diabetes and Hypoglycemia
Objectives
1. Compare and contrast the two major types of diabetes mellitus.
2. Analyze the four kinds of factors that influence the development of diabetes mellitus.
4. Summarize the acute and long-term complications and results of poorly controlled diabetes mellitus.
5. Identify sources of support and information for people with diabetes and their families.
1. Teach a newly diagnosed person with diabetes about the disease, treatment, and self-care.
3. Interpret laboratory tests used in the diagnosis and management of diabetes mellitus.
5. Teach a patient how to recognize and self-treat hypoglycemia.
Key Terms
basal insulin (p. 863)
bolus dose (p. 863)
correction dose (p. 863)
diabetic nephropathy (p. 860)
diabetic neuropathy (p. 876)
endogenous (ĕn-DŎJ-ĕn-ŭs, p. 857)
exogenous (ĕks-ŎJ-ĕn-ŭs, p. 857)
gastroparesis (găs-trō-pă-RĒ-sĭs, p. 876)
glucometer (p. 867)
glycemic control (glī-SĒ-mĭk, p. 859)
glycosuria (glī-cōs-Ū-rē-ă, p. 859)
hyperglycemia (hī-pĕr-glī-SĒ-mē-ă, p. 859)
incretin mimetics (p. 866)
insulin resistance (p. 857)
insulin-to-carbohydrate ratios (p. 860)
ketoacidosis (kē-tō-ă-sĭ-DŌ-sĭs, p. 857)
medical nutrition therapy (MNT) (p. 860)
metabolic syndrome (p. 875)
neuroglycopenia (nū-rō-GLĪ-kŏ-PĒ-nē-ă, p. 876)
polydipsia (pŏl-ē-DĬP-sē-ă, p. 859)
polyphagia (pŏl-ē-FĀ-jă, p. 859)
polyuria (pŏl-ē-Ū-rē-ă, p. 859)
 http://evolve.elsevier.com/deWit
http://evolve.elsevier.com/deWit
Diabetes mellitus and hypoglycemia
Diabetes Mellitus
Diabetes mellitus is a disturbance in metabolism and use of glucose that is secondary to a malfunction of the beta cells of the pancreas. Beta cells are responsible for making insulin. Because insulin is involved in the metabolism of carbohydrates, proteins, and fats, diabetes mellitus is not limited to a disturbance of glucose homeostasis; however, intolerance to glucose is an underlying issue, regardless of the type of diabetes.
Diabetes mellitus, the most common form of diabetes, is a deficiency of the pancreatic hormone insulin, which results in the body’s failure to metabolize sugars and starch. Sugars accumulate in the blood and urine, and the by-products of alternative fat metabolism disturb the acid-base balance of the blood, causing a risk of convulsions and coma.
Types of Diabetes Mellitus
Nearly 23.6 million Americans (approximately 8% of the population) have diabetes mellitus, and millions more have diabetes and do not know it. The cost of treating diabetes in the United States is approximately $132 billion a year (Luger & Chabanuk, 2009).
Table 38-1 summarizes the major characteristics of various forms of diabetes mellitus. Type 1 diabetes—formerly known as insulin-dependent diabetes mellitus (IDDM)—accounts for about 5% to 10% of all cases. Type 1 diabetes occurs when the body’s immune system destroys beta cells. There is no known way to prevent type 1 diabetes. Persons who have type 1 diabetes require injections of exogenous (from outside the body) insulin to maintain life, because they produce little or no endogenous (inside the body) insulin on their own. In general, persons with type 1 diabetes are more prone to a serious complication, ketosis, associated with an excess production of ketone bodies, leading to ketoacidosis (metabolic acidosis). Moreover, type 1 diabetes is more likely to appear early in life. In fact, type 1 diabetes was formerly called juvenile diabetes or ketosis-prone diabetes because of its typical early onset and potential for ketoacidosis.
Table 38-1
Clinical Categories of Diabetes Mellitus and Characteristics
| Type (Former Names) | Characteristics |
| Type 1 (insulin dependent; IDDM; juvenile diabetes; juvenile-onset) | Little or no endogenous insulin produced. New patients can be any age but usually are young. Patient must receive exogenous insulin and follow prescribed diet and exercise program. Renal, cardiovascular, retinal, and neurologic complications likely if disease is not kept under tight control. |
| Type 2 (non–insulin-dependent; NIDDM; adult-onset diabetes; maturity-onset diabetes) | Rarely develop ketosis; may develop hyperglycemic, hyperosmolar nonketotic syndrome (HHNS). Patients vary in need for exogenous insulin. New patients are usually over 30 and most are obese. Disorder often responds to diet and exercise. |
| Latent autoimmune diabetes (LADA) (slow onset type 1 diabetes or type 1.5 diabetes; type 1 diabetes, according to the World Health Organization) | Usually not overweight, have no signs of metabolic syndrome, and may have a history of autoimmune disease. Demonstrate rapid failure of oral hypoglycemic drugs. Insulin should be started within 1 year of diagnosis. |
| Prediabetes (impaired glucose tolerance and impaired fasting glucose) | Glucose levels higher than normal but lower than those with diabetes. Are at high risk for atherosclerotic disease and cardiovascular problems. Progression to diabetes is not inevitable. Weight loss and increased physical activity can delay or prevent diabetes and return blood glucose levels to normal. |
| Gestational diabetes | Occurs only during pregnancy. After pregnancy, women with gestational diabetes have 20%-50% chance of developing diabetes within 5-10 years. |
| Statistical risk of diabetes | Those who have had impaired glucose tolerance in the past but have normal glucose tolerance now; prediabetes; latent diabetes; subclinical diabetes. Those who are predisposed to diabetes because of family history, age, race, or obesity. |
Type 2 diabetes—formerly called non–insulin-dependent diabetes mellitus (NIDDM)—makes up 90% to 95% of all known cases of diabetes. Type 2 diabetes is believed to begin with insulin resistance, which is a situation whereby insulin interaction with glucose becomes less efficient, and therefore fat metabolism is abnormal. As the need for insulin rises, the pancreas gradually loses the ability to produce it. Type 2 diabetes has a tendency to develop later in life than does type 1, and patients with type 2 rarely develop diabetic ketoacidosis. Box 38-1 lists the signs and symptoms of type 1 and type 2 diabetes. Factors associated with development of type 2 diabetes are listed in Box 38-2.
Latent autoimmune diabetes (LADA) is a type 1 diabetes, according to the World Health Organization. Other names for this condition are “slow onset type 1 diabetes” or “type 1.5 diabetes.” It is believed that the presence of islet cell antibodies in the blood (which are not present in healthy individuals) will eventually destroy the beta cells, and insulin production will cease. Patients with LADA are usually not overweight, have no signs of metabolic syndrome, and may have a history of personal or familial autoimmune disease. The diagnosis is based on three criteria: (1) onset after age 30, (2) islet cell antibodies circulating in the blood, and (3) insulin is not required sooner than 6 months after diagnosis. Patients can be misdiagnosed as type 2 diabetes, and rapid failure of oral hypoglycemic drugs suggests LADA. Evidenced-based management suggests that metformin can be used in the early phase and insulin should be started within 1 year of diagnosis; both may offer some protective effects against the destruction of the beta cells, whereas sulfonylureas may hasten destruction of beta cells and therefore are not recommended (Kapustin,  2008).
2008).
Gestational diabetes may occur as a result of the stress of pregnancy. It may be treated with diet, oral hypoglycemia agents, or insulin. After delivery, the condition must be reevaluated; approximately 5% to 10% of women with gestational diabetes go on to be diagnosed with type 2 diabetes after delivery. The baby also carries an increased risk of type 2 diabetes later in life.
Etiology and Pathophysiology
At least four sets of factors influence the development of diabetes mellitus: genetic, metabolic, microbiological, and immunologic. In a recent study funded by the National Institutes of Health (2010) scientists used computer analysis to evaluate 226 environmental factors affecting diabetes. They found that a pesticide derivative (PCB) was strongly associated with developing diabetes, and that the nutrient beta-carotene served a protective role.
Genetic factors are included in the etiology of diabetes because diabetes tends to run in families. It is known that the risk of having some form of diabetes increases in proportion to the number of relatives who are affected, the genetic closeness of the relatives, and the severity of their disease.
Metabolic factors involved in the etiology of diabetes are many and complex. Emotional or physical stress can unmask an inherited predisposition to the disease, probably as a result of glucogenesis induced by increased production of hormones from the adrenal cortex (especially the glucocorticoids). Perhaps even more significant than metabolic factors is the association of type 2 diabetes and obesity. About 80% of type 2 diabetes patients are obese (greater than 20% above their ideal body weight), and there is a higher incidence of type 2 diabetes in persons who lead a sedentary life and eat a high-calorie diet. With weight reduction and increased physical activity, blood glucose can be restored to normal levels and maintained there—hence the importance of diet and exercise in the management of type 2 diabetes. In type 2 diabetes there also seems to be a relationship to aging and a reduction in the function of the pancreatic beta cells and how they synthesize insulin.
Some forms of type 1 diabetes may be related to the viral destruction of beta cells. There are known cases in which children developed type 1 diabetes after having had a recent viral infection. The mumps or coxsackie virus is thought to be the trigger. Evidence that supports viruses as causative factors include:
Signs, Symptoms, and Diagnosis
The American Diabetes Association (ADA) recommends screening all adults, especially if overweight, for type 2 diabetes starting at age 45, to be repeated every 3 years: either hemoglobin A1c (A1C or HbA1c), fasting plasma glucose (FPG), or 2-hour 75-g oral glucose tolerance test (OGTT) are appropriate screening methods. The ADA recommends that screening begin at an earlier age, and at more frequent intervals, if the person has one or more risk factors associated with type 2 diabetes (ADA, 2009).
In addition to laboratory tests (see Chapter 36), the health care provider depends on clinical signs and symptoms of diabetes mellitus to establish a diagnosis. The classic symptoms of diabetes mellitus, regardless of type, are related to an elevated blood glucose level, or hyperglycemia. Hyperglycemia increases the concentration of the intravascular fluid, raising its osmotic pressure and pulling water from the cells and interstitial fluid into the blood. This causes cellular dehydration and the loss of glucose (glycosuria), electrolytes, and water in the urine. Cellular dehydration causes thirst and a resultant increased intake of water (polydipsia) and diuresis with increased urination (polyuria). Hunger (polyphagia) is the result of the body’s effort to increase its supply of energy foods, even though the intake of more carbohydrates does not meet the energy needs of the cells.
Fatigue and muscular weakness occur because the glucose needed for energy is not metabolized properly. Weight loss in patients with type 1 diabetes occurs for two reasons: (1) the loss of body fluid; and (2) in the absence of sufficient insulin, the body begins to metabolize its own proteins and stored fat. The oxidation of fats is incomplete and fatty acids are converted into ketone bodies: beta-hydroxybutyric acid, acetoacetic acid, and acetone. When the kidney is unable to handle accumulated ketones in the blood, ketosis occurs. The overwhelming presence of the strong organic acids in the blood lowers the pH and leads to a severe and potentially fatal acidosis. The metabolism of body protein when insulin is not available causes an elevated blood urea nitrogen (BUN) level. This is because the nitrogen component of protein is discarded when the body metabolizes its own protein to obtain the glucose it needs.
People with diabetes are prone to infection, delayed healing, and vascular diseases. Poor control of diabetes makes the person prone to develop an infection. The propensity for infection is thought to be partly a result of decreased normal function of leukocytes and abnormal phagocyte function. Another contributing factor to infection and delayed healing probably is decreased blood supply to the tissues because of atherosclerotic changes in the blood vessels. An impaired blood supply means a deficit in the protective cells brought by the blood to a site of injury.
It is believed that the neurologic, vascular, and metabolic complications of diabetes predispose the person to infections by allowing organisms to enter tissues that are normally better defended and less accessible. For example, a neurogenic bladder predisposes the patient to stagnant urine and accumulations of bacteria, and a leg ulcer resulting from peripheral vascular disease is without the protection of the skin as a barrier to organisms.
Management of Diabetes
There is no cure for diabetes mellitus; the goal is to maintain blood glucose and lipid levels within normal limits and to control these factors to prevent complications. Studies have demonstrated that there are benefits of tight glycemic control (control of glucose in the blood) for people with both type 1 and type 2 diabetes. Patients attempting tight control follow an intensive therapy plan of blood glucose testing and insulin injections, three or more times a day, or they use an insulin pump. There are some risks associated with perfect control of blood glucose levels, and “tight control” is not indicated for every patient. The most serious control issue is hypoglycemia, or insulin reaction. In a recent study (Finfer &  Delaney, 2008), tight glucose control was associated with increased mortality rate in critically ill patients. American Diabetes Association guidelines now recommend glucose targets of 140 to 180 mg/dL for critical patients. For the noncritically ill, premeal target is 140 mg/dL or less and random readings should be 180 mg/dL or less. The health care provider will adjust these parameters for the individual patient.
Delaney, 2008), tight glucose control was associated with increased mortality rate in critically ill patients. American Diabetes Association guidelines now recommend glucose targets of 140 to 180 mg/dL for critical patients. For the noncritically ill, premeal target is 140 mg/dL or less and random readings should be 180 mg/dL or less. The health care provider will adjust these parameters for the individual patient.
Research has demonstrated that lowering A1C levels to 7% is associated with decreased microvascular complications (eye, kidney, and nerve diseases) of diabetes. If the patient is elderly and very frail or if the life expectancy is short, the American Geriatrics Association recommends an A1C of 8% (Lee,  2009).
2009).
The protocol for control of diabetes mellitus is highly individualized and depends on the type of diabetes a person has, age, general state of health, ability to follow the prescribed regimen, and acceptance of responsibility for managing illness, along with a host of other factors.
The overall goal of diabetes management is achieved when fasting blood glucose stays within normal limits, A1C tests show that blood glucose has stayed within normal limits from one testing period to the next, the patient’s weight is normal, blood lipids remain within normal limits, and the patient has a sense of health and well-being.
Diet
Diet is the cornerstone of diabetic treatment. Weight gain is common in persons with type 2 diabetes, because of high caloric intake and decreased availability of endogenous insulin to fully use ingested food. Weight gain can make a patient with type 2 diabetes more insulin resistant. In many cases, people with type 2 diabetes can control their blood glucose by reducing caloric intake and increasing physical exercise. There is no such thing as a “typical” person with diabetes, and because diabetes is an unstable and changing process, each patient’s needs will change from time to time. A person can eat the “perfect” breakfast 3 days in a row, which results in a “perfect” postprandial (after-meal) blood glucose value, only to eat the very same breakfast the next day and have a high blood glucose measurement. This can be frustrating for the patient. The strategies that are effective in managing diabetes can be altered by many factors (e.g., stress, illness, activity, health beliefs), and the strategies that are effective for one person with diabetes may not be effective for someone else.
Medical nutrition therapy (MNT) is now recommended for all persons with either type 1 or type 2 diabetes. A registered dietitian (RD) or a certified diabetes educator (CDE) performs an in-depth assessment of type of diabetes, height-to-weight ratio, usual dietary intake, food preferences, exercise level, and daily schedule. A range of interventions are considered when designing a plan that is individualized for the patient. These interventions include: reduced energy and fat intake, carbohydrate counting, simplified meal plans, healthy food choices, individualized meal planning strategies, exchange lists, insulin-to-carbohydrate ratios (adjusting insulin doses to match carbohydrate intake), physical activity, and behavioral strategies (American  Dietetic Association, 2009). In general, MNT is geared toward providing adequate nutrition with sufficient calories to maintain normal body weight and control of cholesterol, and to adjust the intake of food so that blood glucose is kept within safe limits.
Dietetic Association, 2009). In general, MNT is geared toward providing adequate nutrition with sufficient calories to maintain normal body weight and control of cholesterol, and to adjust the intake of food so that blood glucose is kept within safe limits.
Meal plans generally include a consistent carbohydrate intake, with 45 to 60 g of carbohydrate per meal (ADA, 2009). If the patient chooses to eat sweets, 10% to 35% of total intake does not have a negative effect, but sweets would count as carbohydrates. Proteins should make up 15% to 20%; for patients with diabetic nephropathy (kidney disease secondary to high blood glucose level), protein intake of 1 g/kg of body weight is recommended. Meals should include 14 g of fiber per 1000 kilocalories, which is the recommendation for the general public. Reduction of saturated fats, trans fats, and dietary cholesterol also improves cardiovascular outcomes (American Dietetic Association, 2009).
Association, 2009).
Cultural preferences must be considered when devising meal plans. One of the most effective means of helping a person with diabetes follow the prescribed diet is by teaching about food values and how they affect diabetes. Initially, three or four teaching sessions performed by the RD or CDE lasting 45 to 90 minutes are recommended, with annual follow-up as a minimum. The ADA and the American Dietetic Association have worked together to devise simplified methods of calculating a diabetic diet and planning meals for a person with diabetes. Organizations such as the ADA and the Joslin Diabetes Center, affiliated with Harvard Medical School, have instructive material (see Online Resources on p. 877).
Exercise
Physical exercise is an important part of managing diabetes. Muscular activity improves glucose utilization for energy and improves circulation. In addition to lowering blood glucose levels by “burning up” the glucose, exercise makes the insulin receptors on cells more sensitive to the hormone, and thus improves utilization of the available glucose. Because diabetic control also considers blood lipid levels, exercise contributes to that control by reducing triglyceride levels and increasing high-density lipoprotein (HDL) levels.
The exercise program should be designed for the individual patient. The plan should consider the age and overall physical condition of the patient, ability to carry out the exercises regularly, and how well controlled the diabetes is. For some patients a brisk walk of 1 or 2 miles daily is as much exercise as they can tolerate. Others may be able to perform more strenuous exercises, but they must be cautioned against extremes, especially if they are taking insulin. Exercise can rapidly lower blood glucose levels and cause serious hypoglycemia.
All exercise programs should begin with milder forms of exercise and gradually increase until the patient’s level of tolerance or the desired therapeutic effect is reached. A program should not be started until the blood glucose is under control. The exercise program should be planned so that the exercises are performed at the same time every day, preferably after a meal, when the blood glucose is highest. Blood glucose should be checked before beginning to exercise. The patient is encouraged to wear a medical-alert bracelet (Figure 38-1) and to exercise with a friend who knows the signs and symptoms of hypoglycemia and how to treat it.

Increasing Food Intake During Exercise
During moderate exercise (such as brisk walking, bowling, or vacuuming) 5 g of simple carbohydrate should be consumed at the end of 30 minutes and at 30-minute intervals during the continued activity. (A food example with 5 g of simple carbohydrate is 1 tsp honey.) Jogging, swimming, or scrubbing floors should be preceded by consumption of 15 to 20 g of complex carbohydrate plus protein 15 to 30 minutes before beginning the exercise, and then, if the activity continues for more than 30 minutes, 10 g of simple carbohydrate should be taken every 30 minutes. Vigorous exercise (such as fast jogging, skiing, or playing tennis) requires intake of 30 to 40 g of complex carbohydrate plus protein 15 to 30 minutes ahead of time and then 10 to 20 g of simple carbohydrate intake every 30 minutes after the first half-hour.
Performing exercise when insulin or an oral antidiabetic agent is at its peak of action can bring on an acute hypoglycemic reaction. Eating a piece of fruit before even light exercise, if done between meals, also can help prevent hypoglycemia in people with type 1 diabetes. Once a patient begins to follow a regular exercise program, the insulin dosage and diet may need to be revised. In general, the patient may need to take less insulin and to increase caloric intake with regular exercise. Keeping a daily record of exercise, along with weight, insulin dosage, and blood glucose levels, can help motivate the patient to continue exercise.
Oral Hypoglycemic Agents
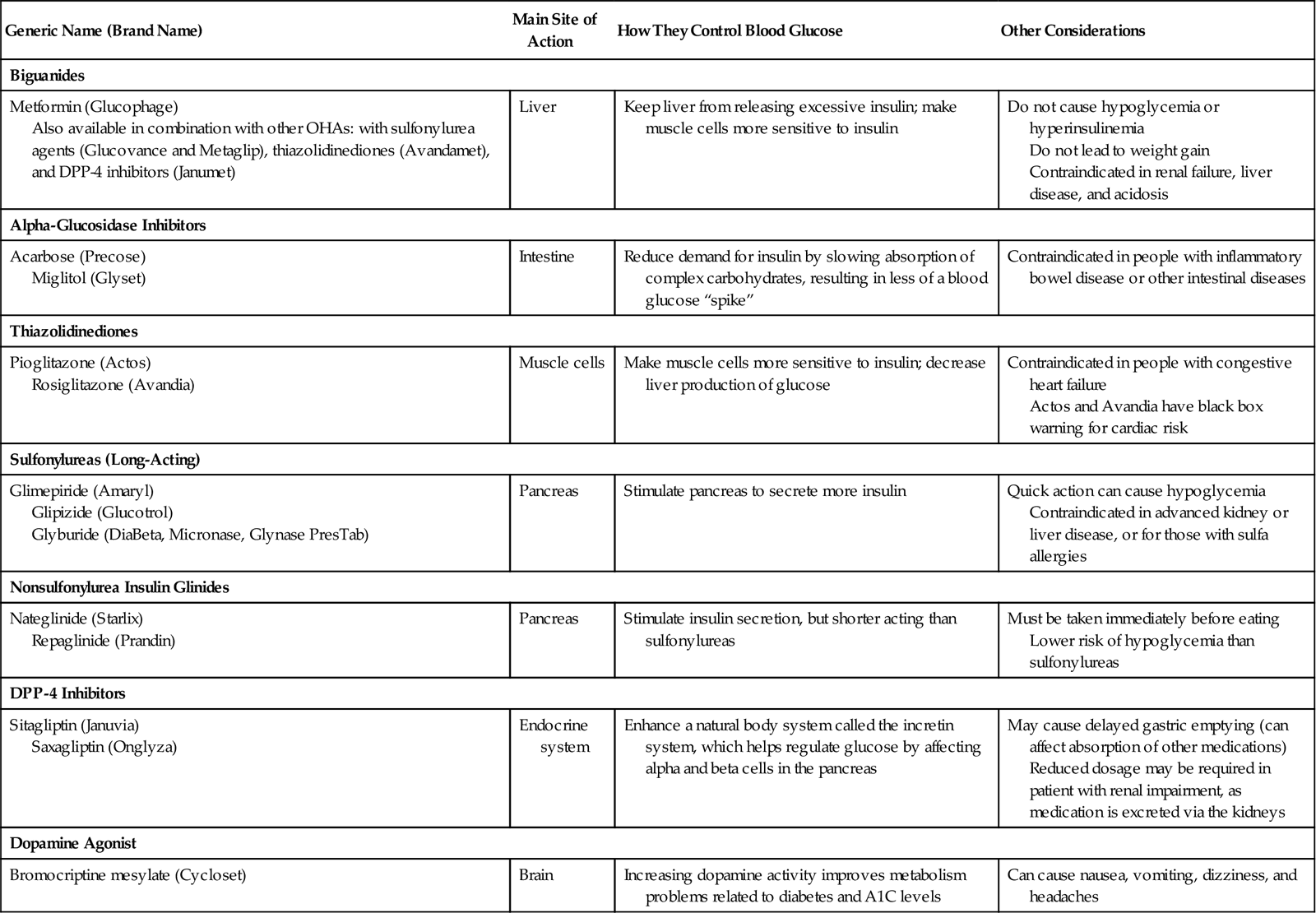
Oral hypoglycemic agents (OHAs) or antidiabetic agents may be prescribed for patients with type 2 diabetes to manage their blood glucose levels. These medications are not a form of oral insulin; pharmacologically, they are from completely different classes of medications. There are now six major categories of OHAs that act in different ways to help achieve blood glucose control. Information about these medications can be found in Table 38-2.
 Table 38-2
Table 38-2
| Generic Name (Brand Name) | Main Site of Action | How They Control Blood Glucose | Other Considerations |
| Biguanides | |||
| Metformin (Glucophage) Also available in combination with other OHAs: with sulfonylurea agents (Glucovance and Metaglip), thiazolidinediones (Avandamet), and DPP-4 inhibitors (Janumet) | Liver | Keep liver from releasing excessive insulin; make muscle cells more sensitive to insulin | Do not cause hypoglycemia or hyperinsulinemia Do not lead to weight gain Contraindicated in renal failure, liver disease, and acidosis |
| Alpha-Glucosidase Inhibitors | |||
| Acarbose (Precose) Miglitol (Glyset) | Intestine | Reduce demand for insulin by slowing absorption of complex carbohydrates, resulting in less of a blood glucose “spike” | Contraindicated in people with inflammatory bowel disease or other intestinal diseases |
| Thiazolidinediones | |||
| Pioglitazone (Actos) Rosiglitazone (Avandia) | Muscle cells | Make muscle cells more sensitive to insulin; decrease liver production of glucose | Contraindicated in people with congestive heart failure Actos and Avandia have black box warning for cardiac risk |
| Sulfonylureas (Long-Acting) | |||
| Glimepiride (Amaryl) Glipizide (Glucotrol) Glyburide (DiaBeta, Micronase, Glynase PresTab) | Pancreas | Stimulate pancreas to secrete more insulin | Quick action can cause hypoglycemia Contraindicated in advanced kidney or liver disease, or for those with sulfa allergies |
| Nonsulfonylurea Insulin Glinides | |||
| Nateglinide (Starlix) Repaglinide (Prandin) | Pancreas | Stimulate insulin secretion, but shorter acting than sulfonylureas | Must be taken immediately before eating Lower risk of hypoglycemia than sulfonylureas |
| DPP-4 Inhibitors | |||
| Sitagliptin (Januvia) Saxagliptin (Onglyza) | Endocrine system | Enhance a natural body system called the incretin system, which helps regulate glucose by affecting alpha and beta cells in the pancreas | May cause delayed gastric emptying (can affect absorption of other medications) Reduced dosage may be required in patient with renal impairment, as medication is excreted via the kidneys |
| Dopamine Agonist | |||
| Bromocriptine mesylate (Cycloset) | Brain | Increasing dopamine activity improves metabolism problems related to diabetes and A1C levels | Can cause nausea, vomiting, dizziness, and headaches |
Patients receiving OHAs should know that these medications do not eliminate the need for following their diet and exercise program. Some may be under the impression that if they go off their diet and indulge themselves, they can just take more pills to compensate. Others who have been on a diet and exercise program for a time and then have an OHA prescribed for them think it is acceptable to stop planning their meals and exercising regularly. All OHAs are capable of producing gastric irritation, nausea, vomiting, and diarrhea. Liver damage with jaundice, bone marrow depression, and allergic skin reactions may result in some patients.
Insulin Therapy
Insulin therapy can be prescribed for patients with either type 1 or type 2 diabetes. The goal of insulin therapy is to closely mimic basal insulin, which is the amount of insulin that would normally be produced by the pancreas throughout the day to maintain a healthy blood sugar level between meals. The pancreas also produces extra insulin after meals (postprandial). The health care provider can use a variety of rapid-acting, short-acting, intermediate-acting, and long-acting insulins that best suit the individual patient (Table 38-3). A single daily injection of intermediate- or long-acting insulin, or a combination insulin (such as Humulin 70/30 that combines short- and intermediate-acting insulins), can be used for some patients. The multiple daily injection (MDI) regimen is more often prescribed and offers the advantage of being more physiologically appropriate. MDI combines short- and intermediate-acting insulins, injected two or more times a day. The patient could also be placed on an intensified regimen. This regimen relies on the patient’s ability to accurately perform blood glucose monitoring. The basal dose, again, would be intermediate- or long-acting insulin. A bolus dose, or correction dose, of short- or rapid-acting insulin is used to manage elevations in blood glucose and bring the next blood glucose measurement into range.
 Table 38-3
Table 38-3
Common Types of Insulins: Onset, Peak, and Duration of Action
| Preparation | Brand Name | Onset (Hr) | Peak (Hr) | Duration (Hr) |
| Rapid Acting | ||||
| Insulin aspart injection | NovoLog | 0.25 | 1-3 | 3-5 |
| Insulin lispro injection | Humalog | 0.25 | 0.5-1.5 | 5 |
| Insulin glulisine injection | Apidra | 0.3 | 0.5-1.5 | 3-4 |
| Short Acting | ||||
| Regular human insulin injection | Humulin R | 0.5 | 2-4 | 5-7 |
| Novolin R | 0.5 | 2.5-5 | 8 | |
| Buffered regular human insulin injection | Velosulin BR | 0.5 | 1-3 | 8 |
| Intermediate Acting | ||||
| Isophane insulin NPH | Humulin N Novolin N | 1.5 | 4-12 | 16-24+ |
| ReliOn N | 1.5 | 4-12 | 24 | |
| Insulin zinc suspension (Lente) | Novolin L | 1 | 6-8 | 5.7-24 |
| Insulin detemir injection | Levemir | 1 | 6-8 | 5.7-24 |
| Long Acting | ||||
| Insulin glargine injection | Lantus | 2-4 | None | 24 |
| Combination Insulin | ||||
| 70% Insulin aspart protamine suspension/30% insulin aspart injection | NovoLog Mix 70/30 | 0.25 | 1-4 | 24 |
| 75% Insulin lispro protamine suspension/25% insulin lispro injection | Humalog Mix 75/25 | 0.25 | 1-2 | 24 |
| 70% Human insulin isophane suspension (NPH)/30% human insulin injection (regular) | Humulin 70/30 Novolin 70/30 ReliOn 70/30 | 0.5 | 2-12 | 24 |
| 50% Human insulin isophane suspension (NPH)/50% human insulin injection (regular) | Humulin 50/50 Novolin 50/50 | 0.5 | 3-5 | 24 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



















 every insulin dose must be verified by another nurse as it is drawn up, every time. This habit will also help you meet the National Patient Safety Goal to increase the safety of administering medications.
every insulin dose must be verified by another nurse as it is drawn up, every time. This habit will also help you meet the National Patient Safety Goal to increase the safety of administering medications.