Homeostatic alteration in water and sodium is frequently encountered in patients with cancer (Fojo, 2005), and hyponatremia (see Chapter 29) occurs more frequently than hypernatremia (Murphy-Ende, 2006a). Sodium, the major extracellular cation, is crucial for maintaining volume stability. A normal serum sodium level for adults and children is 135 to 145 mEq/L (or mmol/L). The term hypernatremia refers to a serum sodium level greater than 145 mEq/L. Hypernatremia is an infrequent electrolyte abnormality.
Body water and electrolyte balance is the result of numerous physiologic mechanisms. Two major fluid and electrolyte transport systems support sodium and water balance and serum osmolality; passive transport systems and active transport systems. Passive transport systems do not expend energy; these are composed of the processes of diffusion, filtration, and osmosis. Diffusion is based on the principle that molecules and ions flow freely, but randomly, from an area of higher concentration to an area of lower concentration across a biologic membrane. Diffusion depends on the concentration gradients of solutes and is permitted or enhanced by the permeability of a cell membrane. Filtration is the movement of a solute and a solvent from one side of a membrane to another side caused by hydrostatic pressure differences between the two sides of the membrane. This is most noticeable when a patient develops right-side heart failure and blood backs up into the systemic venous circulation, causing venous and capillary hydrostatic pressures to rise. As a result, water moves from the capillaries into the interstitial spaces in the periphery, causing visible edema, or third spacing. Osmosis is the movement of a solvent from an area of lower concentration to an area of higher concentration through a semipermeable membrane that separates the solutions. In this process, fluids move into and out of the cell. The concentration of solutes dissolved in body fluids, therefore, affects the movement of water. This is known as osmotic pressure or water pulling pressure (Metheny, 2000).
The intracellular sodium level is 10 mEq/L, a stark contrast to the extracellular sodium concentration of 135 to 145 mEq/L. Therefore, maintaining sodium in its proper intracellular fluid (ICF) and extracellular fluid (ECF) concentrations requires an active transport system. Energy is constantly expended using cellular adenosine triphosphate (ATP) to power the sodium-potassium pump in every body cell to maintain homeostasis by moving sodium out of the cell and potassium into the cell, against their respective concentrations gradients (Metheny, 2000).
Increased serum sodium is always synonymous with increased serum osmolality. The osmolality of a solution is determined by the number of solute particles per kilogram of water. The following equation defines serum osmolality:
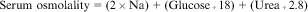
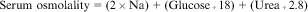
Serum osmolality is regulated tightly between 275 and 290 mOsm/kg, primarily through the influence of vasopressin. Extracellular fluid primarily consists of sodium salts, glucose, and urea. Therefore an increased serum osmolality is not always related to increased sodium levels, because the osmolality may be increased as a result of an increase in other serum solutes. However, under normal conditions, glucose and urea solutes contribute minimally to serum osmolality (Fall, 2000).
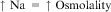
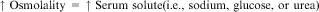
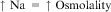
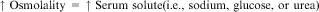
Numerous mechanisms work to maintain body water and sodium balance. Because sodium is largely restricted to the ECF, deficits or excesses of total body sodium are characterized by signs of ECF volume depletion or overload, respectively. The serum sodium concentration does not necessarily change with deficits or excesses of total body sodium. The main determinant of the plasma sodium concentration is plasma water content, which itself is determined by water intake, insensible losses, and urinary dilution (Reynolds et al., 2006). The plasma water, or ECF volume, is regulated by four major control mechanisms: arginine vasopressin (antidiuretic hormone [ADH]), the renin-angiotensin-aldosterone system, the baroreceptor reflex, and volume receptors (Murphy-Ende, 2006a).
Vasopressin system malfunction can produce observable problems, specifically central diabetes insipidus (DI), nephrogenic DI, and syndrome of inappropriate antidiuretic hormone (SIADH). Central and nephrogenic DI may lead to hypernatremia, whereas SIADH leads to hyponatremia.
Under normal conditions, ADH is released from the posterior pituitary when osmoreceptors in the hypothalamus sense an increased serum osmolality. ADH tends to reduce diuresis and increase water retention at the distal tubules of the kidneys, so that fluid will move (by osmosis) from inside the cell into the extracellular spaces; this leaves the cell dehydrated, and the plasma more dilute. Cells in the central nervous system are especially vulnerable to a sudden shift in fluid, which may manifest as neurologic signs or symptoms in the patient (Box 26-1).
BOX 26-1
NEUROLOGIC SIGNS AND SYMPTOMS OF HYPERNATREMIA
• Thirst
• Hypertension
• Low-grade fever
• Mental confusion, disorientation
• Tremors, seizures
• Muscle rigidity and weakness
• CNS irritability: restlessness, agitation
• Muscle cramps, muscle twitching, increased deep tendon reflexes (DTRs)
The thirst center plays an important role in the regulation of serum sodium. The thirst center, which is located in the hypothalamus, consists of neuronal cells, which function as osmoreceptors. Thirst is promoted when the osmotic pressure in the cerebrospinal fluid of the third ventricle or in the circulating extracellular fluid increases. Intracellular dehydration in hypernatremia, and hypercalcemia, causes increased osmolar concentration of the extracellular fluid. Another stimulus that causes thirst is increased production of angiotensin II (Murphy-Ende, 2006a). Thirst is a common symptom in cancer patients and may be related to decreased fluid intake, increased fluid output, poor fluid transport, medications, and mouth breathing. Pathologic conditions that can cause thirst include aldosteronism, chronic glomerulonephritis, DM, DI, hyperparathyroidism, hyperthyroidism, multiple sclerosis, SIADH, primary polydipsia, and terminal condition. (Murphy-Ende, 2006b).
An excess of aldosterone leads to hypernatremia through the complex interaction of hormones and renal function. Under normal conditions the renin-angiotensin-aldosterone system plays an important role in regulating blood volume. Sympathetic stimulation (acting via beta-1 adrenoceptors), renal artery hypotension, and decreased sodium delivery to the distal tubules stimulates the release of renin by the juxtaglomerular cells of the kidney. Renin then travels to the liver and is converted to angiotensin I, which is converted to angiotensin II in the lungs. Angiotensin II travels to the adrenal glands and stimulates the production of aldosterone. Aldosterone is a steroid hormone that acts on the kidneys to increase reabsorption of sodium, which leads to water retention and ultimately to increases in fluid volume and sodium levels. Angiotensin II also stimulates the release of ADH to further enhance fluid retention. The renin-angiotensin-aldosterone cycle is crucial for responding to a low serum sodium level; however, disease states and treatments that lead to an excess of aldosterone may also lead to an excess of sodium. In fact, 65% of patients with primary hyperaldosteronism have solitary aldosterone-producing adrenal adenomas (White, 1994).
EPIDEMIOLOGY AND ETIOLOGY
Four major age-related changes predispose the elderly to dehydration and hypernatremia: a decrease in total body water, an altered sense of thirst, a decrease in the renal urine concentrating ability, and a decrease in the effectiveness of antidiuretic hormone (Arinzon et al., 2005). Frail nursing home residents and hospitalized patients are prone to hypernatremia, because they often depend on others to meet their water requirements (Androgué & Madias, 2000). Hypernatremia occurs in about 1% of all hospitalized patients and in 9% of patients in intensive care (Tisdall et al., 2006). For this reason, monitoring for hypernatremia has been recommended as a measure for ICU quality of care (Polderman et al., 1999), and it may be an indicator of nursing home neglect (Himmelstein et al., 1983).
Sustained hypernatremia can occur only when thirst or access to water is impaired; therefore the groups at highest risk are individuals with altered mental status, intubated patients, infants, and the elderly (Adrogué & Madias, 2000). Oncology patients often have significant water losses, caused by febrile illness, respiratory distress, or gastrointestinal losses, which increase their risk of dehydration and fluid and electrolyte abnormalities.
In the acute care setting, the most common cause of hypernatremia is excessive parenteral administration of sodium solutions (Palevsky et al., 1996). In most patients outside the acute care setting, hypernatremia is caused by water loss in excess of solute where recent fluid loss history may be significant for fevers, sweating, or recent respiratory infection in the setting of altered thirst or altered ability to self-quench a thirst (Fall, 2000). Other causes of hypernatremia include saltwater near-drowning, extreme salt ingestion, and excessive amounts of adrenocortical hormones, such as occurs in Cushing’s syndrome and hyperaldosteronism. Primary hypodipsia may result from destruction of the thirst center or osmoreceptors in the hypothalamus. Central DI also results from a defect in the secretion of ADH and presents in the same scenarios as primary hypodipsia. These conditions have been described in a variety of disorders, including craniopharyngiomas, hypophysectomy, primary or metastatic tumors (most commonly breast and lung) of the hypothalamus, CNS infections or other primary or metastatic brain tumors, cerebrovascular disease/vascular lesions, granulomatous diseases, and head trauma (Kugler & Hustead, 2000; Palevsky et al., 1996).
Essential hypernatremia, a variant of primary hypodipsia, can be seen with tumors that invade the lateral hypothalamus. In this disorder, ADH release as a result of osmotic stimuli is impaired, but ADH response to volume and other nonosmotic stimuli is normal. Patients are euvolemic and asymptomatic (Fig. 26.1) (Kapoor & Chan, 2001).
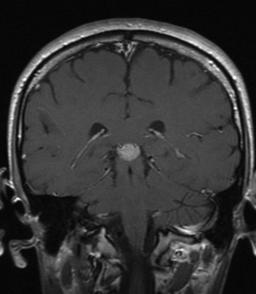 |
| Fig. 26.1A gadolinium-enhanced MRI revealing a pineal tumor (contrast-enhancing lesion seen in the center of the brain) with enlargement of the pituitary stalk. This 23-year-old man’s initial symptom was diabetes insipidus, which appeared 4 months before his diagnosis of germ cell tumor. He has had episodic hypernatremia from his initial presentation and throughout his chemotherapy and radiation. His central DI is managed with DDAVP. |
Hypokalemia and hypercalcemia are the two electrolyte disorders most likely to affect sodium homeostasis, because these conditions reduce the water permeability of the renal collecting ducts by inhibiting adenylate cyclase activation in response to ADH (Kapoor & Chan, 2001). Diseases that may lead to chronic tubulointerstitial disease (e.g., systemic lupus erythematosus, sickle cell anemia, multiple myeloma) and congenital defects may cause nephrogenic DI (Kugler & Hustead, 2000; Palevsky et al., 1996).
Numerous medications may be associated with hypernatremia, including antacids with sodium bicarbonate, antibiotics (e.g., ticarcillin disodium-clavulanate potassium [Timentin]), salt tablets, sodium bicarbonate injections (e.g., during cardiac arrest), intravenous sodium chloride solutions, and sodium polystyrene sulfonate (Kayexalate). Medications that specifically may cause NDI include lithium, amphotericin B, demeclocycline, methoxyflurane, and foscarnet (Kugler & Hustead, 2000; Palevsky et al., 1996). In addition, ingestion of large amounts of licorice leads to increased secretion of aldosterone, causing retention of sodium and water (Shibata, 2000).
RISK PROFILE
• Cancers involving the brain: Hypothalamic (craniopharyngioma, germinoma, meningioma), pituitary (suprasellar extension); other primary or metastatic tumors (i.e., lung, breast, leukemia, lymphoma) of the brain involving the supraoptic or paraventricular nuclei. Malignancy of the kidney or adrenal glands (i.e., renal cell carcinoma, sarcoma), multiple myeloma, and malignant ascites (Reynolds et al., 2006; Kapoor & Chan, 2001; Kugler & Hustead, 2000; Shulz, 1998; Palevsky et al., 1996; White, 1994).
• Conditions: Infants and elderly individuals unable to ingest fluids at will. Congestive heart failure; kidney damage; coma; Cushing’s syndrome; excessive fluid losses; uncontrolled diabetes mellitus that causes solute diuresis; fever; head injury; neurosurgery; chronic hypokalemia or hypercalcemia; pregnancy; psychosis; diabetes insipidus that causes urine-concentrating defects; mental or physical disability; sickle cell nephropathy; central nervous system infections, such as TB, syphilis, mycoses, toxoplasmosis, encephalitis, and chronic meningitis; granulomas (neurosarcoid, histiocytosis, Wegener’s disease); hypertonic dialysis; and electrolyte abnormalities (hypercalcemia, hypokalemia) (Reynolds et al., 2006; Kugler & Hustead, 2000; Schulz, 1998; Palevsky et al., 1996; ).
• Environmental factors: Near-drowning in saltwater, residence in nursing home, severe burns (Schulz, 1998; Himmelstein et al., 1983).
• Foods: Licorice, high-sodium foods, high-protein tube feedings (Achinger et al., 2006; Shibata, 2000; Schulz, 1998; Palevsky et al., 1996).
• Medications: Catecholamines; ethanol; reserpine; morphine; chlorpromazine; phenytoin; lithium carbonate; demeclocycline; methoxyflurane; cisplatin; sodium bicarbonate; sodium chloride; Aldomet; Apresoline; sodium-containing antibiotics/antifungals (e.g., carbenicillin, ticarcillin, and amphotericin B); hypertonic saline administration; foscarnet; clozapine; aminoglycosides; ifosfamide; and analgesics (i.e., analgesic nephropathy) (Reynolds et al., 2006; Kugler & Hustead, 2000; Schulz, 1998; Palevsky et al., 1996; ).
PROGNOSIS
The mortality rate associated with hypernatremia varies widely, depending on the severity of the condition and the rapidity of onset. However, differentiating the contribution of hypernatremia to mortality from the contribution of underlying illnesses is difficult (Adrogué & Madias, 2000). For example, coexisting medical conditions, such as hyperosmolar hyperglycemic nonketotic syndrome, negatively affect mortality. Mortality rates vary from 16% to 60%, depending on the patient population (Palevsky et al., 1996). A retrospective study by Polderman and colleagues (1999) found that hospital-acquired hypernatremia was associated with a higher mortality rate (32%) than hypernatremia present on admission to the ICU (20.3%). This mortality rate may be worse because of delays in diagnosis or inappropriate treatment (Chassagne et al., 2006).
Arinzon and colleagues (2005) evaluated elderly patients in long-term care who developed acute illness. They found that disturbances in sodium concentrations were predictors of bad outcomes. However, in this study the bad outcomes were related to the underlying disease burden and not to the underlying changes in plasma sodium, the time of its development, advanced age, gender, or coexisting changes in the plasma potassium level.
PROFESSIONAL ASSESSMENT CRITERIA (PAC)
| Hypovolemic State | Euvolemic State | Hypervolemic State | |
| Vital Signs | |||
| Temperature | Normal or elevated | Normal | Normal |
| Blood pressure | Decreased | Normal | Increased |
| Pulse | Elevated | Normal | Elevated |
| Respiratory rate | Normal | Normal | Tachypneic |
| Central venous pressure | <2 mm Hg | 2-6 mm Hg | >6 mm Hg |
| History | |||
• Extremely excessive oral and/or IV intake • Fluid losses from GI tract • Intubation or coma, limiting intake • Tumors or trauma to hypothalamus, pituitary, or adrenal glands • Kidney disease or trauma • Mental impairment (psychosis, inability to advocate for self) • Increased serum osmolality (hyperglycemia, high-protein tube feedings) | • Excessive oral and/or IV sodium intake
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree

Get Clinical Tree app for offline access

| ||