Fluids, Electrolytes, Acid-Base Balance, and Intravenous Therapy
Objectives
1. Recall the various functions fluid performs in the body.
2. Identify the body’s mechanisms for fluid regulation.
4. Distinguish the signs and symptoms of various electrolyte imbalances.
5. Discuss why the elderly have more problems with fluid and electrolyte imbalances.
6. Recognize the disorders that cause specific fluid and electrolyte imbalances.
7. Compare the major causes of acid-base imbalances.
8. State interventions to correct an acid-base imbalance.
9. Discuss the steps in managing an intravenous infusion.
10. Describe the measures used to prevent the complications of intravenous therapy.
11. Identify intravenous fluids that are isotonic.
1. Assess patients for signs of dehydration.
2. Correctly assess for and identify edema and signs of overhydration.
3. Apply knowledge of normal laboratory values in order to recognize electrolyte imbalances.
4. Carry out interventions to correct an electrolyte imbalance.
5. Determine if a patient has an acid-base imbalance.
6. Carry out measures to prevent the complications of intravenous therapy.
Key Terms
acidosis (ăh-sĭ-DŌ-sĭs, p. 41)
active transport (ĂK-tĭv, p. 34)
aldosterone (p. 32)
alkalosis (ăl-kă-LŌ-sĭs, p. 44)
anions (ĂN-ī-ŏnz, p. 40)
antidiuretic hormone (ADH) (ăn-tĭ-dī-ū-RĔT-ĭk HŎR-mōn, p. 31)
ascites (ăh-SĪ-tēz, p. 41)
atrial natriuretic peptide (ANP) (p. 32)
carpopedal spasm (spăzm, p. 44)
cations (KĂT-ī-ŏnz, p. 40)
dehydration (dē-hī-DRĀ-shŭn, p. 34)
diffusion (dĭ-FŪ-zhŭn, p. 32)
edema (ĕh-DĒ-mă, p. 39)
electrolytes (ĕh-LĔK-trō-līts, p. 40)
extracellular (ĕks-tră-SĔL-ū-lăr, p. 31)
filtration (fĭl-TRĀ-shŭn, p. 33)
hydrostatic pressure (hī-drō-STĂ-tĭk PRĔ-shŭr, p. 33)
hypercalcemia (hī-pĕr-kăl-SĒ-mē-ăh, p. 45)
hyperchloremia (hī-pĕr-klŏr-Ē-mē-ăh, p. 45)
hyperkalemia (hī-pĕr-kă-LĒ-mē-ăh, p. 44)
hypermagnesemia (hī-pĕr-măg-nĕ-SĒ-mē-ăh, p. 45)
hypernatremia (hī-pĕr-nā-TRĒ-mē-ăh, p. 41)
hyperphosphatemia (hī-pĕr-fŏs-fă-TĒ-mē-ăh, p. 45)
hypertonic (hī-pĕr-TŎN-ĭk, pp. 33, 51)
hyperventilation (hī-pĕr-vĕn-tĭ-LĀ-shŭn, p. 48)
hypervolemia (hī-pĕr-vō-LĒ-mē-ăh, p. 39)
hypocalcemia (hī-pō-kăl-SĒ-mē-ăh, p. 44)
hypochloremia (hī-pō-klŏr-Ē-mē-ăh, p. 45)
hypodermoclysis (hī-pō-dĕrm-ōk-LĪ-sĭs, p. 56)
hypokalemia (hī-pō-kă-LĒ-mē-ăh, p. 44)
hypomagnesemia (hī-pō-măg-nĕ-SĒ-mē-ăh, p. 45)
hyponatremia (hī-pō-nă-TRĒ-mē-ăh, p. 40)
hypophosphatemia (hī-pō-fŏs-făw-TĒ-mē-ăh, p. 45)
hypotonic (hī-pō-TŎN-ĭk, pp. 33, 51)
hypovolemia (hī-pō-vō-LĒ-mē-ăh, p. 39)
hypoxemia (hī-pŏk-SĒ-mē-ăh, p. 48)
insensible (p. 34)
interstitial (ĭn-tĕr-STĬSH-ăl, p. 32)
intracellular (ĭn-tră-SĔL-ū-lăr, p. 31)
intravascular (ĭn-tră-VĂS-cū-lăr, p. 32)
ions (ī-ŏnz, p. 32)
isotonic (ī-sō-TŎN-ĭk, p. 33, 50)
ketoacidosis (kē-tō-ă-sĭ-DŌ-sĭs, p. 48)
osmolality (ŏz-mō-LĂ-lĭ-tē, p. 40)
osmosis (ŏz-MŌ-sĭs, p. 32)
stridor (STRĪ-dŏr, p. 49)
tetany (TĔT-ă-nē, p. 44)
transcellular (trăns-SĔ-lū-lăr, p. 32)
turgor (TŬR-gŏr, p. 34)
 http://evolve.elsevier.com/deWit/medsurg
http://evolve.elsevier.com/deWit/medsurg
Over half of the human body’s weight is water. Throughout life there is a gradual decline in the amount of body water. An infant’s body is approximately 77% water, and an elderly person’s body is about 45% water. The elderly and the very young are more likely to experience severe consequences with even minor changes in their fluid balance. Fatty tissue does not contain as much water as other tissues; thus the greater the amount of fat in the body, the less the percentage of body water. Maintaining a healthy weight is important to regulating the body’s percentage of water. Keeping body fluids within a normal range is necessary because for every cell of every organ, life processes take place within fluid. The nutrients needed for life, reproduction, and the normal functioning of a cell must be dissolved or suspended in water. Moreover, the largest part of each cell is fluid. For all of the cell’s life processes to take place, there must be a continuous exchange of water, glucose, oxygen, nutrients, electrolytes, and waste products. The four main functions of water in the body are to:
1. Be a vehicle for the transportation of substances to and from the cells.
2. Aid heat regulation by providing perspiration, which evaporates and cools the body.
3. Assist in maintenance of hydrogen (H+) balance in the body.
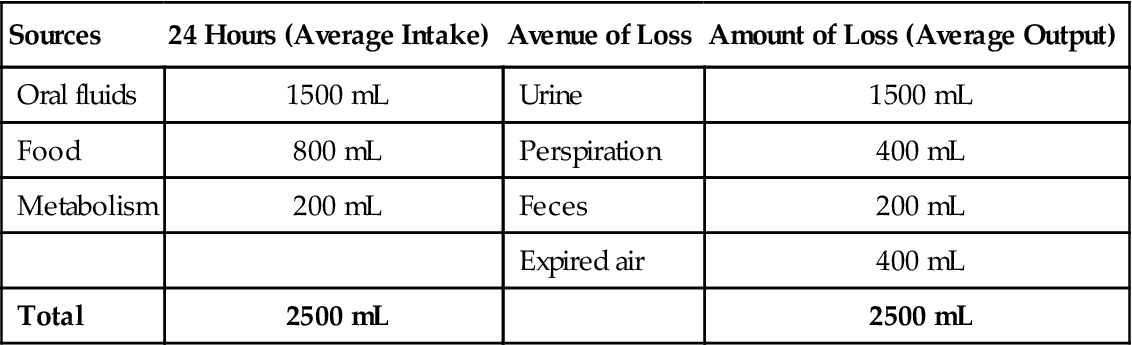
Table 3-1 shows sources of water and avenues of a body’s water loss.
Table 3-1
Sources of Water and Avenues of Water Loss
| Sources | 24 Hours (Average Intake) | Avenue of Loss | Amount of Loss (Average Output) |
| Oral fluids | 1500 mL | Urine | 1500 mL |
| Food | 800 mL | Perspiration | 400 mL |
| Metabolism | 200 mL | Feces | 200 mL |
| Expired air | 400 mL | ||
| Total | 2500 mL | 2500 mL |
Adapted from deWit, S.C. (2009) Fundamental Concepts and Skills for Nursing (3rd ed.). Philadelphia: Saunders, p. 436.
Distribution and Regulation of Body Fluids
Pathophysiology
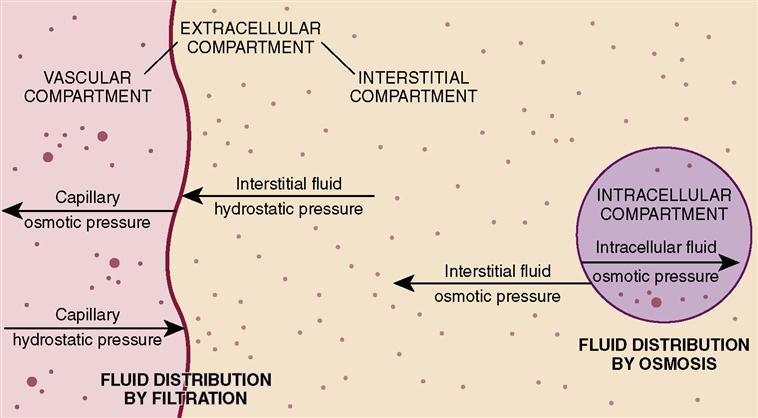
Body fluids are continually in motion, moving in and out of the blood and lymph vessels, through the spaces surrounding the cells, and through the bodies of the cells themselves. Fluid within the cell is considered to be in one compartment (intracellular) and fluid outside the cell in another (extracellular) (Figure 3-1). The three types of extracellular fluid (ECF) and body fluid distribution are shown in Box 3-1. Excretion of the body’s fluid is mainly achieved via the kidney. Control of fluid balance is managed by:
Pain, nausea, and stress can also cause the release of ADH by the pituitary. When the ECF volume is low, or when sodium concentration is elevated, the adrenal cortex releases aldosterone, which causes reabsorption of sodium from the renal tubules. The renin-angiotensin-aldosterone system regulates the release of aldosterone. Renin is released when there is decreased blood flow to the kidney. Baroreceptors in the atrium of the heart detect fluid overload and stimulate the myocardium to release atrial natriuretic peptide. Atrial natriuretic peptide helps protect the body from fluid overload.
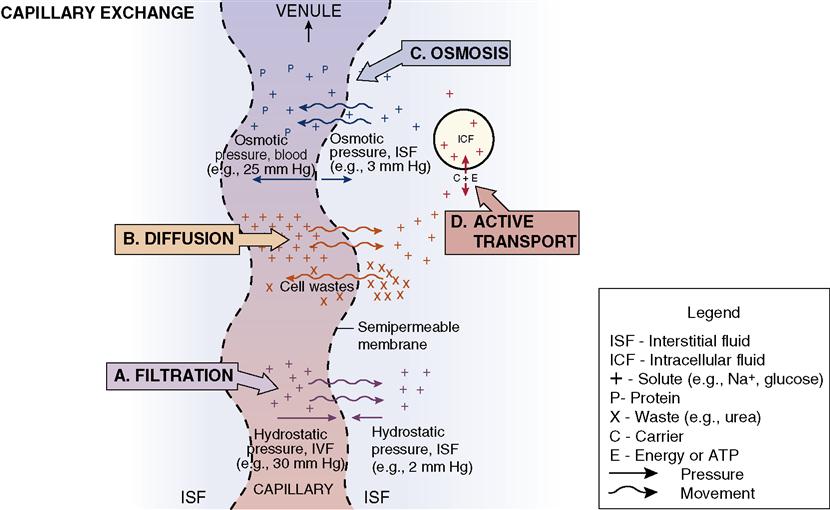
To be normally distributed within the body, water and the substances suspended or dissolved in water must move from compartment to compartment. As blood flows through the capillaries, fluid and solutes can move into the interstitial spaces, where nutrients and wastes can be exchanged by the cells of the body. Several processes accomplish the movement of fluids, electrolytes, nutrients, and waste products back and forth across the cell membranes (Figure 3-2).
Movement of Fluid and Electrolytes
Passive Transport
Diffusion
Diffusion is the process by which substances move back and forth across the membrane until they are evenly distributed throughout the available space. As the plasma moves along a capillary, large amounts of fluid filter through pores in the capillary walls. The fluid moves into and out of the capillaries by filtering through the permeable capillary wall or cell membrane walls. There is a capillary hydrostatic pressure inside the capillary that pushes against the membrane of the capillary. When the solution on one side of the membrane is more concentrated than the solution on the other side of the membrane, the particles in the more concentrated solution travel through the membrane to the less concentrated side in an attempt to equalize the concentration of the two solutions. Diffusion is possible because of kinetic motion, which diffuses the molecules in the intracellular fluid (ICF) and the plasma. The molecules literally bounce off one another, mixing and stirring the body fluids.
Diffusion is a spontaneous mixing and moving that allows the exchange of molecules, ions (electrically charged particles), cellular nutrients, wastes, and other substances dissolved or suspended in body water. The direction of water flow depends on which side of the membrane has the greatest concentration of solutes. Substances will move from a high to a low concentration until the concentration on both sides of the membrane is equal. This is called movement down a concentration gradient. Glucose, oxygen, carbon dioxide, water, and other small ions and molecules move by diffusion, which is a process of equalization.
Diffusion may occur by movement along an electrical gradient as well. The attraction between particles of opposite charge and the repellent action between particles of like charge comprise an electrical gradient. Many intracellular proteins have a negative charge that tends to attract the positively charged sodium and potassium ions from the ECF.
Osmosis
Osmosis is the movement of pure solvent (liquid) across a membrane. Water moves by osmosis. When there are differences in concentration of fluids in the various compartments, osmotic pressure (what holds fluid in the vascular space) will move water from the area of lesser concentration of solutes to the area of greater concentration until the solutions in the compartments are of equal concentration. The process takes place via a semipermeable membrane—a membrane that allows some substances to pass through but prevents the passage of other substances. Fluid moves between the interstitial and intracellular and the interstitial and intravascular compartments by osmosis.
When living cells are surrounded by a solution that has the same concentration of particles, the water concentration of the ICF and the ECF will be equal. Such a solution is termed isotonic (of equal solute concentration). If cells are surrounded by a solution that has a greater concentration of solute than the cells, the water in the cells will move to the more concentrated solution and the cells will dehydrate and shrink. The solution is hypertonic (of greater concentration) in relation to the cells. If the cells are surrounded by a solution that has less solute than the cells, the solution is hypotonic (of less concentration) in relation to the cells. The particles within the cells exert osmotic pressure and draw water inward through the semipermeable membrane. The cells swell from the extra fluid (overhydrate). These concepts are important to the administration of intravenous fluids (see discussion later in this chapter). Solutions are classified as isotonic, hypertonic, or hypotonic according to their concentration of electrolytes and other solutes.
Filtration
Filtration is the movement of water and solutes through a semipermeable membrane due to a pushing force on one side of the membrane. The pumping action of the heart creates hydrostatic pressure (pressure exerted by fluid) within the capillaries. Hydrostatic pressure causes fluid to press outward on the vessel. Water and electrolytes move through the capillary wall to the interstitial fluid. Filtration occurs in the kidney, where waste substances and excess water are eliminated.
Active Transport
In contrast to diffusion, osmosis, and filtration, active transport requires cellular energy, which can move molecules into cells regardless of their electrical charge or the concentrations already in the cell. Active transport may move substances from an area of lower concentration to an area of higher concentration. The energy source for the process is adenosine triphosphate (ATP). ATP is produced during the complex metabolic processes in the body’s cells. Enzyme reactions metabolize carbon chains of sugars, fatty acids, and amino acids, yielding carbon dioxide, water, and high-energy phosphate bonds. Amino acids, glucose, iron, hydrogen, sodium, potassium, and calcium are moved through the cell membrane by active transport. The “sodium pump” is the mechanism by which sodium and potassium are moved into or out of the cell via active transport.
Fluid Imbalances
Pathophysiology
Healthy people maintain fluid balance by drinking sufficient fluids and eating a balanced diet each day. Solid foods contain up to 85% water, and water is also produced in the body as a by-product of metabolism. The healthy kidney balances the amount of substances entering and leaving the blood, helping to maintain normal concentrations of fluid and electrolytes. Illness affects fluid balance in many ways. The patient may be unable to ingest food or liquids, there may be a problem with absorption from the intestinal tract, or there may be a kidney impairment that affects excretion or reabsorption of water and electrolytes. Any disease that affects circulation (e.g., heart failure) will ultimately affect the distribution and composition of body fluids. Extra fluid is lost when the metabolic rate is accelerated, such as occurs in fever, thyroid crisis, burns, severe trauma, and states of extreme stress. Perspiration can account for a fluid loss of up to 2 L/hr in an adult. For every degree of fever on the Celsius scale, an insensible (unaware of) water loss of 10% may occur. Perspiration and water lost in respiration are insensible losses. When the weather is hot and dry, water loss from the body is greater. Patients on mechanical ventilators, those with rapid respirations, and those with severe diarrhea or excessive amounts of fistula drainage also lose greater quantities of water. Any seriously ill patient is at risk for a fluid and electrolyte imbalance.
A fluid imbalance exists when there is an excess (too much) or a deficit (too little) of water in the body. When this occurs, there will be an accompanying imbalance in the substances dissolved in body water. When considering sodium imbalances, it is important to remember that water follows sodium in the body, through osmosis. The sodium concentration causes an osmotic pull, and water will go to where the sodium concentration is highest.
Deficient Fluid Volume
Those at risk for deficient fluid volume are patients who are unable to take in sufficient quantities of fluid because of impaired swallowing, extreme weakness, disorientation or coma, or the unavailability of water, and patients who lose excessive amounts of fluid through prolonged vomiting, diarrhea, hemorrhage, diaphoresis (sweating), excessive wound drainage, or diuretic therapy.
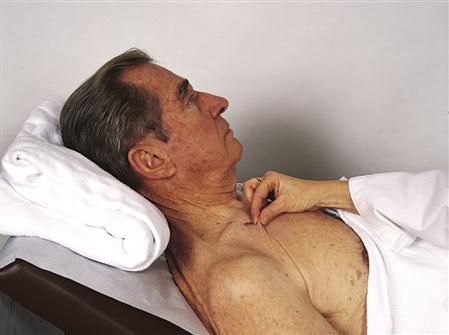
When a fluid deficit occurs, water moves from the cells into the interstitial and intravascular spaces. This movement of water out of the cells causes dehydration of the cells. Dehydration is treated by administering fluid orally or intravenously. For patients who will be unable to take in fluids or food on their own for an extended period, a feeding tube must be placed or total parenteral nutrition (TPN) started (see Chapter 30). Signs and symptoms of dehydration are presented in Box 3-2. Turgor (degree of elasticity) is checked by gently pinching up the skin over the abdomen, forearm, sternum, forehead, clavicle, or thigh (Figure 3-3). In a person with normal fluid balance, the pinched skin will immediately fall back to normal when released. If a fluid deficit is present, the skin may remain elevated or tented for several seconds. However, since pinching the skin to measure fluid deficit also measures skin elasticity, this test is not a valid indicator of fluid status in the elderly—whose skin is often inelastic and routinely tents when pinched. In the infant, dehydration is evident by sunken fontanels.
Many elderly people rely on laxatives and enemas to clear the bowel. This practice can cause fluid volume deficit along with sodium and potassium loss. Fluid volume deficit contributes to constipation and orthostatic hypotension with related dizziness and falls, and makes the person more susceptible to infection.
A furrowed, dry tongue that is not the result of drug therapy indicates a fluid deficit. If the person has fever, this adds to the fluid loss. Because of the fluid and accompanying electrolyte losses, the person may become confused. Offering the patient small amounts of liquid and electrolyte solution frequently—if fluid can be kept down—helps prevent additional problems.
Nursing Management
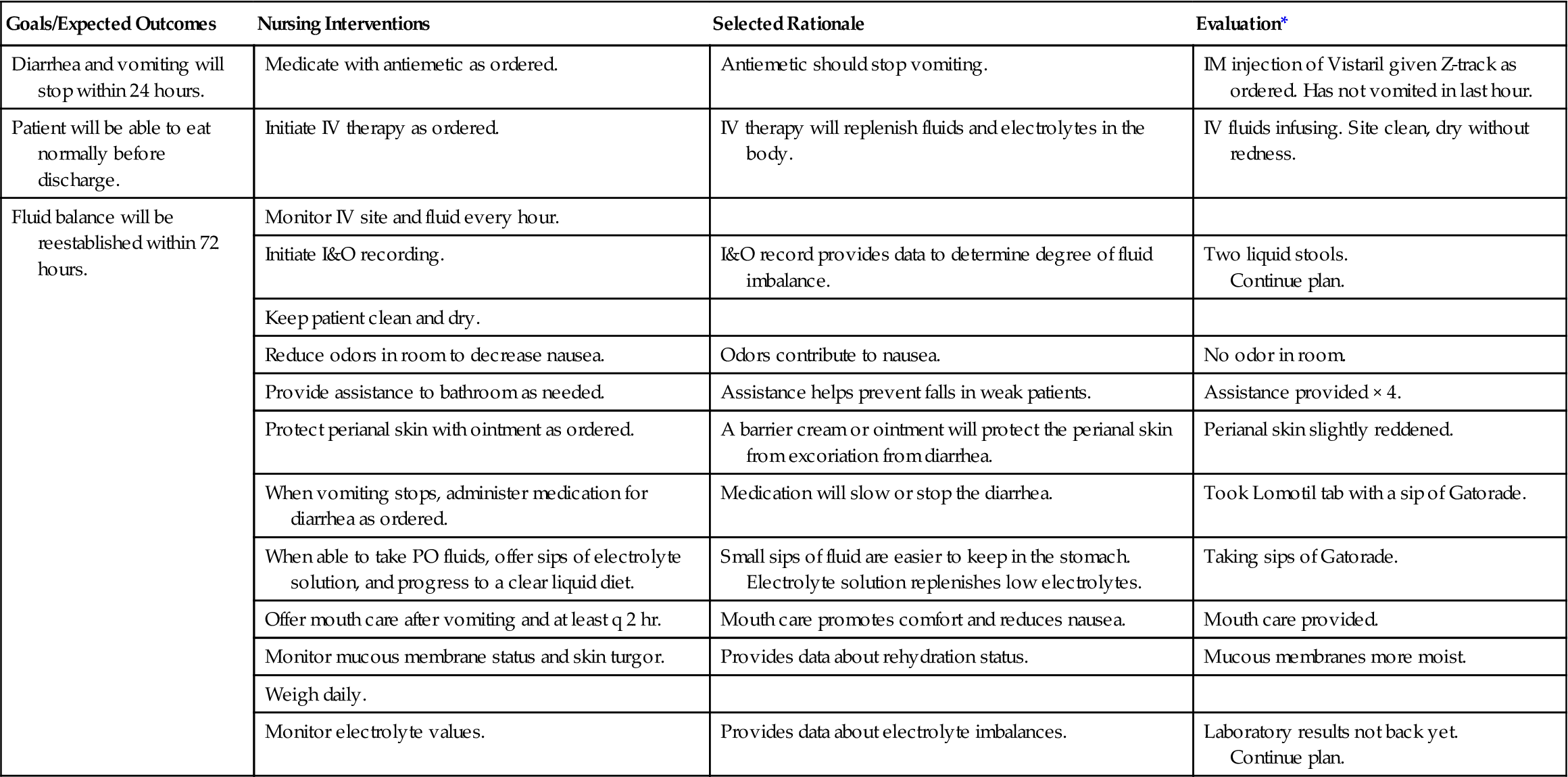
Provide an adequate fluid intake for those who are unable to do this for themselves. Include the patient’s preferences for liquids on the plan of care. The patient should receive fruit juices, bouillon, and any other nutritious liquid tolerated (Nursing Care Plan 3-1).
A frequent cause of excessive fluid loss is abnormally rapid excretion of intestinal fluids, such as that which occurs from vomiting and diarrhea.
Nausea and Vomiting
Nausea is a feeling of discomfort or an unpleasant sensation vaguely felt in the epigastrium and abdomen. It is a symptom of illness and is often accompanied by a tendency to vomit. Nausea is experienced when nerve endings in the stomach and other parts of the body are irritated. Irritated nerve endings in the stomach send messages to the part of the brain that controls the vomiting reflex; however, nerve cells in other parts of the body can trigger the same response. Pain can trigger the nausea-vomiting mechanism. Nausea and vomiting are an automatic response of the involuntary autonomic nervous system to unpleasant stimuli.
Nausea and vomiting may occur from gastrointestinal irritation from foods, viruses, radiation of the mucosa, and some drugs and other chemicals. Certain types of anesthetics may trigger nausea, as may pregnancy.
The patient may complain of nausea or feeling “sick to my stomach,” of queasiness, abdominal pain, epigastric discomfort or burning, and vomiting. The patient may also exhibit pallor; mild diaphoresis; cold, clammy skin; excessive salivation; and the patient may attempt to remain quiet and motionless. If vomiting occurs, the vomitus should be observed for odor, color, contents (e.g., undigested food), and amount. Noting and recording vomiting patterns, conditions that trigger vomiting, and quality of nausea as described by the patient can be helpful in planning treatment.
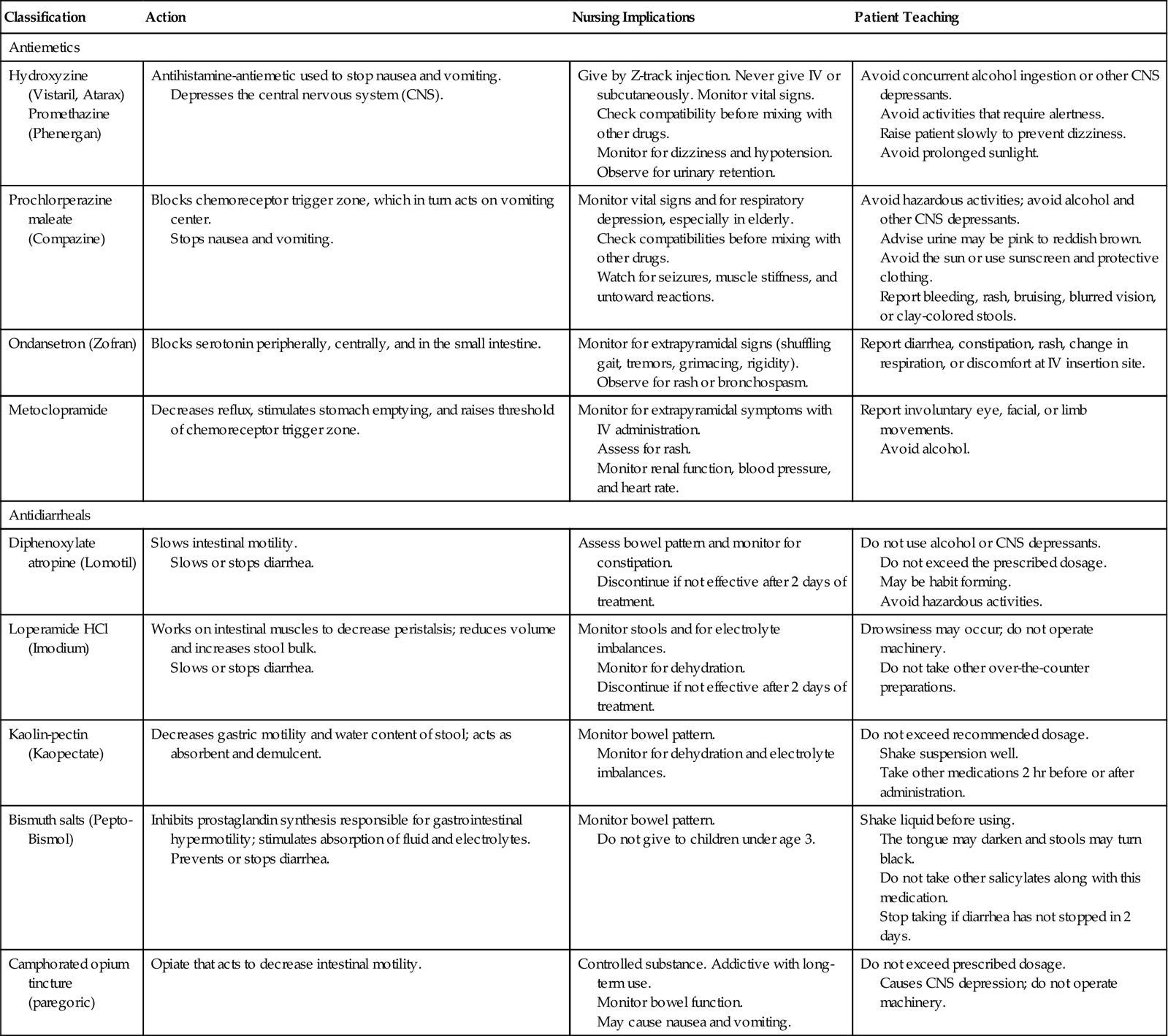
Medical treatment for nausea consists of administering one of the antiemetic drugs (Table 3-2). Antihistamines, sedatives and hypnotics, anticholinergics, phenothiazines, and other drugs are used to control nausea and vomiting. The patient is given nothing by mouth (kept NPO) until vomiting has stopped; afterward, the patient is started back on clear liquids, progressing slowly to a regular diet. Sips of carbonated drinks are usually tolerated well, at first.
 Table 3-2
Table 3-2
Drugs Commonly Prescribed for Vomiting and Diarrhea
| Classification | Action | Nursing Implications | Patient Teaching |
| Antiemetics | |||
| Hydroxyzine (Vistaril, Atarax) Promethazine (Phenergan) | Antihistamine-antiemetic used to stop nausea and vomiting. Depresses the central nervous system (CNS). | Give by Z-track injection. Never give IV or subcutaneously. Monitor vital signs. Check compatibility before mixing with other drugs. Monitor for dizziness and hypotension. Observe for urinary retention. | Avoid concurrent alcohol ingestion or other CNS depressants. Avoid activities that require alertness. Raise patient slowly to prevent dizziness. Avoid prolonged sunlight. |
| Prochlorperazine maleate (Compazine) | Blocks chemoreceptor trigger zone, which in turn acts on vomiting center. Stops nausea and vomiting. | Monitor vital signs and for respiratory depression, especially in elderly. Check compatibilities before mixing with other drugs. Watch for seizures, muscle stiffness, and untoward reactions. | Avoid hazardous activities; avoid alcohol and other CNS depressants. Advise urine may be pink to reddish brown. Avoid the sun or use sunscreen and protective clothing. Report bleeding, rash, bruising, blurred vision, or clay-colored stools. |
| Ondansetron (Zofran) | Blocks serotonin peripherally, centrally, and in the small intestine. | Monitor for extrapyramidal signs (shuffling gait, tremors, grimacing, rigidity). Observe for rash or bronchospasm. | Report diarrhea, constipation, rash, change in respiration, or discomfort at IV insertion site. |
| Metoclopramide | Decreases reflux, stimulates stomach emptying, and raises threshold of chemoreceptor trigger zone. | Monitor for extrapyramidal symptoms with IV administration. Assess for rash. Monitor renal function, blood pressure, and heart rate. | Report involuntary eye, facial, or limb movements. Avoid alcohol. |
| Antidiarrheals | |||
| Diphenoxylate atropine (Lomotil) | Slows intestinal motility. Slows or stops diarrhea. | Assess bowel pattern and monitor for constipation. Discontinue if not effective after 2 days of treatment. | Do not use alcohol or CNS depressants. Do not exceed the prescribed dosage. May be habit forming. Avoid hazardous activities. |
| Loperamide HCl (Imodium) | Works on intestinal muscles to decrease peristalsis; reduces volume and increases stool bulk. Slows or stops diarrhea. | Monitor stools and for electrolyte imbalances. Monitor for dehydration. Discontinue if not effective after 2 days of treatment. | Drowsiness may occur; do not operate machinery. Do not take other over-the-counter preparations. |
| Kaolin-pectin (Kaopectate) | Decreases gastric motility and water content of stool; acts as absorbent and demulcent. | Monitor bowel pattern. Monitor for dehydration and electrolyte imbalances. | Do not exceed recommended dosage. Shake suspension well. Take other medications 2 hr before or after administration. |
| Bismuth salts (Pepto-Bismol) | Inhibits prostaglandin synthesis responsible for gastrointestinal hypermotility; stimulates absorption of fluid and electrolytes. Prevents or stops diarrhea. | Monitor bowel pattern. Do not give to children under age 3. | Shake liquid before using. The tongue may darken and stools may turn black. Do not take other salicylates along with this medication. Stop taking if diarrhea has not stopped in 2 days. |
| Camphorated opium tincture (paregoric) | Opiate that acts to decrease intestinal motility. | Controlled substance. Addictive with long-term use. Monitor bowel function. May cause nausea and vomiting. | Do not exceed prescribed dosage. Causes CNS depression; do not operate machinery. |
Nursing Management
Have the patient lie down and turn his head to one side, or have the patient sit and lower his head between the legs so that vomitus is not aspirated into the respiratory tract. Hold an emesis basin close to the side of the face. Use a cool, damp washcloth to wipe the patient’s face and the back of the neck. Have the patient breathe through the mouth. Provide mouth care after the episode. Sucking on ice chips helps reduce nausea in some patients. A quiet, cool, odor-free environment helps calm nausea. If nausea and vomiting persist, observe for dehydration.
Diarrhea
Diarrhea is defined as the rapid movement of fecal matter through the intestine. During diarrhea, patients absorb nutrients poorly and lose water and electrolytes. These electrolytic substances—especially the potassium needed by the body to prevent alkalosis—are lost in large amounts by patients with diarrhea.
Major diarrhea is related to local irritation of the intestinal mucosa, especially irritation caused by infectious agents, such as Salmonella, Clostridium difficile, and Escherichia coli; by gastrointestinal flu; and by chemicals. Chronic and prolonged diarrhea is typical of such disorders as ulcerative colitis, irritable bowel syndrome, allergies, lactose intolerance, and nontropical sprue. Obstruction to the flow of intestinal contents, such as from a tumor or a fecal impaction, also can produce diarrhea. Considerable potassium and sodium are lost during diarrhea.
To rest the intestines and stomach of a patient with acute diarrhea, limit the intake of foods. Once oral feedings are allowed, begin clear liquids and progress to bland liquids and then solid foods of increased calories and high-protein, high-carbohydrate content. Give rehydrating solutions containing glucose and electrolytes first. Avoid iced fluids, carbonated drinks, whole milk, roughage, raw fruits, and highly seasoned foods.
Medications prescribed for diarrhea depend on the cause of the disorder and the length of time the condition has been present (see Table 3-2). Mild cases usually respond well to kaolin and bismuth preparations (e.g., Kaopectate), which coat the intestinal tract and make the stools more firm. Bismuth subsalicylate (e.g., Pepto-Bismol) is the recommended treatment for “traveler’s diarrhea”; given in advance of travel, bismuth subsalicylate may prevent this type of diarrhea. Diarrhea caused by infections may be treated with drugs that are specific for the causative organism. Depending on the organism responsible, it is sometimes advisable to allow the toxins to be eliminated naturally from the body, and so drugs may not be given initially. Diarrhea is characterized by frequent watery bowel movements, abdominal cramping, and general weakness. Diarrheic watery stools often contain mucus and are blood streaked. It is the consistency rather than the number of stools per day that is the hallmark of diarrhea. In some cases the number can be as high as 15 to 20 liquid stools. If the condition is chronic, the patient can suffer from dehydration, malnutrition, and anemia. Bowel sounds are likely to be gurgling and tinkling sounds that come in waves and are hyperactive. Note and record the number of stools during the shift and the characteristics of each stool and any associated pain.
Nursing Management
Nursing measures for diarrhea aim to provide physical and mental rest, to prevent unnecessary loss of water and nutrients, protect the rectal mucosa, and eventually replace lost fluids. Diarrhea can be associated with nervous tension and anxiety. The patient often is embarrassed by the condition and inconvenienced by frequent trips to the bathroom or the need to request a bedpan. This emotional stress only serves to make the condition worse. Help break the vicious cycle by maintaining a calm and dignified manner, accepting and understanding the patient’s behavior, and providing privacy and a restful environment for the patient.
Excess Fluid Volume
An excessive amount of body water usually occurs first in the extracellular compartment because this is where water enters and leaves the body. When people become ill, they may receive more water than they excrete, which can happen if they receive IV fluid too quickly, are given tap water enemas, or are persuaded to drink more fluids than they can eliminate. If these events happen, the patient will suffer a fluid volume excess. When any of these conditions is present, the patient is likely to suffer from water intoxication.
Impaired elimination, such as occurs in renal failure, is a major cause of fluid volume excess (see Box 3-2). An objective measure of water excess and circulatory overload is the hematocrit. The hematocrit measures the percentage of red blood cells in a volume of whole blood. When fluid volume excess occurs, hypervolemia (excessive blood volume) may also occur. Hypervolemia elevates blood pressure.
Urine concentration provides another clue to the fluid status. Urine concentration is commonly measured by specific gravity and compared with the specific gravity of distilled water, which is 1.000. Urine contains urea, electrolytes, and other substances, so its specific gravity will exceed 1.000 and ranges between 1.003 and 1.030. The average range is 1.010 to 1.025.
Edema
Edema is associated with the retention of water, sodium, and chloride and defined as an accumulation of freely moving interstitial fluid (fluid surrounding cells). Look for puffy eyelids and swollen hands. Edema also can occur in body cavities, as in the peritoneal cavity (ascites) and the cranial cavity. The accumulation of body fluids can affect almost all of the tissue spaces (generalized edema). Alternatively, fluid accumulation can affect a limited area (localized edema). Generalized edema occurs when the body’s mechanisms for eliminating excess sodium fail. Edema becomes life threatening when accumulated fluids overload the circulatory system, as in congestive heart failure, and when fluids accumulate in the lungs, as in pulmonary edema.
Four general causes of edema are (1) a loss of plasma proteins, (2) obstruction of lymphatic circulation, (3) an increase in capillary permeability, and (4) an increase in capillary hydrostatic pressure.
Increased hydrostatic pressure causes pulmonary edema. A loss of plasma proteins decreases osmotic pressure in the vascular system causing fluid to leak from the vessels, leading to edema. A tumor or infection can damage a lymph node, or lymph nodes may be removed during cancer surgery. When lymph nodes are removed during mastectomy surgery, lymph may accumulate in the tissues resulting in lymphedema.
When an inflammatory response or infection occurs, histamine and other chemical mediators are released from the cells involved in the tissue injury. These chemicals cause increased capillary permeability, and more fluid moves into the interstitial spaces. Proteins leak into the interstitial spaces also, decreasing the osmotic pressure in the capillaries. Protein in the interstitial spaces holds fluid there rather than moving that fluid back into the capillaries. When fluid shifts from the vascular space (from the plasma) to the interstitial space, dehydration and hypovolemia (too little blood volume) can occur. The occurrence of the shift of fluid is termed third spacing and may occur with extensive trauma, burns, peritonitis, intestinal obstruction, nephrosis, sepsis, or cirrhosis of the liver in which there is an increase in capillary hydrostatic pressure or increased capillary membrane permeability.
Localized edema often occurs with inflammation. Localized edema usually is nonpitting, does not come and go, and is characterized by tight, shiny skin that is stretched over a hard and red area. Causes of localized edema include trauma, allergies, burns, obstruction of lymph flow, and liver failure.
Dependent edema is noted in the feet, ankles, and lower legs, or in the sacral region of patients confined to bed or chair. Dependent edema is an effect of gravity and therefore can be somewhat relieved by elevating the affected body part 18 inches (or above heart level, when possible) and by repositioning the patient frequently. Pitting edema is common in patients with dependent edema. The name is derived from the fact that a pit or depression can be created by pressing a fingertip against the swollen tissue. To check for pitting edema, press your thumb into the patient’s skin at a bony prominence, such as the tibia or malleolus, and hold for 5 seconds. If the depression, or “pit,” remains for a while after the pressure is released, the patient has pitting edema. Assessing the severity and progress of pitting edema in the feet and ankles (pedal edema) can be done more accurately by rating the findings and comparing assessments from one shift to another (Figure 3-4).
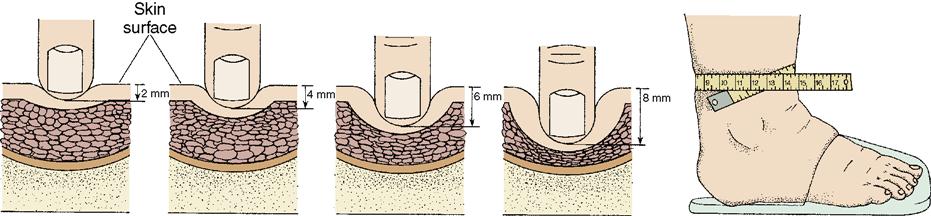
The scale used for rating pedal edema is:
| 1+ | Mild pitting—slight indentation with no swelling of the leg |
| 2+ | Moderate pitting—indentation subsides quickly |
| 3+ | Deep pitting—indentation remains for a short time; leg looks swollen |
| 4+ | Very deep pitting—indentation lasts a long time and leg is very swollen |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







 Nursing Care Plan 3-1
Nursing Care Plan 3-1