CHAPTER 25. Central Venous Access Devices
Care, Maintenance, and Potential Complications
Lisa Gorski, MS, HHCNS-BC, CRNI®, FAAN, Roxanne Perucca, MS, CRNI® and Mark R. Hunter, RN, CRNI®∗
Care and Maintenance, 495
Postinsertion-Related Complications, 505
Nursing Considerations, 512
Summary, 513
Central venous access devices (CVADs) are essential to infusion therapy, needed for short-term administration of irritating IV solutions and medications in acute care settings and for short- and long-term infusion therapy in home health and other outpatient settings. Yet, it is important to recognize that all patients with CVADs, commonly called central lines, are at risk for infection, occlusion, and other complications. Outcomes of hospital-associated central line infections include increased length of hospital stay and increased cost, with non–inflation-adjusted costs varying from $3,700 to $29,000 per infection (Marschall, Mermel, Classen et al, 2008). Less is known about the risk of infection as well as other complications in non-ICU acute care units and in alternative care settings, such as outpatient and home care. The risk of all complications is decreased when hand hygiene is practiced and when central lines are appropriately maintained and continually assessed for potential problems, allowing early intervention.
CARE AND MAINTENANCE
Care and maintenance of CVADs includes ongoing assessment of the catheter site and infusion system, site care, dressing changes, catheter stabilization, changing injection caps, and catheter flushing to maintain patency. Implanted venous access ports must be accessed and regularly assessed, and the infusion nurse must also address pain issues during port access. Other CVAD-related care and maintenance activities include blood sampling from the CVAD for laboratory studies, culturing for suspected infection, and removing the catheter as soon as it is no longer needed. For patients living at home with a CVAD, the nurse must educate the patient to check the device and report any signs of complications, and how to manage activities of daily living with a CVAD in place. The goal of care is safe administration of infusion therapy with the absence of complications and removal of the CVAD when no longer needed.
CVAD ASSESSMENT AND MONITORING
Monitoring of CVADs is encompassed in routine patient assessment and when the CVAD is used for medication or solution administration. The monitoring process includes a review of the patient’s medical record. Laboratory results and vital signs are reviewed for indications of infection such as an increase in the number of white blood cells and elevated temperature. The need for the central line is reviewed daily to ensure prompt catheter removal when the device is no longer needed for infusion therapy. Longer duration of central venous catheter (CVC) placement is associated with increased risk of catheter-associated infection. Catheter duration beyond 5 to 7 days significantly increases the cumulative risk of infection (Safdar and Maki, 2005).
A visual inspection is conducted of the entire infusion system from the solution container, progressing down the administration set to the insertion site of the central line. The solution container and administration set should be visually assessed for clarity of the infusate, for integrity of the system (i.e., leakage), and for an expiration date. The solution container and administration sets should be labeled and changed according to organizational policies and procedures.
Assessment of the central line includes the insertion site, catheter tract, and adjacent skin. The infusion nurse must differentiate between the different types and placements of CVADs, whether nontunneled (e.g., subclavian, external/internal jugular, femoral site insertion), peripherally inserted (PICC), tunneled, or implanted port. Regardless of the CVAD type, on a regular basis the insertion site is assessed for integrity of the dressing; for any signs of infection including erythema, drainage, swelling, or induration; and for any other complications such as catheter migration. For patients with tunneled catheters, the tunnel is also assessed for any pain, swelling, drainage, or erythema. The portal pocket for implanted devices is assessed for the same. Beyond the immediate catheter placement site, the adjacent skin, neck area, and extremity on the placement side of the central line are assessed for signs of venous thrombosis. These signs and symptoms may include arm, shoulder, or neck swelling; chest area, limb, jaw, or ear pain; and dilated collateral veins over the arm, neck, or chest. For patients with peripherally inserted central catheters (PICCs), the mid-arm circumference measurement may be regularly assessed (Gorski, 2005); however, the Infusion Nursing Standards of Practice do not make specific recommendations for this practice (INS, 2006a). Minimally, a baseline arm circumference may be helpful for later comparison, if deep venous thrombosis (DVT) of the upper arm is suspected. Upper extremity DVT is commonly caused by CVADs (Kearon et al, 2008).
The external length of the catheter should be documented at the time of placement for future comparison in monitoring for potential catheter dislodgment and migration. If leakage of parenteral solutions from the catheter insertion site is noted, catheter integrity should be evaluated immediately. Erythema, drainage, and pain, if noted, should be monitored closely. If drainage is present, the amount, color, and consistency should be noted and reported to the physician. A culture of the drainage is generally ordered.
Frequency of site assessment is dependent upon patient condition and organizational policies (for example, with every shift in an acute care setting). For home care patients, the site should be assessed with every home visit and patients should be taught to inspect their site at least every day (Gorski, 2005). Documentation of central line assessment should be completed in the patient’s medical record and should include the parenteral solutions infusing, the location of the device, type of dressing utilized, any complications noted, notification of physicians, and follow-up plans. Patient monitoring in relation to the CVAD is summarized in Box 25-1.
Box 25-1
MONITORING PATIENT WITH A CVAD
1. Know type and features of the CVAD.
• Tunneled
• Nontunneled
• Peripherally inserted
• Implanted port
• Catheter features
• Valved
• Nonvalved
• Number of lumens
2. Review the medical record.
• Laboratory results
• Vital signs
• Continued need for the CVAD
3. Assess the solution container and administration set.
• Clarity of the infusate (e.g., particles)
• Solution expiration date
• Integrity of the system
• Solution container and administration set are labeled
4. Assess the CVAD.
• Insertion site dressing
• Insertion site
• Drainage
• Pain or tenderness
• Erythema or induration
• Swelling
5. Communicate any problems or evidence of complications to the physician and/or the health care team.
6. Document assessment in patient’s record.
• CVAD location
• Type of dressing
• Site assessment
• Presence of any complications
• Any actions taken
SITE CARE AND DRESSING CHANGES
Site care is performed regularly in conjunction with dressing changes. Aseptic technique is required when providing site care; this includes hand hygiene and use of sterile gloves and mask when dealing with CVADs (INS, 2006a). The CDC Guidelines for the Prevention of Intravascular Catheter-Related Infections recommend 2% chlorhexidine as the preferred skin disinfectant (O’Grady, Alexander, Dellinger et al, 2002) for patients older than 2 months of age. Chlorhexidine preparations have residual antibacterial activity that persists for hours after application and maintain activity in the presence of organic matter, and they have no or minimal systemic absorption. In a meta-analysis of studies that compared chlorhexidine gluconate with povidone-iodine solutions for catheter site care, the incidence of bloodstream infections was significantly reduced when chlorhexidine gluconate was used (Chaiyakunapruk et al, 2002). Povidone-iodine, tincture of iodine (rarely used), and 70% alcohol are considered acceptable disinfectants by both INS (2006a,b) and the CDC (O’Grady et al, 2002). Povidone-iodine is generally used for infants. An important aspect of site disinfection is to allow the antiseptic solution to fully dry before applying a sterile dressing.
Maintaining a clean, dry, and occlusive dressing is important to protect the catheter insertion site and reduce the risk for infection (Figure 25-1). A dressing is placed at the time of CVAD insertion and is regularly changed in conjunction with site care, which includes site assessment and skin disinfection. The dressing should also be replaced if it is loosened or dislodged or if blood or drainage is present. Implanted ports require a dressing until the area is healed following the implantation procedure and when accessed with a noncoring needle for intermittent or continuous infusions.
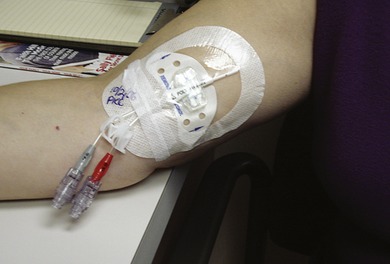 |
| FIGURE 25-1 PICC dressing with StatLock® stabilization. |
For tunneled catheters in the acute care setting, the dressing regimen is generally the same as that for a nontunneled catheter. In the outpatient setting, if the tunnel is well-healed, no dressing may be required. In a randomized, controlled study of 78 patients with cancer, there were no significant differences in sepsis rates among patients with newly inserted tunneled central venous catheters who were randomized either to a gauze dressing or to no dressing starting at 21 days after catheter insertion (Olson et al, 2004). If the patient is not immunosuppressed and healing at the insertion site is complete, site care may be limited to daily inspection and cleansing with soap and water while bathing.
Dressing choices include the transparent semipermeable membrane (TSM) dressing or a simple gauze dressing. In a systematic review of controlled trials that compared the effects of gauze and tape versus transparent dressings, there was no evidence of difference in the incidence of infectious complications between dressing types (Gillies et al 2003). However, the studies were from small samples and there was a high level of uncertainty regarding risk for infection related to the type of dressing. The authors stressed the need for more research.
Based on limited research and evidence that does not support one choice over another, the type of dressing should be selected based on patient preference and needs. The Infusion Nursing Standards of Practice (INS, 2006a) and the CDC guidelines (O’Grady et al, 2002) recommend changing the TSM dressing at least every 7 days and changing gauze dressings every 48 hours. In the event of drainage, site tenderness, other signs of infection, or loss of dressing integrity, the dressing should be changed sooner, allowing the opportunity to closely assess, cleanse, and disinfect the site. Advantages to a TSM dressing include the ability to continually visualize the insertion site without disturbing the dressing as well as cost-effectiveness because of less frequent dressing changes. For example, with home health patients or outpatients, a weekly home or outpatient visit for site care and dressing change is cost-effective and convenient for the patient. However, the patient should be instructed to check the catheter insertion site every day and immediately report any changes, such as redness or drainage or pain at the site.
Gauze dressings are an appropriate choice for the patient who experiences site drainage, perspires excessively, or has a sensitivity reaction to TSM dressings. A gauze dressing is used on a newly placed CVAD when there is bleeding, for at least 24 hours. Often, the transparent dressing is used to secure the gauze dressing. Use of gauze under a TSM dressing is not an uncommon practice, and there is often a misconception, especially among non–infusion nurses, that the dressing is then a TSM dressing and is changed every 7 days. If gauze is used under the TSM dressing, it is considered a gauze dressing and is changed every 48 hours (INS, 2006a).
Antiseptic dressings, such as the chlorhexidine-impregnated foam dressing, are being used more often as research supports their benefits. One such product (BIOPATCH®) is a small, round disk that is placed around the catheter at the exit site, covered with a transparent dressing, and changed every 7 days according to the manufacturer’s recommendations. In a meta-analysis, chlorhexidine-impregnated foam dressings were found to be effective in reducing bacterial colonization at both vascular and epidural sites and were identified with a trend toward reduced catheter-associated bloodstream and central nervous system–related infections (Ho and Litton, 2006). The studies included short-term catheters. In a small, randomized trial of adult patients on a hematology unit undergoing chemotherapy, application of chlorhexidine-impregnated foam dressings reduced the incidence of exit site and tunnel infections but there was no difference in the number of catheter-associated bloodstream infections (CABSIs) (Chambers, Sanders, Patton et al, 2005). There is a lack of research addressing long-term catheters and whether an antiseptic-impregnated dressing has any impact on infection outcome. The CDC did not make recommendations for use of chlorhexidine sponge dressings in 2002 (O’Grady et al, 2002), citing it as an unresolved issue. Evidence-based recommendations from the Infectious Diseases Society of America (IDSA)/Society for Healthcare Epidemiology of America (SHEA) for hospitals recommend their use in the following situations (Marschall et al, 2008):
• The hospital has unacceptably high central line–associated bloodstream infection (CLABSI) rates, despite basic infection prevention measures.
• Patients have limited venous access and a history of CLABSIs.
• There is heightened risk for severe consequences from a CLABSI (e.g., a recently placed prosthetic heart valve).
While the BIOPATCH has been the most commonly used product, there are new and alternative products emerging at the time of publication of this text including the TegadermTM CHG. Silver dressings are being promoted as an alternative antiseptic dressing. However, in an in vitro study, researchers found that the chlorhexidine-impregnated foam dressing had larger zones of inhibition than silver dressings and that it was the only dressing effective against Candida (Bhende and Rothenburger, 2007). Clearly, this is an area of emerging research.
Before changing a dressing, the patient’s medical record should be reviewed for previous history, allergies, condition of the catheter-skin junction site, and dressing material used. The catheter insertion site should be assessed and palpated for redness, tenderness, or drainage. Before proceeding with the dressing change, it is important to teach the patient about the rationale for the dressing change to alleviate concerns and anxiety. Use of a central line dressing kit standardizes the dressing change procedure and improves time efficiency by eliminating the need to gather individual supplies (Figure 25-2). The dressing change procedure begins with removal of the old dressing. This is accomplished by lifting the edge of the dressing beginning at the catheter hub and gently pulling the dressing perpendicular to the skin toward the insertion site. Removing the dressing in this direction will prevent catheter dislodgment should the catheter adhere to the dressing.
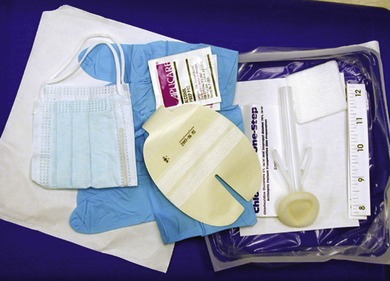 |
| FIGURE 25-2 Central line dressing kit. |
PROCEDURE FOR SITE CARE AND DRESSING CHANGE
BEFORE BEGINNING PROCEDURE
1. Wash hands.
2. Assemble equipment.
3. Don sterile gloves and other personal protective equipment (PPE).
4. Use aseptic technique and observe Standard Precautions throughout procedure.
SITE CARE AND DRESSING CHANGE
1. Remove dressing from VAD insertion site.
2. Inspect site and catheter.
3. Disinfect catheter-skin junction using antiseptic solution.
• Using friction, apply antiseptic solution.
• If using alcohol, apply friction for a minimum of 30 seconds.
• If using chlorhexidine gluconate, use friction according to manufacturer’s labeled use and directions.
• Only one application is necessary.
• Prepared site will be approximately the size of the dressing (i.e., 2- to 4-inches in diameter).
• Allow antiseptic solution to air-dry (do not blow or blot dry).
• Repeat twice as necessary depending on antiseptic solution.
4. Dress access site.
POST-DRESSING CHANGE
1. Discard used supplies.
2. Remove gloves.
3. Wash hands.
4. Label new dressing.
5. Document in patient’s permanent medical record.
When completed, the dressing is labeled with the date, time, and initials of the clinician performing the dressing change. Documentation in the patient’s medical record should include site assessment, skin disinfectant used, dressing material, catheter stabilization method (e.g., manufactured device, sterile tapes, sutures), patient response, and specific nursing actions taken to resolve or prevent adverse reactions.
CATHETER STABILIZATION
Catheter stabilization, also called catheter securement, is increasingly recognized as an important intervention in reducing the risk for phlebitis, infection, catheter migration, and catheter dislodgment. When the catheter is stabilized, there is less movement of the catheter in and out of the insertion site, and the catheter is less likely to be dislodged. Studies examining catheter stabilization have primarily focused on peripheral IV catheters and peripherally inserted central catheters (PICCs). The Infusion Nursing Standards of Practice recommends the use of manufactured catheter stabilization or securement devices as preferred over other methods such as sterile tapes and surgical strips. Such devices consist of an adhesive pad and a mechanism to hold the catheter and administration tubing to the pad (e.g., StatLock® [Bard Access Systems, Covington, GA], Grip-Lok [Zefon International, Ocala, FL]).
Promoted by both health care worker safety issues and clinical research, suturing is no longer recommended for catheter securement because of the risk for health care provider needlestick injuries (OSHA, n.d.). Sutures may also be associated with increased risk for infection. In one prospective randomized study, 170 patients with PICCs were randomized either to sutures or to the placement of a manufactured securement device (Yamamoto, Soloman, Soulen et al, 2002). Significant findings included shorter time to achieve PICC securement (compared to suturing) and fewer PICC-related bloodstream infections in the group using the manufactured securement device; there was one needlestick injury in the suture group.
The use of catheter stabilization devices requires education emphasizing proper use of the product, maintenance of aseptic technique, and catheter stabilization during the placement procedure. There are several commercially available manufactured stabilization devices. Use of the StatLock® stabilization device can be seen in Figure 25-1. It is imperative that the infusion nurse understands the organizational procedures and incorporates specific manufacturer’s directions for use and frequency of device replacement.
IMPLANTED PORT ACCESS
A noncoring needle is used to access an implanted port, and the smallest size needle to accommodate the infusion should be used. Smaller needles prolong the life of the port and will also be less painful during access. Standard needles are never used to access the port as they will core a hole in the silicone septum of the port, potentially leading to leakage. If the port is accessed for a continuous infusion, the Infusion Nursing Standards of Practice (INS, 2006a) recommends that the noncoring needle is changed at least every 7 days. Aseptic technique, including wearing a mask and attention to skin disinfection, is imperative during the port access procedure. A sterile transparent semipermeable membrane (TSM) dressing is used to cover the accessed needle and administration set. Many nurses place gauze to support the wings of the needle and as long as the gauze does not obscure or cover the catheter-skin insertion site, it is not considered a gauze dressing, and the TSM dressing can be changed at least every 7 days according to the Infusion Nursing Standards of Practice (INS, 2006a).
When accessing an implanted port, consideration should be made for use of local anesthesia based upon patient assessment and tolerance to the procedure. While many patients are not concerned about port needle access, some are very anxious and fearful about this procedure. With every patient, assessment of pain, feelings and fear about IV-related procedures, and preferences for pain control are essential and should be incorporated into the care plan. There are an increasing number of effective topical anesthetic creams available. Depending on the product used, the time for effective anesthesia may vary from a few minutes to 1 hour. Other pain management strategies include distraction or relaxation techniques and use of ice over the port site for a few minutes before site preparation and access (Gorski, 2005).
It is critical that patients are educated in daily assessment and monitoring of the site, especially when a noncoring needle is left in place with a continuous infusion. It is not uncommon to deliver infusions of antineoplastic agents, including vesicants, through an implanted port in ambulatory patients at home. Patients should be taught to check the dressing daily, how to dress and undress to avoid pulling at the needle site, and to make sure women’s bra straps do not rub over the accessed area. Instruction should also include immediately stopping the infusion pump and calling the nurse to report any signs or symptoms of pain, burning, stinging, or soreness at the site, and calling the agency immediately if there is any wetness, leaking, or swelling noted at the site (Gorski, 2005). See Box 25-2 for an example of a patient education tool.
Box 25-2
PATIENT EDUCATION TOOL: IMPLANTED PORT BASED INFUSIONS
1. Patient Instructions: Intravenous (IV) Infusion through an Implanted Port
An implanted port is a type of IV that is placed in a large vein in your chest. As you know, your port is placed completely beneath the skin. A special needle is used to enter the port through your skin.
You will be receiving an IV infusion of medication through your port. This means that the needle placed in the port must stay in place until you have received all
of your IV medication. Your medication will be infused with a pump.
Name of medication and dose: ___________________________
Duration of infusion: ___________________________
Size of needle in port: ___________________________
Type of IV pump: ___________________________
Your home care nurse will start the infusion. It is important that you help in making sure that your IV medication is safely infused. While your infusion is running, read these instructions and keep them handy in your home as a reminder.
2. If you feel any of the following, turn off your pump and call your nurse right away:
• Pain
• Stinging
• Burning
• Other soreness at port site
3. Look at the dressing over the port every day. If you notice any wetness, swelling, or redness or if the needle seems to be out of place, turn off your pump and call your nurse right away.
4. Pick up and carry your IV pump every time you get up to walk.
5. Dress and undress carefully to avoid pulling tubing at the port needle.
6. For women: make sure bra straps do not rub over port needle area.
7. If you accidentally drop your pump or if the tubing is accidentally pulled at the needle in your port, call your home care nurse right away for further instructions.
From Wheaton Franciscan Home Health & Hospice, Milwaukee, Wisc.
CATHETER FLUSHING AND LOCKING
The Infusion Nursing Standards of Practice (INS, 2006a) state that vascular access devices shall be flushed at established intervals to promote and maintain patency and prevent the mixing of incompatible medications and solutions. Maintaining patency in a CVAD requires either a continuous infusion or periodic flushing. Catheter flushing presents the most controversy, as research has not demonstrated the optimal flushing solution or frequency. Prevention or treatment of catheter-associated infection using antimicrobial solutions is a controversial area of practice and is addressed in the final paragraphs of this section.
There is considerable variance in the types of flushing solution as well as the amounts and frequency used in clinical practice; in addition, there are many unanswered questions about the “optimal” flush to maintain catheter patency. In 2008 INS released Flushing Protocols, a user-friendly tool to give recommendations for flushing solution and frequency for all venous access devices based on the type of infusion (INS, 2008). The Protocols give direction to both saline flushes and heparin “locking” (Table 25-1). Heparin locking refers to instilling dilute heparin in the unused catheter to prevent blood clotting. The Protocols were based on the Infusion Nursing Standards of Practice and a review of existing literature. The Infusion Nursing Standards of Practice (2006a) recommends the following:
| Min, minimum; NA, not applicable; PIV, peripheral IV; Postadmin, postadministration; Preadmin, preadministration. | ||||||
| ∗All CVADs require a minimum of a 10-mL syringe for flushing and locking. | ||||||
| From Infusion Nurses Society: Flushing protocols, Norwood, Mass, 2008, Author. | ||||||
| Device | Intermittent | Parenteral nutrition | Blood product administration | Blood draws | Flushing with no therapy | Heparin locking |
|---|---|---|---|---|---|---|
| PIV | Min 2 mL | NA | Preadmin 2 mL Postadmin 10 mL | NA | At least q12 hr | NA |
| Midline | Min 3 mL | NA | Preadmin 3 mL Postadmin 10 mL | NA | At least q12 hr | 3 mL of 10 units/mL heparin |
| PICC | Min 5 mL | 5 mL | Preadmin 5 mL Postadmin 10 mL | Predraw 5 mL Postdraw 10 mL | Nonvalved: at least q24 hr Valved: at least weekly | 5 mL of 10 units/mL heparin |
| Nontunneled | Min 5 mL | 5 mL | Preadmin 5 mL Postadmin 10 mL | Predraw 5 mL Postdraw 10 mL | Nonvalved: at least q24 hr Valved: at least weekly | 5 mL of 10 units/mL heparin |
| Tunneled | Min 5 mL | 5 mL | Preadmin 5 mL Postadmin 10 mL | Predraw 5 mL Postdraw 10 mL | Nonvalved: at least 1-2 times per week Valve: at least weekly | 5 mL of 10 units/mL heparin |
| Port | Min 5 mL | 5 mL | Preadmin 5 mL Postadmin 10 mL | Predraw 5 mL Postdraw 10 mL | Accessed nonvalved: at least 1-2 times per week Valved: at least weekly Deaccessed: at least monthly | 3-5 mL of 100 units/mL heparin |
• The flushing volume should be at least twice the internal volume of the central venous access device and injection cap.
• Preservative-free 0.9% sodium chloride flushing solutions should be used to ensure and maintain patency of CVADs at established intervals.
• 0.9% Sodium chloride with preservatives should not be administered to neonates and pediatric patients; if used with adult patients, the volume used should not exceed 30 mL per day.
• Flushing with a heparin solution should occur to ensure and maintain patency of CVADs at established intervals. The heparin concentration should not be in amounts that cause systemic anticoagulation. Concentrations of 1 to 10 units/mL should be used with neonate and pediatric patients.
• Single-use flushing systems are recommended to reduce the risk of contamination and infection.
For central lines, the methods of flushing valved and non-valved CVADs are differentiated in the INS Flushing Protocols (INS, 2008). A valved catheter has an intricate valve(s) within the catheter, either at the distal or at the proximal end of the catheter. When the catheter is not in use the valve reduces the backflow of blood into the catheter and reduces the risk of air embolism by remaining closed. Valved catheters can be found in PICCs, nontunneled and tunneled catheters, and implanted ports. Weekly flushing with preservative-free 0.9% sodium chloride is recommended to maintain catheter patency. For the nonvalved, intermittently used CVAD, INS (2008) recommends “locking” the catheter with low-concentration heparin as follows:
• PICC and nontunneled CVAD: Daily heparin lock with 5 mL (10 units/mL)
• Tunneled CVAD: Twice weekly heparin lock with 5 mL (10 units/mL)
• Implanted port: Monthly heparin lock with 3 to 5 mL (100 units/mL)
Catheter locking to maintain patency remains a controversial and underresearched area. Many acute care hospitals and alternative sites (home care, outpatient) use preservative-free 0.9% sodium chloride without heparin to lock catheters and maintain catheter patency. While such organizations cite internal data supporting the maintenance of catheter patency, there is a lack of published data to support this practice. The move to use preservative-free 0.9% sodium chloride results from concern over heparin-induced thrombocytopenia, concern over heparin supporting microbial growth, periodic heparin supply issues, and use of positive/neutral pressure injection caps/valves.
As some drugs are incompatible with preservative-free 0.9% sodium chloride and/or heparin, it is important for the nurse to know drug and solution compatibilities. Incompatibility is defined as incapable of being mixed or used simultaneously without undergoing chemical or physical changes or producing undesirable effects (Weinstein, 2007). Signs of incompatibility may include visible precipitation, haze, gas bubbles, and cloudiness. Some precipitates may be microcrystalline, smaller than 50 microns, and not apparent to the unaided eye. Refer to Table 25-2 for a list of drug incompatibilities with NaCl, dextrose, and heparin. The “SASH” method (saline-administration-saline-heparin) is used whenever heparin is used for catheter locking and is outlined in Box 25-3.
| ∗Consult with pharmacy for information on drugs not listed in this table. | |||||
| From Infusion Nurses Society: Flushing protocols, Norwood, Mass, 2008, Author. | |||||
| NaCl | DEXTROSE | HEPARIN | |||
|---|---|---|---|---|---|
| Generic | Brand | Generic | Brand | Generic | Brand |
Aldesleukin Amphotericin B cholesteryl sulfate Amphotericin B Amphotericin B lipid complex Amphotericin B liposomal Dantrolene sodium Daunorubicin liposomal Dihydroergotamine mesylate Epoetin alfa Filgrastim Immune globulin Liposomal doxorubicin Methoxamine Mycophenolate mofetil HCl Nitroprusside sodium Norepinephrine bitartrate Oxaliplatin Propafenone Propofol Quinupristin/dalfopristin Trimetrexate glucuronate | Proleukin Amphotec Fungizone Abelcet AmBisome Dantrium DaunoXome D.H.E. 45 Procrit, Epogen Neupogen Gammunex Doxil Vasoxyl CellCept Nitropress Levophed Eloxatin Rythmol Diprivan Synercid Neutrexin | Baclofen Bupivacaine Cladribine Clonidine Dantrolene Daptomycin Dihydroergotamine Interferon alfa-2 Itraconazole Levothyroxine sodium Methadone HCl Phenytoin Streptomycin Tenecteplase Treprostinil sodium | Lioresal Marcaine Leustatin Duraclon Dantrium Cubicin D.H.E. 45 Intron A Sporanox Synthroid Dolophine Dilantin Streptomycin TNKase Remodulin | Alteplase Amikacin Amobarbital Amphotericin B cholesteryl sulfate Amphotericin B deoxychoate Atropine Cefmatazole Chlordiazepoxide Ciprofloxacin Clarithromycin Codeine Cytarabine Daunorubicin HCl Diazepam Doxorubicin HCl Doxycycline hyclate Droperidol Drotrecogin alfa Ergonovine maleate Erythromycin lactobionate Filgrastim Gentamicin sulfate Haloperidol decanoate Haloperidol lactate Hyaluronidase Hydrocortisone sodium phosphate Hydroxyzine HCl Idarubicin HCl Kanamycin sulfate Levofloxacin Levorphanol tartrate Methylprednisolone Mitoxantrone HCl Morphine sulfate Nesiritide recombinant Norepinephrine bitartrate Orphenadrine citrate Pentamidine Phenytoin sodium Polymyxin B sulfate Prochlorperazine edisylate Promethazine HCl Quinupristin/dalfopristin | Activase Sodium Amytal Amphotec Fungizone Librium Cipro Biaxin Tarabine Cerubidine Valium Adriamycin Inapsine Xigris Methergine Neupogen Haldol decanoate Haldol Hydase Solu-Cortef Levaquin Levo-Dromoran Solu-Medrol Novantrone Natrecor Levophed Dilantin Compazine Phenergan Synercid |
Box 25-3
SASH METHOD (SALINE-ADMINISTRATION-SALINE-HEPARIN)
• Perform hand hygiene. • Assemble supplies. • Disinfect catheter injection cap with 70% alcohol using friction. • Connect 0.9% sodium chloride filled syringe to injection cap, maintaining asepsis. • Slowly aspirate until positive blood return is obtained. • If resistance is met or there is no blood return, the infusion nurse should take further steps to assess patency. Never forcibly flush the catheter. • Flush with 0.9% sodium chloride and disconnect syringe. • Disinfect catheter injection cap with 70% alcohol using friction. • Connect IV administration set of medication/solution to injection cap. • Administer medication. • Disconnect medication from injection cap and cover end of IV administration set with sterile cap. • Disinfect catheter injection cap with 70% alcohol using friction. • Connect 0.9% sodium chloride filled syringe to injection cap maintaining asepsis and flush catheter. • Disinfect catheter injection cap with 70% alcohol using friction. • Connect heparin syringe to injection cap maintaining asepsis and lock catheter. • Disconnect syringe from injection cap. • Discard used supplies in appropriate receptacles. • Remove gloves; perform hand hygiene. • Document in medical record. |  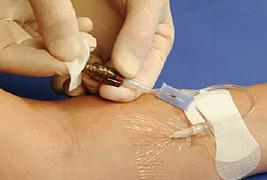 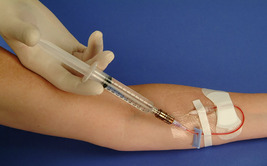   |
Adapted from Infusion Nurses Society: Policies and procedures for infusion nursing, ed 3, Norwood, Mass, 2006b, Author.
If resistance is met or an absent blood aspirate is noted, the nurse should take further steps to assess patency. The catheter should never be forcibly flushed. Pressures in excess of 40 pounds per square inch (psi) may cause catheter rupture with possible embolization (Weinstein, 2007). A wide-barrel syringe (diameter of 10 mL), which exerts less than 10 psi, is recommended for use by many manufacturers of CVADs to avoid excessive pressure and subsequent catheter damage when administering medication and flushing the catheter.
The future will change as ongoing research not only attempts to answer the questions of optimal routine flushing and locking solutions but also discovers novel ways to use flush solutions for eradication of microorganisms within the catheter lumen. Hadaway (2006) proposed an expanded purpose for flushing with antimicrobial solutions (e.g., ethylenediaminetetraacetate [EDTA] and ethanol)—reducing the bacterial biofilm that forms on catheter surfaces. Research is in progress to examine if such novel flush solutions can treat catheter-associated infection and prevent the need for catheter removal and replacement. Some clinicians recommend routine catheter locking with antibiotic or antimicrobial solutions. The 2002 CDC guidelines (O’Grady et al, 2002) state that antibiotic lock solutions should not be routinely used to prevent central line infections but could be used in special circumstances, such as for a patient with repeated infections despite optimal maximal adherence to aseptic technique. The IDSA/SHEA (Marschall et al, 2008) recommends antimicrobial locks for central lines as a preventive strategy in patients with limited venous access and a history of recurrent CLABSIs and in patients with heightened risk for severe consequences from a CLABSI (e.g., recently placed prosthetic heart valve).
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access
