The Neurologic System*
Objectives
1. Define the vocabulary particular to problems of the nervous system.
2. Discuss the differences in the action of sympathetic and parasympathetic nervous systems.
3. Identify four specific ways in which a nurse can contribute to preventing neurologic disorders.
5. Become familiar with the techniques used for assessment of the nervous system.
1. Gather a pertinent history for a patient with a nervous system problem.
2. Demonstrate a “neuro” check.
Key Terms
accommodation (p. 488)
aphasia (ă-FĀ-zhă, p. 495)
Babinski’s reflex (p. 480)
calculi (KĂL-kū-lī, p. 494)
caloric testing (kăl-Ō-rĭk, p. 480)
clonus (KLŌ-nŭs, p. 480)
decerebrate posturing (dē-SĔR-ē-brāt, p. 487)
decorticate posturing (dē-KŎR-tĭ-kāt, p. 487)
delirium (dĕ-LĬR-ē-ŭm, p. 495)
dysphagia (dĭs-FĀ-jē-ă, p. 493)
hemiparesis (hĕm-ē-pă-RĒ-sĭs, p. 492)
hemiplegia (hĕm-ĭ-PLĒ-jă, p. 492)
nystagmus (nĭs-TĂG-mŭs, p. 487)
quadriplegic (kwŏd-rĭ-PLĒ-jĭk, p. 492)
tetraplegia (TĔT-ră-PLĒ-jă, p. 492)
 http://evolve.elsevier.com/deWit/medsurg
http://evolve.elsevier.com/deWit/medsurg
Overview of Anatomy and Physiology of the Neurologic System
How is the nervous system organized?
• The functional unit of the nervous system is the neuron, which consists of a cell body, dendrites, and an axon (Figure 22-1). Neurons react to stimuli, conduct impulses, and influence other neurons. There are afferent and efferent neurons.
• The CNS is made up of the brain and spinal cord (Figure 22-2).
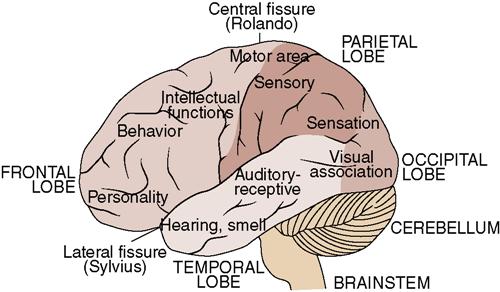
•  The brain is divided into the cerebrum, diencephalon, cerebellum, and brainstem, which each perform various functions. Table 22-1 lists the functions of the various divisions of the brain.
The brain is divided into the cerebrum, diencephalon, cerebellum, and brainstem, which each perform various functions. Table 22-1 lists the functions of the various divisions of the brain.
• The brainstem consists of the midbrain, pons, and medulla.
• The different parts of the brain control various functions (Figure 22-3).
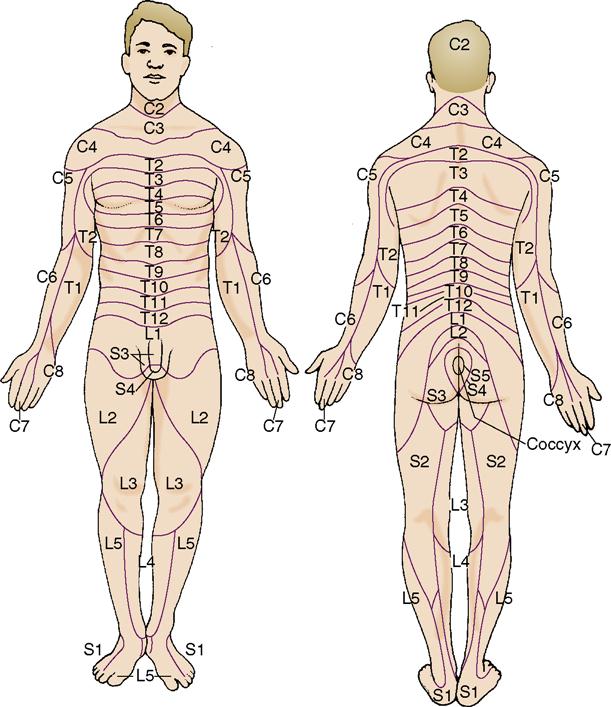
• The PNS is composed of the sensory organs—eyes, ears, taste buds, olfactory receptors, and touch receptors—12 pairs of cranial nerves, and 31 pairs of spinal nerves and ganglia that link the sensory organs, muscles, and other parts of the body to the brain and spinal cord. The distribution pathways of the spinal nerves are called dermatomes (Figure 22-4).
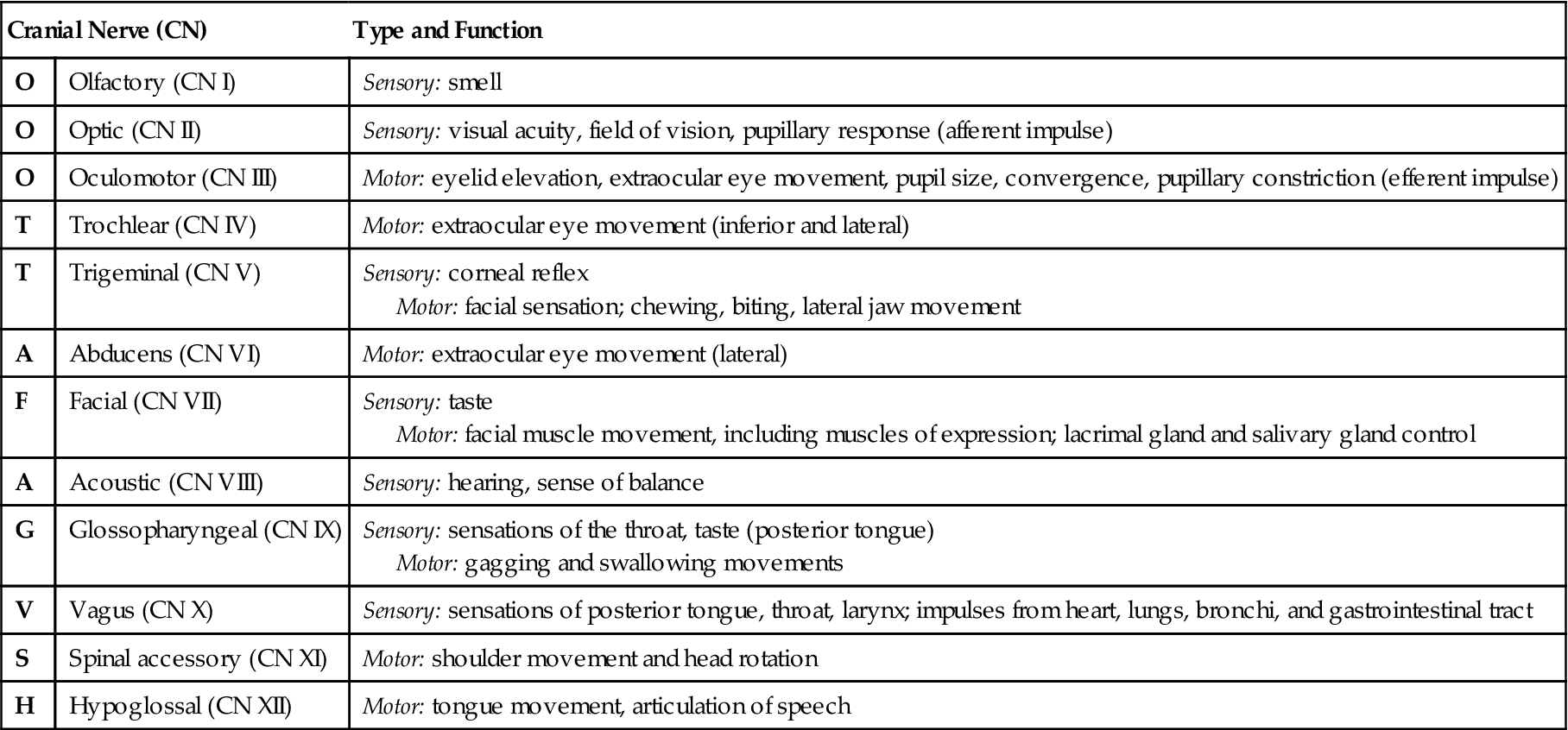
• There are 12 pairs of cranial nerves, some of which  are sensory nerves and others of which are motor nerves (Table 22-2).
are sensory nerves and others of which are motor nerves (Table 22-2).
• The spinal cord extends from the medulla to the level of the first lumbar vertebra.
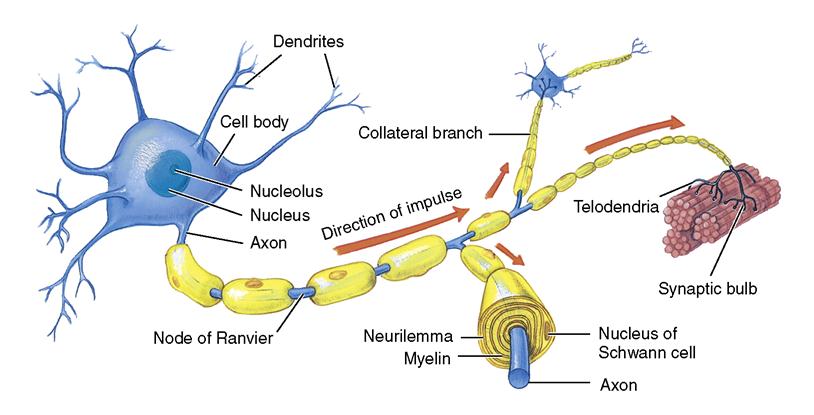
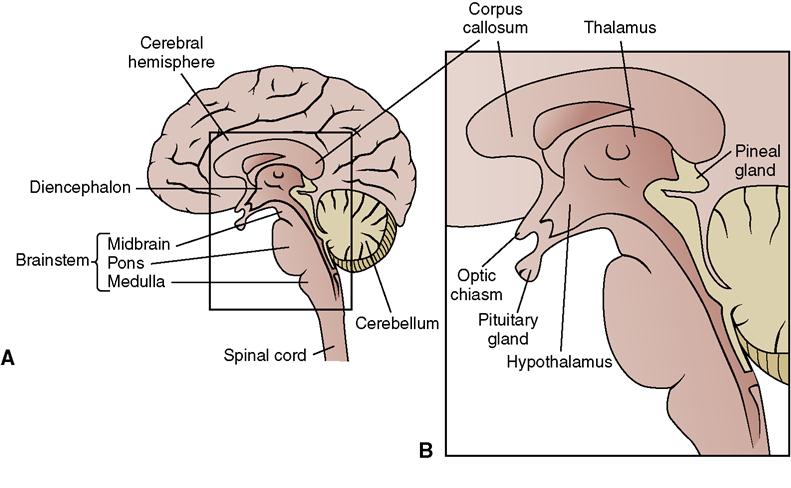
Table 22-1
Functions of the Divisions of the Brain
| Division | Function |
| Cerebrum | Center of intellect and consciousness. |
| Receives and interprets sensory information; controls voluntary movements and certain types of involuntary movements; responsible for thinking, learning, language capability, judgment, and personality; stores memories. | |
| Cerebellum | Responsible for coordination of movement, posture, and muscle tone that are the mechanisms of balance. |
| Diencephalon | Consists of two parts. |
| Thalamus | Relay center between spinal cord and cerebrum. |
| Hypothalamus | Controls body temperature, appetite, and water balance; links nervous and endocrine systems. |
| Brainstem | Consists of three parts. |
| Midbrain | Mediates visual and auditory reflexes; controls cranial nerves III and IV and certain eye movements. |
| Pons | Links connecting various parts of the brain; helps regulate respiration. |
| Medulla oblongata | Contains reticular formation that regulates heartbeat, respiration, and blood pressure; controls center for swallowing, coughing, sneezing, and vomiting; relays messages to other parts of the brain. |
Table 22-2
The Cranial Nerves and Their Functions
| Cranial Nerve (CN) | Type and Function | |
| O | Olfactory (CN I) | Sensory: smell |
| O | Optic (CN II) | Sensory: visual acuity, field of vision, pupillary response (afferent impulse) |
| O | Oculomotor (CN III) | Motor: eyelid elevation, extraocular eye movement, pupil size, convergence, pupillary constriction (efferent impulse) |
| T | Trochlear (CN IV) | Motor: extraocular eye movement (inferior and lateral) |
| T | Trigeminal (CN V) | Sensory: corneal reflex Motor: facial sensation; chewing, biting, lateral jaw movement |
| A | Abducens (CN VI) | Motor: extraocular eye movement (lateral) |
| F | Facial (CN VII) | Sensory: taste Motor: facial muscle movement, including muscles of expression; lacrimal gland and salivary gland control |
| A | Acoustic (CN VIII) | Sensory: hearing, sense of balance |
| G | Glossopharyngeal (CN IX) | Sensory: sensations of the throat, taste (posterior tongue) Motor: gagging and swallowing movements |
| V | Vagus (CN X) | Sensory: sensations of posterior tongue, throat, larynx; impulses from heart, lungs, bronchi, and gastrointestinal tract |
| S | Spinal accessory (CN XI) | Motor: shoulder movement and head rotation |
| H | Hypoglossal (CN XII) | Motor: tongue movement, articulation of speech |
How does the peripheral nervous system interact with the central nervous system?
• The reflex arc is a simple conduction pathway that utilizes a receptor (a sensory neuron centered in the spinal cord) and a motor neuron located in an effector (skeletal muscle). A stimulus travels from the sensory receptor through the spinal cord and back to the effector, causing action (Figure 22-5).
• Reflex arcs are important to most functions of the body, including maintaining an upright position.
• Sympathetic and parasympathetic nerves have opposite effects on many organs (Table 22-3).
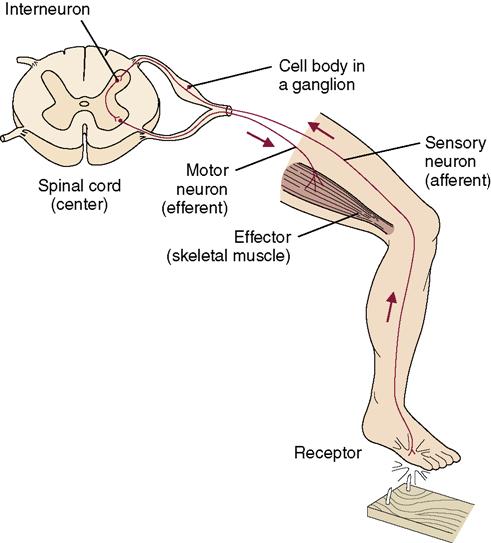
Table 22-3
Autonomic Effects on Various Organs of the Body
| Organ | Effect of Sympathetic Stimulation | Effect of Parasympathetic Stimulation |
| Eye | ||
| Pupil | Dilated | Constricted |
| Ciliary muscle | Slight relaxation (far vision) | Constricted (near vision) |
| Glands: nasal, lacrimal, parotid, submandibular, gastric, pancreatic | Vasoconstriction and slight secretion | Stimulation of copious secretion (containing many enzymes for enzyme-secreting glands) |
| Sweat glands | Copious sweating (cholinergic) | Sweating on palms of hands |
| Apocrine glands | Thick, odoriferous secretion | None |
| Blood vessels | Most often constricted | Most often little or no effect |
| Heart | ||
| Muscle | Increased rate Increased force of contraction | Slowed rate Decreased force of contraction (especially of atria) |
| Coronaries | Dilated (β2); constricted (α) | Dilated |
| Lungs | ||
| Bronchi | Dilated | Constricted |
| Blood vessels | Mildly constricted | Dilated |
| Gut | ||
| Lumen | Decreased peristalsis and tone | Increased peristalsis and tone |
| Sphincter | Increased tone (most times) | Relaxed (most times) |
| Liver | Glucose released | Slight glucose synthesis |
| Gallbladder and bile ducts | Relaxed | Contracted |
| Kidney | Decreased output and renin secretion | None |
| Bladder | ||
| Detrusor | Relaxed (slight) | Contracted |
| Trigone | Contracted | Relaxed |
| Penis | Ejaculation | Erection |
| Systemic arterioles | ||
| Abdominal viscera | Constricted | None |
| Muscle | Constricted (α-adrenergic) Dilated (β-adrenergic) Dilated (cholinergic) | None |
| Skin | Constricted | None |
| Blood | None | |
| Coagulation | Increased | None |
| Glucose | Increased | None |
| Lipids | Increased | None |
| Basal metabolism | Increased up to 100% | None |
| Adrenal medullary secretion | Increased | None |
| Mental activity | Increased | None |
| Piloerector muscles | Contracted | None |
| Skeletal muscle | Increased glycogenolysis Increased strength | None |
| Fat cells | Lipolysis | None |
From Guyton, A.C., & Hall, J.E. (2006). Textbook of Medical Physiology with Student Consult Access (11th ed.). Philadelphia: Elsevier Saunders, p. 754.
How is the central nervous system protected?
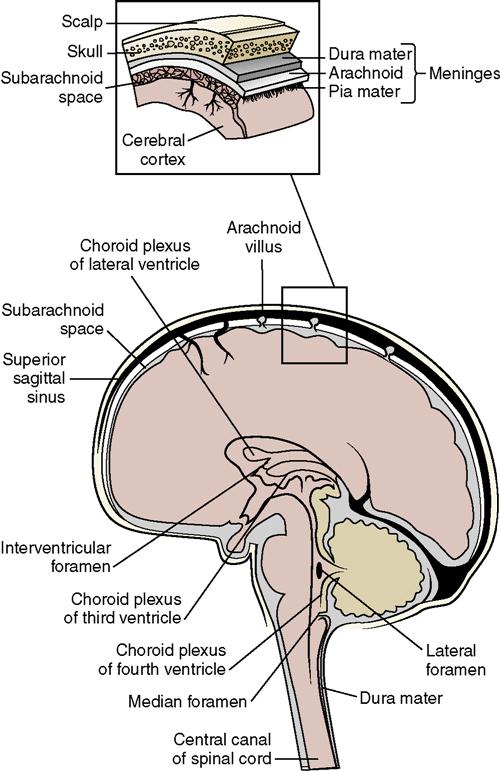
• The subarachnoid space is located between the pia mater and the arachnoid membrane and is where the cerebrospinal fluid (CSF) circulates (Figure 22-6).
• CSF serves to cushion and protect the brain and spinal cord. It is formed continuously as a filtrate from the blood in specialized capillary networks in the choroid plexus, located in the ventricles of the brain. It is reabsorbed by the arachnoid villi of the arachnoid membrane at the same rate at which it is formed. The volume of CSF normally stays constant (see Figure 22-6).
How do nerves conduct impulses?
• The stimulus travels from one neuron to another across a synapse (the space between two neurons).
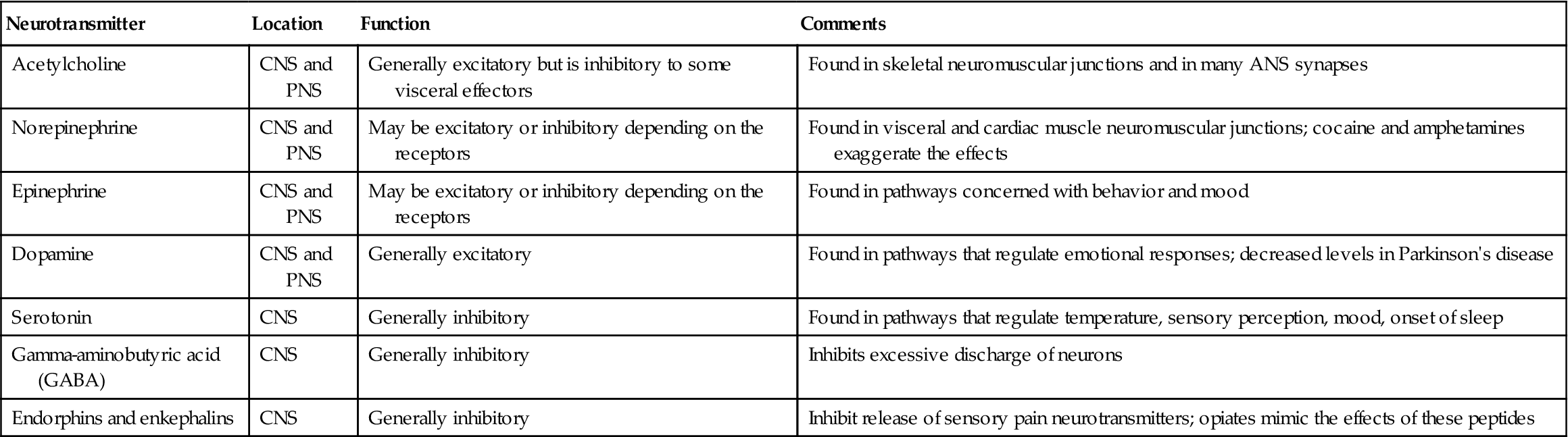
• A neurotransmitter secreted by the neuron is necessary for transmission of an impulse across the synapse (Table 22-4). Acetylcholine, dopamine, and norepinephrine are the major neurotransmitters.
Table 22-4
Neurotransmitters that Affect Transmission of Nerve Impulses
| Neurotransmitter | Location | Function | Comments |
| Acetylcholine | CNS and PNS | Generally excitatory but is inhibitory to some visceral effectors | Found in skeletal neuromuscular junctions and in many ANS synapses |
| Norepinephrine | CNS and PNS | May be excitatory or inhibitory depending on the receptors | Found in visceral and cardiac muscle neuromuscular junctions; cocaine and amphetamines exaggerate the effects |
| Epinephrine | CNS and PNS | May be excitatory or inhibitory depending on the receptors | Found in pathways concerned with behavior and mood |
| Dopamine | CNS and PNS | Generally excitatory | Found in pathways that regulate emotional responses; decreased levels in Parkinson’s disease |
| Serotonin | CNS | Generally inhibitory | Found in pathways that regulate temperature, sensory perception, mood, onset of sleep |
| Gamma-aminobutyric acid (GABA) | CNS | Generally inhibitory | Inhibits excessive discharge of neurons |
| Endorphins and enkephalins | CNS | Generally inhibitory | Inhibit release of sensory pain neurotransmitters; opiates mimic the effects of these peptides |
ANS, autonomic nervous system; CNS, central nervous system; PNS, peripheral nervous system.
From Applegate, E. (2010). The Anatomy and Physiology Learning System (4th ed.). Philadelphia: Elsevier Saunders, p. 177.
What are the special characteristics of the nervous system?
What changes occur in the nervous system with aging?
Causative factors involved in neurologic disorders
Many factors can affect neurologic function, including genetic and acquired developmental disorders. Infections and inflammation, benign and malignant tumors, vascular or neuromuscular degeneration, and metabolic and endocrine disorders all can cause damage to or interfere with normal function of the nervous system. Chemical or physical trauma often causes permanent damage to the brain or spinal cord. Box 22-1 lists by category the most common neurologic disorders in the adult.
Prevention of neurologic disorders
The nervous system coordinates all sensory and motor activities by receiving, interpreting, and relaying messages that are vital to the proper performance of all the body’s activities. Respiratory, circulatory, digestive, and endocrine functions all depend on an intact and normally functioning autonomic nervous system.
Nurses can help prevent neurologic problems in many ways. The goals for Healthy People 2020 encourage health protection through education about safety and responsible self-care.
Teaching the dangers of recreational drug use, such as the possibility of stroke from the use of “crack” cocaine and the potential for accidents while under the influence of some drugs, are other areas for public education. Informing the public about the damaging effect of too much alcohol on brain cells, as well as the increased incidence of alcohol-induced accidents, is another area for education.
Promoting immunizations to protect from tetanus, measles, poliomyelitis, and infectious diseases that may cause high fever and resultant brain damage is an area in which nurses can be effective. Control of hypertension and cholesterol can reduce the number of strokes and the damage they cause. Teaching people to recognize the symptoms of stroke and to seek early treatment may prevent permanent disability.
Pesticides and various chemicals in household and work environments can cause neurologic toxicity and damage. Parkinson’s disease has been particularly more prevalent in farm workers and others who handle pesticides or work where pesticides are frequently used (Science Daily Ed., 2009). Read information on containers of pesticides carefully. Wear long-sleeved clothing and gloves when spraying pesticides in the garden. Spray in quiet, nonwindy conditions. Wash hands and exposed skin with soap and water afterward. Change clothes and launder the clothing worn after pesticide spraying.
Evaluation of neurologic status
The complete neurologic examination performed by the health care provider systematically measures the ability of the body to perform its myriad motor and sensory functions. Mental acuity, memory, and emotional stability also are assessed. A complete neurologic examination is a very long procedure and may be performed in stages over several days. However, gross assessment of the cranial nerves, coordination and balance, muscle strength, and reflexes is standard for every patient with a neurologic complaint. Nurses sometimes do all or part of the examination.
Cranial Nerves
The 12 cranial nerves (CNs; designated as CN I through CN XII) control both sensory and motor activities within various parts of the body (see Table 22-2). Table 22-5 tells how to perform a basic assessment of cranial nerve function.
Table 22-5
Quick Gross Assessment of Major Cranial Nerves
| Cranial Nerve Tested | Quick Method of Testing* |
| Olfactory | Have patient smell a sample of ground coffee, perfume, and pickle juice. |
| Optic | Test visual acuity with a Snellen eye chart. Test visual fields by asking patient to hold the head still and identify items on various areas of a chart. |
| Oculomotor, trochlear, and abducens | Assess pupil size, direct and consensual constriction, and accommodation. Assess the cardinal fields/directions of gaze. |
| Trigeminal | Ask patient to clamp jaw shut, open the mouth against resistance, open the mouth widely, move the jaw from side to side, and make chewing motions. Test sensation by placing a hot and then a cold item on various portions of the face. Ask whether item is warm or cold. |
| Facial | Observe the face for symmetry; ask patient to smile, frown, raise the eyebrows, tightly close the eyes, whistle, show the teeth, and puff out the cheeks. |
| Vestibulocochlear (or acoustic) | Whisper from varying distances and locations behind the patient and ask what was said. Test equilibrium with Romberg’s test: ask patient to stand with feet only slightly apart and eyes closed. See if there is swaying of the body. |
| Glossopharyngeal and vagus | Ask patient to open mouth wide and say “Ah.” Place tongue depressor on first third of tongue to flatten it and observe movement of the uvula and palate. They should rise symmetrically with the uvula at midline. Assess gag reflex by touching each side of the pharynx; there should be a brisk response. Have patient swallow a bit of water. |
| Spinal accessory | Ask patient to elevate the shoulders with and without resistance, turn the head to each side, resist attempts to pull the chin back toward the midline, and push the head forward against resistance. |
| Hypoglossal | Ask patient to open mouth wide, stick out tongue, and rapidly move it from side to side and in and out. Watch for deviation from midline. Apply pressure to cheek and ask patient to push tongue against hand to check for strength. |
*These maneuvers do not check for every function of these cranial nerves, but will provide data indicating whether a more thorough assessment is needed.
Coordination and Balance
This portion of the neurologic examination evaluates functions controlled by the higher centers of the brain, the cerebrum and cerebellum. The patient is asked to stand with her feet together and to close her eyes (Romberg’s test). If the sense of balance is normal, a steady posture will be maintained and there will not be swaying from side to side. Next ask the patient to walk across the room, and assess the gait. Next stand in front of the patient, hold up a finger, and ask the patient to touch your finger and then her own nose; move your finger to different locations in front of the patient. This tests both the ability to follow directions and coordination.
Neuromuscular Function Testing
Groups of large muscles are tested for strength and coordination. Evaluate the patient’s gait while walking, hand grip strength, and arm and leg strength as the patient pushes against resistance. More sophisticated tests include electromyography (Table 22-6 on pp. 481 to 484).
Table 22-6
Diagnostic Tests for Neurologic Disorders
| Test | Purpose | Description | Nursing Implications |
| Skull and spine radiographs | To detect fractures, bone loss, and other bony abnormalities | Radiographs are taken of the desired area from various angles. | Explain that the test is noninvasive. Advise that various positions will need to be assumed. |
| Lumbar puncture (spinal tap) (see Figure 22-11) | To determine if CSF pressure is elevated; to determine if there is a blockage to the flow of CSF; to inject medications; to obtain fluid for chemical analysis and culture | Physician performs a sterile puncture into the arachnoid space, using local anesthetic, between L3 and L4 or L4 and L5; opening pressure is obtained; fluid is aspirated and placed in sterile test tubes labeled 1, 2, and 3. Fluid is analyzed for color, pH, cell count, protein, chloride, and glucose; a culture is usually done. Local anesthetic is used. | Procedure requires a signed consent form. Obtain sterile lumbar puncture tray, local anesthetic, sterile gloves, and tape. Assist patient into position with back bowed, head flexed on chest, and knees drawn up to the abdomen. Patient may be lying or sitting. Assist patient to maintain position and to hold still during procedure. Reassure patient and provide emotional support. Personnel must wear a mask during procedure. Postprocedure: Appropriately label tubes with patient data afterward and transport them to the laboratory immediately. Keep patient flat in bed to reduce headache for 1 hr or longer after procedure and encourage fluid intake unless contraindicated. Observe the site for signs of drainage and inflammation. |
 Electroencephalography (EEG) Electroencephalography (EEG) | To detect abnormal brain wave patterns that are indicative of specific diseases, such as seizure disorder, brain tumor, CVA, head trauma, and infection; to determine cerebral death | May be performed while patient is asleep, drowsy, or undergoing stimulation such as hyperventilation or rhythmic bright light. Test may be done at the bedside or in the EEG lab. Tracing is taken with patient in reclining chair or lying down. Electrodes are applied to the scalp with an electrode paste. Test takes 45 min-2 hr. | Explain purpose of test to the patient; assure her she will not receive an electrical shock, the test is not painful, and that the machine does not determine intelligence or read her mind. Hair should be clean and dry. No sleeping pills or sedatives the night before test; check with physician regarding other drugs to be held; restrict coffee, tea, caffeine, and alcohol for 24-48 hr. If a sleep EEG is ordered, patient may need to be kept up most or all of the night before the test; do not keep NPO, as hypoglycemia can affect the test. Postprocedure: Wash hair to remove the electrode paste. Nothing touches the patient during the procedure. |
| Magnetoencephalography (MEG) | To pinpoint the area of the brain damaged by a stroke, where seizure activity is originating, or location of other injury or disorder | A biomagnetometer machine detects the small magnetic fields generated by neurons. | Postprocedure: Care is the same as for Electroencephalography (see above). |
| Electromyography (EMG) | To measure electrical activity of skeletal muscle at rest and during voluntary activity to determine abnormalities in muscular contraction; helpful in diagnosing neuromuscular, peripheral nerve, and muscular disorders | With the patient sitting in a chair or lying on a table, needle electrodes are inserted in selected muscles. Tracings of electrical activity are taken with the muscles at rest, then with various voluntary activities that produce muscle contraction. The test takes 1–2 hr depending on how many muscles are tested. | Procedure requires a signed consent form. Explain the procedure to the patient; tell her that there is discomfort when the electrodes are placed. Check with physician regarding medications to be withheld; muscle relaxants, cholinergics, and anticholinergics can influence test result. There is no food or fluid restriction. If serum enzymes are ordered, they should be drawn before the EMG. |
| Myelography | To detect spinal lesions, intervertebral disk problems, tumors, or cysts | Contrast medium is injected into the spinal canal, and fluoroscopic examination and radiographs are made. The study is contraindicated if the patient has increased ICP. The patient is placed prone and strapped to the x-ray table for the spinal puncture; as the contrast medium is injected, the table is tilted. After the test, if oil-based medium was used, it is withdrawn. The patient is kept in bed with head of bed elevated 60 degrees or flat depending on the contrast medium used. Procedure takes 1 hr. Rarely performed. | Procedure requires a signed consent form. Explain what to expect. Patient may feel a warm flush when contrast medium is injected. Bowel evacuation regimen may be ordered the night before. Keep NPO for 4-8 hr before procedure. Check for medications to be withheld before and for 48 hr after test. Assess for allergy to iodine or shellfish. Dress in myelogram pajamas; administer preoperative sedative or analgesic if ordered. Postprocedure: Monitor VS q 30 min × 2 hr, then q 1 hr × 4 hr. Assess pulses and sensation in extremities; monitor urinary output; catheterize as ordered if patient cannot void in 8 hr; encourage increased fluid intake. Observe for signs of meningitis. |
| Computed axial tomography (CAT or CT scan) Xenon CT Intrathecal contrast-enhanced CT | To examine the brain from many different angles, obtaining a series of cross-sectional images that provide views from three dimensions To identify hematomas, tumors, cysts, hydrocephalus, cerebral atrophy, obstruction to CSF flow, and cerebral edema | May be done with or without contrast dye enhancement. Patient lies on a narrow table with her head cradled and is moved so that her head is inside the circular opening of the machine. A security strap is wrapped snuggly around her. CT scanner produces a narrow x-ray beam. Various clicking and whirring noises are heard as the machine rotates the scanner for different views. The test takes 45 min-1½ hr. A lumbar puncture is needed for an intrathecal contrast-enhanced CT. | Procedure requires a signed consent form. Explain the procedure and what patient will see, hear, and feel. If contrast dye is used, the patient will feel a warm flush and have a metallic taste in her mouth as it is injected. If contrast dye is used, patient should be NPO for 3-4 hr before test to prevent vomiting. Assess for allergy to iodine or shellfish. Remove all hairpins, jewelry, and metal from the head and neck. Patient may need to be sedated if she is prone to claustrophobia; the table can be uncomfortable for those with arthritis or back problems. She will be able to communicate with the machine’s operator. Postprocedure: Care is the same as for lumbar puncture (see above). |
| Cerebral angiography | To visualize the structure of the cerebral arteries to determine the presence of stricture, tumor, aneurysm, thrombus, or hematoma | Radiopaque liquid is injected through a catheter inserted into the common carotid artery, and a series of radiographs is taken. Fluoroscopy is used during the procedure. Digital subtraction angiography (DSA) is done by using a computer along with the angiography procedure. Test takes 1-2 hr. | Procedure requires a signed consent form. Assess for allergy to iodine and shellfish. Explain procedure; patient will be supine on x-ray table; local anesthetic will be used to introduce the catheter; an IV line will be started in case of need for emergency drugs; patient will feel a flush as the dye is injected. Patient should be NPO 8-12 hr before test; anticoagulants are discontinued beforehand. May be given preprocedure sedative, antihistamine, or steroid to decrease possibility of allergic reaction to dye. Postprocedure: Assess for bleeding at catheter site; assess distal pulses; perform neurologic checks; monitor VS q 15 min × 2 hr, then q 1 hr × 4 hr or until stable. Assess for dysphagia and respiratory distress that could indicate internal bleeding in the neck. Activities are restricted for 24 hr. |
| Radionuclide imaging (brain scan) | To detect an intracranial mass: tumor, abscess, hematoma, or aneurysm | A radioisotope is administered IV. Abnormal tissue usually absorbs more of the isotope than normal tissue. After a 1- to 3-hr waiting period for absorption, a scintillation scanner is used to image the brain. The test takes 30 min-1 hr. | Explain the procedure; patient will sit or lie on a table; the scanner makes clicking noises; the amount of radioactivity is very low and is not dangerous to the patient or others. Patient will need to lie or sit still during the scanning. A drug may be given the night before to block uptake of the radioactive element by the thyroid and salivary glands. There is no food or fluid restriction; no special aftercare. |
| Magnetic resonance imaging (MRI) | To visualize soft tissue without the use of contrast media or ionizing radiation; provides excellent images of soft tissue, eliminating bone; can visualize lesions undetected by CT scan To detect white matter areas in nervous system that represent demyelination, as in multiple sclerosis | An electromagnet is used to detect radiofrequency pulses produced by alignment of hydrogen protons in the magnetic field. Computer produces tomographic images with high contrast of area studied. Cannot be used in the presence of metal. Is quite expensive. A contrast agent often is used for better visualization and definition of specific structures. | Inform patient that the test is painless; no dietary restrictions. Remove all metal objects before test. Screen the patient for hidden sources of metal, such as bullet fragments, iron filings, aneurysm clips. MRI is contraindicated for patients with pacemakers. Patient must be still during test. Explain that body part to be imaged is moved inside large machine; some patients become claustrophobic. Patient will be able to communicate with the machine operator. Requires a signed consent for use of contrast media. |
| Magnetic resonance angiography (MRA) | To evaluate intracranial and extracranial blood vessels and for diagnosing cerebrovascular disease; is rapidly replacing cerebral angiography | Similar to MRI. Uses differing signals of flowing blood to collect data. May be enhanced with use of contrast media. | Explain the need for lying completely still for 1 hr. May require sedation. Screen patient for any metal on body before test. Requires a signed consent for use of contrast media. |
| Magnetic resonance spectroscopy (MRS) | To determine loss of neurons with markers of neuronal integrity (e.g., N-acetyl aspartate) and to study brain diseases | Uses MRI to gather information about chemical composition of brain tissue. | Same as for MRI (see above). |
| Positron emission tomography (PET) | To assess for cell death, damage in brain tissue | Radioactive material is given and provides differing color in areas of cellular activity. | Procedure requires a signed consent form. Explain that two IV lines will be inserted. Patient is to avoid sedatives or tranquilizers before test. Ask to empty bladder before test. Patient may be asked to perform various activities during the test. |
| Single-photon emission computed tomography (SPECT) | To visualize glucose or oxygen metabolism in the brain and to visualize blood flow | Radiolabeled compounds are injected and their single-photon emissions are scanned. Images are made of the accumulated radiolabeled compounds. | Same as for PET (see above). |
| Ultrasound arteriography (Doppler flow studies) | To study flow and determine areas of constriction or obstruction in cerebral arteries. To detect arterial spasm | Noninvasive test. Doppler image scanning device is used with computer to visualize anatomy of major cerebral arteries. | Tell patient that the test is noninvasive and painless. A small Doppler wand is positioned over particular “window” areas on the skull (temples), and with the computer, sound waves are directed so as to produce an image of the interior arteries and their blood flow. No special preparation or aftercare. |
| Carotid duplex Doppler studies | To determine if blood flow in carotid arteries is decreased or blocked | Sound waves graph a picture of blood flow in the carotid arteries. | Explain that the test is noninvasive and painless. Patient will lie flat with head turned to one side and then the other as metal wand passes along the artery. |
| Evoked potential studies | To measure response of the CNS to visual, auditory, or sensory stimulus and helpful in detecting tumor of CN VIII, blindness in infants, or brainstem lesions. Also useful in diagnosing multiple sclerosis | May be done in conjunction with EEG. Electrodes are used to pick up and transmit impulses to a computer while a stimulus is delivered to the patient. Signals are displayed on an oscilloscope, and data are stored for later interpretation. | Explain the procedure to the patient. Visual-evoked potentials: stimulus may be a bright flashing light or checkerboard patterns. Somatosensory-evoked potentials require stimulation of a peripheral sensory nerve with a mild electric shock. Auditory brainstem-evoked potentials use various noises or tone bursts through earphones. Discomfort is minimal. Test takes 30–60 min. |
| Cerebrospinal fluid analysis and culture | To detect abnormalities that are indicative of specific neurologic problems and determine which organism is responsible for infection | CSF is obtained by lumbar puncture. It is analyzed for color, cell count, protein, chloride, and glucose. The fluid is cultured to detect the presence of organisms; if present, an antibiotic sensitivity test is done to determine which drug will best kill the organism. CSF pressure also is measured. Normal CSF values for the adult are: Color: clear Cell count (WBCs): 0-8 mm3 Protein: 14-45 mg/dL Chloride: 118-132 mEq/L Glucose: 40-80 mg/dL Pressure: 70-150 cm H2O or < 20 mm Hg | Follow lumbar puncture procedure. Label the test tubes as 1, 2, and 3 and be certain they are filled with at least 3 mL of CSF in this order. Do not refrigerate the tubes; transport to the laboratory immediately. Maintain Standard Precautions (see Appendix B). |
| PLAC (lipoprotein-associated phospholipase A2; Lp-PLA2) | To detect enzyme marker for increased ischemic stroke risk | This substance is thought to be partly responsible for atherosclerosis formation. | Explain that this is a simple blood test. Results take 7-10 days. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree