Approximately 99% of calcium in the body is bound to bones and teeth. The remaining 1% is found in the serum. Serum calcium exists in an ionized form, a protein-bound form, and a complexed fraction. About half of the total extracellular calcium is ionized, and half is bound mainly to albumin or, to a lesser degree, to other proteins and anions. The ionized form is the biologic component active in the regulation of intracellular and extracellular functions (McDonnell Keenan & Wickham, 2005).
Hypercalcemia occurs when calcium is mobilized from the bone storage areas to the circulation, where it is reflected in total serum calcium values. Serum calcium values are affected by serum protein levels in that every change of total serum albumin of 1 g/dL is associated with a 0.8 mg/dL change in total calcium (Body, 2004). Therefore, because cancer patients frequently have low albumin levels which, in turn, yield falsely low serum calcium measurements, the serum calcium values may need to be corrected for protein concentration to reflect true serum calcium (Table 23-1).
| Step 1 | Step 2 | Step 3 | Step 4 |
|---|---|---|---|
Sample lab reports: Ca++ = 10 mg/dL Albumin = 1.6 g/dL Increase calcium by 0.8 mg/dL for each 1 g/dL of albumin below normal 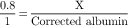 | Correct albumin: Subtract low normal serum albumin level from report level: Normal4.0 g/dL minus—1.6 g/dL reported2.4 g/dL | To correct underreported Ca++: 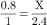 X = 1.92 mg/dL X = 1.92 mg/dL | Corrected Ca++ = Reported Ca++ + Correction factor: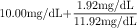 |
Another strategy in these patients is to obtain an ionized calcium measurement, which more accurately denotes hypercalcemia (values greater than 1.35 mmol/L are considered hypercalcemic), but the test is more expensive and less readily available (McDonnell Keenan & Wickham, 2005). In adults, a total serum calcium level (adjusted for protein concentration) greater than 10.5/dL is considered hypercalcemic (Stewart, 2005; Guise & Mundy, 1998). There is no standard hypercalcemia severity scale, but a calcium level greater than 10.5 mg/dL but less than 12 mg/dL may be classified as mild hypercalcemia. Hypercalcemia is considered severe when the calcium level is above 13.5 to 15 mg/dL (Stewart, 2005; Shuey & Brant, 2004; Davidson, 2001).
Normal calcium homeostasis is maintained by negative feedback loop interactions between parathyroid hormone (PTH), calcitriol, an active metabolite of vitamin D (1,25-dihydroxycholecalciferol), and calcitonin. PTH stimulates osteoclastic bone resorption and the release of calcium and phosphate from bone. It stimulates calcium reabsorption and inhibits phosphate reabsorption from the renal tubules. It also stimulates 1,25-dihydroxycholecalciferol production from the kidney, which plays a role in intestinal calcium and phosphate absorption. Calcitonin directly inhibits osteoclastic bone resorption (Guise & Mundy, 1998).
Normal bone undergoes continual and dynamic remodeling by osteoclasts and osteoblasts, which interact in a balance of bone resorption and rebuilding in response to the normal mechanical stressors placed on the bone. The process is orchestrated by numerous growth factors, hormones, and cytokines (Lipton, 2004).
Four primary biologic pathways have been identified in the development of tumor-induced hypercalcemia (TIH). Each pathway may be solely responsible for TIH, or they may interact with varying degrees of complexity (Guise & Mundy, 1998).
Most commonly (in about 80% to 90% of occurrences), TIH is related to the secretion of parathyroid hormone–related protein (PTHrP) by cancer cells (Stewart, 2005; Saunders et al., 2004; Guise & Mundy, 1998). PTHrP is distinctly different from but behaves in a biologically similar manner to PTH (Guise & Mundy, 1998). PTHrP is increased in the presence of factors such as epidermal growth factor, insulin, IGF-I and IGF-II, TGF alpha and beta, angiotensin II, and the src protooncogene. It is decreased by glucocorticoids and 1,25-dihydroxycholecalciferol (Guise & Mundy, 1998). Although PTHrP has been identified in normal tissue and in normal human physiology, tumor-produced PTHrP interacts with PTH receptors in bone and kidney to cause enhanced renal retention of calcium, osteoclast-mediated bone resorption, and increased phosphate excretion in patients with malignancy (Guise & Mundy, 1998). Tumor-secreted PTHrP has been identified in a variety of solid and hematologic malignancies. About 60% of breast cancers secrete PTHrP which, in addition to being a mediator of hypercalcemia, may also play an important part in the pathophysiology of breast cancer metastasis to bone (Guise & Mundy, 1998).
About 20% of TIHs result from factors that increase osteoclastic bone resorption in areas of the bone infiltrated with malignant cells (Stewart, 2005; Saunders et al., 2004). IL-1, IL-6, TNF alpha, and tumor necrosis factor have been identified as agents with the ability to stimulate osteoclastic resorption, with resultant hypercalcemia. Prostaglandin E may play a minor part in metastatic tumor–related bone resorption and hypercalcemia (Guise & Mundy, 1998).
In fewer than 1% of occurrences, TIH may be related to ectopic production of true PTH (Stewart, 2005). Also, some hematologic malignancies (e.g., lymphomas) secrete 1,25-dihydroxycholecalciferol, thereby enhancing osteoclastic bone resorption and increasing intestinal absorption of calcium (Stewart, 2005; Saunders et al., 2004).
EPIDEMIOLOGY AND ETIOLOGY
Malignancy has been reported as the most common cause of hypercalcemia in hospitalized patients. It has also been cited as a frequently undiagnosed and undertreated condition (Lamy et al., 2001). TIH is most commonly observed in breast, lung, and hematologic malignancies, but it has been seen with virtually all malignancies, including melanoma and vulvar, renal, ovarian, aerodigestive, and prostate cancers (Bilenchi et al., 2005; Penel et al., 2005; Wu et al., 2004; des Grottes et al., 2001; Lamy et al., 2001; Guise & Mundy, 1998). Classically, TIH has been observed in 10% to 20% of patients with malignancies (Saunders et al., 2004). However, with the use of bisphosphonates in patients with bone involvement of multiple myeloma and breast cancer, the incidence of hypercalcemia has been empirically observed to be decreasing (although this has not fully been documented by evidence) (Maxwell et al., 2003; Body, 2004; McCloskey et al., 2001).
RISK PROFILE
• TIH occurs in advanced malignancies, most commonly lung, breast, aerodigestive, and hematologic malignancies.
• TIH can be further complicated or influenced by conditions of primary hyperparathyroidism, immobility, dehydration, hyperthyroidism, renal dysfunction, skeletal fracture, acute osteoporosis, vitamin D intoxication, Paget’s disease, tuberculosis, coccidioidomycosis, human immunodeficiency virus (HIV) infection, adrenal insufficiency, and granulomatous disease.
• Infantile hyperphosphatasia can contribute to hypercalcemia in pediatric patients.
• Age and concomitant administration of sedatives or narcotics may enhance the neurologic symptoms associated with hypercalcemia (Stewart, 2005).
• Drugs that can contribute to hypercalcemia include all-transretinoic acid, estrogens and antiestrogens (e.g., tamoxifen), antacids containing calcium, calcium supplements, lithium, and thiazide diuretics.
• An excessive intake of calcium and vitamin D can contribute to TIH in patients whose tumors produce 1,25-dihydroxycholecalciferol (e.g., lymphomas) (Davidson, 2001). Foods rich in calcium and vitamin D include almonds, broccoli, collards, dairy products, fortified orange juice, leafy green vegetables, salmon, sardines, shrimp, tofu, soy beans, and parenteral feedings.
PROGNOSIS
TIH occurs most frequently in patients with advanced malignancies and is considered to be an indicator of a poor prognosis (Penel et al., 2005; Stewart, 2005; Lamy et al., 2001). Treatment of the underlying malignancy is the most important factor in determining the prognosis (Kristensen et al., 1998). In a retrospective study of 84 patients, Siddiqui and colleagues (2002) found that age, presenting symptoms, hemoglobin, platelets, creatinine, and albumin did not predict mortality. Male gender, an underlying diagnosis other than multiple myeloma, and a higher initial serum calcium level at presentation predicted early mortality. Penel and colleagues (2005) found a 72% mortality rate, with a median overall survival of 35 days, in a population of patients with aerodigestive cancers and TIH. Kristensen and coworkers (1998) determined an overall median survival in breast cancer patients of 6.7 months after the first episode of TIH, with higher initial calcium levels being predictive of decreased survival time. Higher calcium levels at presentation may reflect increased severity of the underlying malignancy, which partly explains why successful return to normocalcemia does not seem to improve the prognosis (Kristensen et al., 1998).
PROFESSIONAL ASSESSMENT CRITERIA (PAC)
Presenting symptoms vary according to the serum calcium level and the rate at which the serum calcium became elevated (Clines & Guise, 2005; Stewart, 2005; Body, 2004). For example, a patient who has a rapid rise in calcium to a moderate level of 12.5 mg/dL may show obvious neurologic changes, whereas a patient with a chronic rise in calcium to greater than 14 mg/dL may be relatively symptom free. The symptoms of hypercalcemia and the underlying cause of the TIH may create a cycle effect (Fig. 23.1).
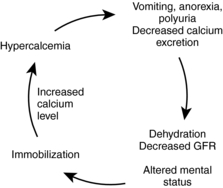 |
| Fig. 23.1Cycle of hypercalcemia. Data from Clines, G. A, & Guise, T. A. (2005). Hypercalcaemia of malignancy and basic research on mechanisms responsible for osteolytic and osteoblastic metastasis to bone. Endocrine-Related Cancer, 12:549-583; Stewart, A. F. (2005). Hypercalcemia associated with cancer. New England Journal of Medicine, 352(4):373-379. |
Classic Symptoms
1. Anorexia
2. Confusion
3. Polydipsia
4. Polyuria
5. Weakness
History
1. Advancing malignant disease
2. Primary parathyroid condition and/or immobility
3. More rarely, history of recent initiation of tamoxifen therapy in breast cancer patients with bone metastasis, creating a “flare” hypercalcemia (Nikolic-Tomasevic et al., 2001)
Vital Signs and Physical Exam
Neurologic and Psychological Changes
A study by Siddiqui and colleagues (2002) found neurologic and psychological changes in about one third of patients at presentation.
1. Altered cognition
2. Disorientation
3. Lethargy
4. Bone pain
5. Muscle fatigue and weakness
6. Hypotonia
7. Nightmares, disturbed sleep
8. Decreased or absent deep tendon reflexes
9. Late symptoms: stupor, coma, and seizures
Renal and Urinary Elimination Symptoms
The same study by Siddiqui and coworkers (2002) found that two thirds of the patients presented with acute renal failure associated with volume depletion.
1. Polyuria
2. Renal calculi
3. Renal failure
Cardiovascular Symptoms
1. Prolonged P-R interval
2. Widened QRS
3. Shortened QT, ST intervals
4. Bradycardia (in rapidly escalating hypercalcemia)
5. Late symptoms: Widened T waves, heart block, ventricular arrhythmias, cardiac arrest, and enhanced sensitivity to digitalis
Gastrointestinal Symptoms
1. Nausea and vomiting
2. Anorexia
3. Polydipsia
4. Increased secretion of gastric acid
5. Constipation/ileus
Diagnostic Tests
1. ECG to detect dysrhythmias
2. Bone scintigraphic scan to determine burden of bony metastasis
Laboratory Tests
1. BUN
2. Creatinine
3. Serum calcium
4. Albumin
5. Total protein
6. Ionized calcium (if doubt exists about validity of total calcium results) (Stewart, 2005)
7. PTH (to rule out co-morbid hyperparathyroidism) (Stewart, 2005)
8. Plasma 1,25(OH)2D (1,25-dihydroxycholecalciferol) in sarcoidosis, granulomatous disorders, and hematologic malignancies (Stewart, 2005)
9. Phosphorus (low with TIH)
10. Potassium (low with TIH)
11. Magnesium (low with TIH) (Milionis et al., 2002)
12. PTHrP (if available)
NURSING CARE AND TREATMENT
The goals of treatment include correction of dehydration, inhibition of bone resorption, an increase in renal excretion of calcium, and treatment of the underlying malignancy. Because antihypercalcemic therapy is considered a palliative therapy that has no ultimate effect on survival (Stewart, 2005), prompt treatment of the malignancy is imperative. However, in cases of advanced, symptomatic malignancy for which no effective cancer treatment is available, a humane approach may be no treatment. Left untreated, patients experience a rapid rise in calcium and resultant unawareness of their condition because of increasing encephalopathy.




1. The most effective approach to long-term management of TIH is medical treatment of the underlying malignancy (surgery, chemotherapy, and/or radiation therapy). Nursing interventions that support these therapies are necessary.
2. Assess vital signs, mental status, neurologic status, hydration status, and GI system status. The frequency of reassessment is determined by the severity of the TIH and the patient’s symptoms. In severe TIH, assessment may need to be done every hour until the patient’s condition becomes stable; frequency then can be reduced to every 4 hours.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access
