Malnutrition and cachexia continue to complicate the course of disease and treatment in patients with cancer. Agreement exists in the literature and among clinicians that malnutrition is a common problem. However, a predictable and measurable definition of malnutrition has yet to be established (Corish & Kennedy, 2000). Elia (2001) has suggested that cancer-related malnutrition be defined as “a state of nutrition in which a deficiency or excess (or imbalance) of energy, protein, and other nutrients causes measurable adverse effects on tissue/body form, function, and clinical outcome.”
Primary cachexia, also known as primary anorexia/cachexia, is a specific syndrome characterized by protein breakdown and/or increased energy expenditure, resulting in anorexia, emaciation, loss of muscle tissue, weakness, and fatigue (Strasser & Bruera, 2002). Cachexia frequently does not respond to nutritional supplements or an increased food intake (Mantovani et al., 2003; Van Halteren et al., 2003). Researchers’ understanding of the pathophysiology of these complications is still evolving. Supportive nutrition interventions consisting of assessment, treatment, and monitoring of the patient’s nutritional status are an expanding area of study to delineate the outcomes for patients with cancer (Ottery, 1995).
PATHOPHYSIOLOGY
Primary Anorexia/Cachexia
Primary anorexia/cachexia syndrome is a metabolic imbalance associated with the chronic inflammatory response generated by the patient’s body (the host) as a result of exposure to malignant cells (Strasser & Bruera, 2002). This imbalance causes an alteration in glucose metabolism, muscle protein synthesis, proteolysis of muscle proteins, and decreased lipogenesis (Van Halteren et al., 2003). Increased energy expenditure in relation to lean body mass is a consequence of this imbalance. The patient often presents with loss of appetite, early satiety, chronic nausea, muscle wasting, loss of fat, overall weight loss, and fatigue (Strasser, 2002).
Proinflammatory cytokines, which are small proteins that can be produced by any cell in the body, play a significant role in the host’s response (Dinarello, 2000). Examples of cytokines commonly associated with primary anorexia/cachexia syndrome are tumor necrosis factor (TNF) and interleukins 1 and 6 (IL-1, IL-6) (Van Halteren et al., 2003). The research suggests that TNF mediates the loss of muscle proteins by causing fragmentation of muscle DNA, which results in muscle cell death (apoptosis) (Carbo et al., 2002). IL-1 appears to support TNF by enhancing TNF’s ability to kill targeted cells (Dinarello, 2000). IL-6 also seems to support TNF and has been implicated in the development of anorexia and the breakdown of fats and proteins (Van Halteren et al., 2003).
Malignant cells not only act as a trigger for the host’s inflammatory response, they also contribute glycoprotein molecules that appear to accelerate muscle breakdown and metabolism (Strasser, 2004). Examples of tumor-generated glycoprotein molecules are proteolysis-inducing factor (PIF) and lipid-mobilizing factor (LMF). PIF contributes to muscle protein breakdown, and LMF contributes to the breakdown of fat. Both substances are detectable in the blood and correlate with weight loss (Fig. 35.1) (Strasser & Bruera, 2002).
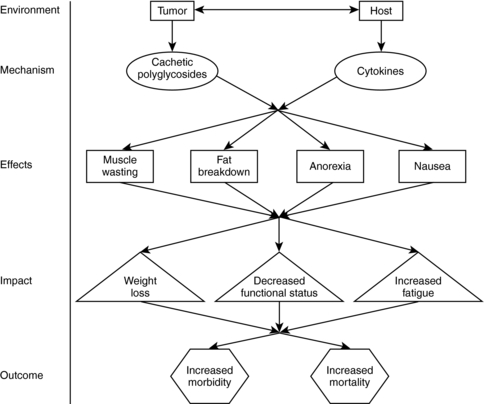 |
| Fig. 35.1Primary anorexia/cachexia syndrome. |
Secondary Anorexia/Cachexia
Secondary anorexia/cachexia is a constellation of conditions that can co-occur with primary anorexia/cachexia. The presence of secondary anorexia/cachexia with primary anorexia/cachexia complicates the presentation, assessment, and management of both syndromes (Strasser, 2002).
The conditions that make up secondary anorexia/cachexia are grouped into three major categories: starvation/malnutrition, loss of muscle mass, and other catabolic states. Multiple etiologies are responsible for the changes associated with each category and the pathophysiology depends on the etiology of the co-morbid conditions involved (Strasser, 2002).
Starvation/malnutrition occurs as a result of decreased oral intake, alteration in gastrointestinal absorption, and/or loss of protein-rich body fluids (Strasser & Bruera, 2002). Examples of commonly encountered contributing factors to starvation and malnutrition in the oncology patient include stomatitis, taste alteration and food aversions, xerostomia, dysphagia, nausea, vomiting, constipation, diarrhea, bowel obstruction, ascites, nephrotic syndrome, pain, dyspnea, depression, and cognitive impairment (Strasser & Bruera, 2002). This is not an all-inclusive list. The significance is that all these conditions contribute to a disruption in the intake of nutrients that, if severe enough, results in significant weight loss and malnutrition.
Loss of muscle mass is another category of secondary anorexia/cachexia. Circumstances associated with loss of muscle mass not previously discussed include prolonged immobility and deconditioning, growth hormone deficiency, aging, and muscle wasting (Strasser & Bruera, 2002). These conditions tend to be found in aging and ill people.
Catabolism is the breakdown of tissue. Catabolic states that may contribute to secondary anorexia/cachexia include infections, treatment with proinflammatory cytokines, chronic heart failure, renal failure, liver failure, diabetes mellitus, and hyperthyroidism (Strasser & Bruera, 2002).
The mechanisms of action of primary anorexia/cachexia and secondary anorexia/cachexia are different. In simple starvation and malnutrition, a form of secondary anorexia/cachexia, oral intake is either voluntarily or involuntarily withheld, depriving the body of needed calories and nutrients. This form of starvation emphasizes fat breakdown and limited protein breakdown, and generally is associated with a reduction in energy expenditure (Strasser, 2002).
The enhanced catabolic state of primary anorexia/cachexia promotes increased breakdown of muscle proteins and fats (Strasser, 2002). The most significant difference between these two syndromes from a management perspective is that increased intake of nutrients does not improve primary anorexia/cachexia (Strasser, 2002). Table 35-1 compares and contrasts the changes associated with primary anorexia/cachexia and simple starvation.
| ALTERATION | ||
|---|---|---|
| Characteristic | Primary Anorexia/Cachexia | Starvation |
| Glucose turnover | ↑ | ↓ |
| Ketone bodies | ↓ | ↑ |
| Acute phase protein synthesis | ↑ | ↔ |
| Muscle protein synthesis | ↓ | ↓ |
| Proteolysis of muscle proteins | ↑↑ | ↑ |
| Lipogenesis | ↓ | ↓ |
| Lipolysis | ↑ | ↑↑ |
| Energy expenditure/lean body mass | ↑ | ↓ |
EPIDEMIOLOGY AND ETIOLOGY
Epidemiology
Nutritional problems are the most common secondary diagnosis for patients with cancer. The incidence varies, depending on the disease’s stage, location, and treatment. Cachexia occurs in 24% of patients in the early stages of advanced cancer and in 80% of patients with cancer in the terminal stages. It is the cause of death in 20% of patients with terminal-stage cancer (Mantovani et al., 2003; Van Halteren et al., 2003; Strasser & Bruera, 2002).
The location of the cancer influences the occurrence of cachexia. Patients with pancreatic cancer commonly present with a history of digestive disturbance and weight loss (Ottery, 1996a). Patients with esophageal cancer complain of difficulty swallowing and a resulting loss of appetite and weight (Woodtli & Van Ort, 1993). Many patients with head and neck cancer undergoing radiation therapy are at risk for anorexia, loss of weight, and the beginning stages of malnutrition and cachexia (Woodtli & Van Ort, 1991).
Treatment approaches also affect the occurrence of malnutrition and cachexia. Patients receiving cytotoxic chemotherapy experience varying digestive symptoms, such as nausea, vomiting, stomatitis, and diarrhea; all of these, when uncontrolled, lead to anorexia, loss of weight, and eventually cachexia (Grant & Kravits, 2000).
Patients undergoing surgery are on restricted intake before and after the surgical procedure. If the surgery involves the mouth, neck, esophagus, and other areas of the gastrointestinal track, limited intake and disrupted absorption may occur, leading to anorexia/cachexia. Surgical procedures support and promote catabolism. As a result of increased catabolism coupled with a stress-related increase in metabolic requirements, surgical patients are susceptible to loss of weight and eventually cachexia (Strasser & Bruera, 2002).
Patients undergoing radiation therapy experience symptoms at the local site of therapy. This could mean a loss of appetite, difficulty swallowing, and lack of digestion and absorption, all of which may lead to anorexia and cachexia (Woodtli & Van Ort, 1993). The more toxic the chemotherapy, the more extensive the surgery, and the larger the radiation field, the more likely the patient is to be vulnerable to anorexia/cachexia.
Etiology
Evidence suggests that the presence of malignant cells in the host stimulates a chronic inflammatory response fueled by cytokines. This creates an imbalance between increased energy needs and the body’s diminished ability to process nutrients to meet those needs. A likely clinical effect of this imbalance is the development of malnutrition with significant risk of progression to anorexia/cachexia (Strasser, 2002).
Etiologies of primary anorexia/cachexia include metabolic abnormalities produced by the relationship of the tumor and host tissues, resulting in a decreased intake of nutrients and increased energy requirements (Strasser, 2002). Secondary anorexia/cachexia is caused by starvation/malnutrition (impaired oral intake and/or impaired gastrointestinal absorption), other catabolic states (chronic infections, poorly controlled diabetes), and loss of muscle mass (prolonged bed rest) (Strasser & Bruera, 2002).
Secondary etiologies are associated with cancers that occur in the gastrointestinal tract, causing direct interference with food intake, digestion, and absorption, and with responses to cancer treatments, such as chemotherapy and radiation therapy. For patients with oral, esophageal, or stomach cancer, early loss of oral intake leads to anorexia/cachexia, followed by weight loss. For patients with cancer of the pancreas, digestive difficulties, including pain, may be the presenting symptom. Co-morbidities, especially if uncontrolled, may lead to anorexia/cachexia. For example, acute infections can increase the catabolic state. Organ failures (heart, lungs, kidneys), poorly controlled diabetes, and hyperthyroidism can lead to secondary anorexia/cachexia in patients with cancer. In addition, with bed rest, a lack of activity ensues, and muscle wasting begins (Strasser & Bruera, 2002).
RISK PROFILE
• Patients with specific cancers:
• Cancer of the head and neck
• Cancer of the esophagus, stomach, and small intestine
• Cancer of the pancreas
• Patients in specific cancer stages:
• Recurrent disease
• Advanced or terminal cancer
• Patients undergoing specific cancer therapies:
• Patients undergoing radical surgical procedures or surgery, especially those involving the head, neck, and gastrointestinal system.
• Patients undergoing radiation therapy that includes the gastrointestinal tract within the treatment field.
• Patients experiencing chemotherapy-associated side effects of nausea, vomiting, diarrhea, and pain, especially of the mouth and gastrointestinal tract.
• Patients undergoing hematopoietic cell transplantation.
• Patients with cancer who have uncontrolled or poorly controlled co-morbidities (e.g., heart disease, renal disease, diabetes).
• Patients with cancer who are on bed rest or are bedridden.
• Patients with cancer who are experiencing psychological or spiritual crises.
PROGNOSIS
The presence of primary anorexia/cachexia has been associated with alterations in functioning, increased symptoms, intolerance for treatment, and impaired clinical outcome (Strasser & Bruera, 2002). Primary anorexia/cachexia has been commonly associated with a loss of 10% or more of the prediagnosis weight within the previous 6 months. Additional research has demonstrated that weight loss alone is not the best predicator of the impact of cachexia. Factors such as weight loss, a decreased food intake, and the presence of an inflammatory response, as measured by the C-reactive protein level, may be useful for assessing the impact of cachexia on outcome (Fearon et al., 2006).
PROFESSIONAL ASSESSMENT CRITERIA (PAC)
Primary anorexia/cachexia is a complex syndrome that may be affected by the presence of co-occurring conditions, including therapies such as chemotherapy, radiation therapy, and surgery. A comprehensive assessment that includes both subjective and objective data is essential for the formulation of an appropriate plan of care. The assessment should be initiated at the first patient contact, and the patient should be reassessed at regularly scheduled intervals throughout treatment. Proactive planning and symptom management helps minimize the impact of changes in nutritional status.
Areas of focus that facilitate the formulation of a comprehensive nutritional profile include the involuntary weight loss history, history of nutritional intake, perceptions of body image, subjective global assessment, symptom distress measures, laboratory values, anthropometric measurements, quality of life measures, and diagnostic tests. Referral to appropriate health care professionals for evaluation of specialized aspects of the patient’s presentation is useful.
Involuntary Weight Loss History
Regular measurement of weight and height is a fundamental component of the assessment. The body mass index (BMI), which is calculated from height and weight measurements, provides an objective measure of chronic protein-energy status (Elia, 2001). Loss of 10% or more of the body weight within 6 months indicates the presence of cachexia and may be associated with a reduced tolerance for treatment and a worsening prognosis (Strasser, 2002). Patient and caregiver reports of subjective experiences of weight loss (e.g., clothes that no longer fit, an awareness of being thinner) should be incorporated into the weight loss history and, when present, should increase the clinician’s sensitivity to the probability that significant weight loss has already occurred.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access
