Cancer chemotherapy has advanced significantly over the past several decades. However, the use of these agents has been limited by significant cardiac toxicities, including, arrhythmias, ischemia, hypertension, myocarditis, pericarditis, cardiomyopathy, and congestive heart failure (Shanholtz, 2001). The most common and well-known toxicity is congestive heart failure. Congestive heart failure (CHF) is defined as “defective cardiac filling and/or impaired contraction and emptying, resulting in the heart’s inability to pump a sufficient amount of blood to meet the needs of the body tissues or to be able to do so only with an elevated filling pressure” (Colucci & Braunwald, 2004).
The contractile units of the heart muscle are the nonmyocytes and the myocytes (Fig. 19.1). Any injury, regardless of mechanism, leads to impaired contractility. Activation of compensatory mechanisms occurs, primarily through neurohormonal stimulation and cytokine activation, in an attempt to maintain adequate cardiac output to tissues. Patients may have asymptomatic left ventricular (LV) dysfunction for months to years (Moser, 1998). Over time, cellular and biochemical changes occur within the heart that are well recognized, including loss of myofilaments, apoptosis, disorganization of the cytoskeleton, disturbances in calcium homeostasis, and alteration in neurohormonal receptor density, signal transduction, and collagen synthesis (Francis, 2001). The term cardiac remodeling refers to ventricular hypertrophy and changes in the size and shape of the ventricle; these are the hallmarks of chronic heart failure and lead to the symptoms that patients experience.
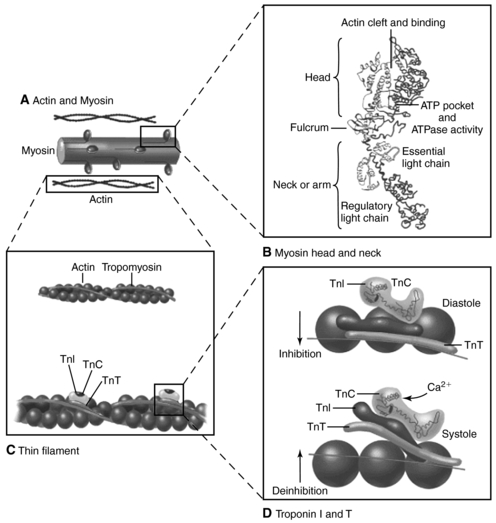 |
| Fig. 19.1Cardiac structure The heart is made up of nonmyocytes and myocytes. A, Nonmyocytes include connective tissue (fibroblasts), vascular smooth muscle cells, and endothelial cells. Nonmyocytes comprise most of the cells of the heart. B, Myocytes are separated by intercalcated disks, which are important for both structural integrity and electrical conduction. These structures are altered by the presence of free oxygen radicals; contractile force is lost, and cell death occurs.(From Zipes, D. P., Libby, P., Bonow, R. O., et al. [2005]. Braunwald’s heart disease: A textbook of cardiovascular medicine, [7th ed.], Philadelphia, W.B. Saunders.) |
Heart failure has many causes, including ischemic heart disease, hypertension, structural abnormalities of the heart, infection, toxins, and idiopathic causes (Box 19-1). In the United States, the most common cause is ischemic heart disease. A recent NHANES epidemiologic study of more than 13,000 patients with no previous history of heart failure estimated that over 60% of heart failure that occurs in the general population can be attributed to coronary artery disease (He et al., 2001). Although toxins, including chemotherapeutic agents, are not a common cause of heart failure in the general population, these drugs have important implications for oncology patients.
BOX 19-1
CAUSES OF HEART FAILURE
Most Common Causes
Coronary artery disease
Hypertension
Valvular heart disease (especially aortic and mitral disease)
Other Causes
Infections: Viruses (including the human immunodeficiency virus), bacteria, parasites
Pericardial diseases
Drugs (e.g., doxorubicin [Adriamycin], cyclophosphamide [Cytoxan], cocaine), alcohol
Connective tissue disease
Infiltrative disease (e.g., amyloidosis, sarcoidosis, hemochromatosis, malignancy)
Tachycardia
Obstructive cardiomyopathy
Neuromuscular disease (e.g., muscular or myotonic dystrophy, Friedreich’s ataxia)
Metabolic disorders (e.g., glycogen storage disease type 2 [Pompe’s disease] and type 5 [McArdle’s disease])
Nutritional disorders (e.g., beriberi, kwashiorkor)
Pheochromocytoma
Radiation
Endomyocardial fibrosis
Eosinophilic endomyocardial disease
High-output heart failure (e.g., intracardiac shunt, atrioventricular fistula, beriberi, pregnancy, Paget’s disease, hyperthyroidism, anemia)
Peripartum cardiomyopathy
Dilated idiopathic cardiomyopathy
In oncology patients, chemotherapeutic agents lead to myocyte damage in specific ways. In anthracycline toxicity, the mechanism of heart failure is thought to be initiated by cell death, or apoptosis, related to the generation of free oxygen radicals (Perik et al., 2004). The free radicals are thought to produce subcellular changes in the myocardium, resulting in myofibril loss with separation of the intercalated disk and dilation of the sarcotubular system, structures essential for normal contraction of the myocyte (Singal & Iliskovic, 1998). Trastuzumab, a monoclonal antibody that binds to the HER2 receptor site, is known to cause heart failure whether used alone or in combination with anthracyclines for metastatic beast cancer. The mechanism is not well understood; however, it is thought to be a complex interaction between inhibition of the cardioprotective effects of the HER2 receptors and ligands with additional stressors and the activation of proinflammatory pathways. These events can lead to early cell death and may play a role in cardiac hypertrophy (Feldman et al. 2000). Although their mechanisms are not understood, other chemotherapeutic agents also can rarely cause cardiotoxicity and ultimately congestive heart failure, including alkylating agents, mitomycin, paclitaxel, and vinca alkaloids (Pai & Nahato, 2000).
EPIDEMIOLOGY AND ETIOLOGY
In a recent metaanalysis of cardiomyopathy in pediatric patients with cancer, the incidence of anthracycline-induced clinical cardiomyopathy ranged from 0 to 16%, and the condition developed at any time during or after therapy (Kremer et al., 2002a). However, in survivors of childhood cancer, the incidence of late cardiac structural abnormalities can be as high as 65% (Lipshultz et al., 1991 and Lipshultz et al., 2005). The incidence of left ventricular dysfunction with the use of trastuzumab alone is 7%; however, when the drug is combined with anthracycline and cyclophosphamide, the incidence can be as high as 28% (Feldman et al., 2000). The combination of anthracycline and mitomycin can result in a 10% incidence of heart failure (Yahalom & Portlock, 2004). Rarely, cardiac tumors can impair ventricular contraction or ventricular filling, leading to symptoms of heart failure.
RISK PROFILE
• Chemotherapy: Single or combination (Table 19-1).
| Generic Name | Trade Name |
|---|---|
| Azacitidine | Vidaza |
| Bevacizumab | Avastin |
| Bicalutamide | Casodex |
| Bortezomib | Velcade |
| Cyclophosphamide | Cytoxan |
| Doxorubicin | Adriamycin, Doxil |
| Estramustine | Emcyt |
| Exemestane | Aromasin |
| Goserelin | Zoladex |
| Idarubicin | Idamycin |
| Ifosfamide | Ifex |
| Imatinib | Gleevec |
| Toremifene | Fareston |
• Mediastinal radiation (Clements et al., 2002).
• Gender: Females are at higher risk (Kremer et al., 2002a), although one study found that males who received concomitant mediastinal radiation also were at higher risk (Clements et al., 2002).
• Higher cumulative dose (Kremer et al., 2002a): A gradual rise in incidence is noted with increased doses; a rate as high as 36% is seen with cumulative doses of doxorubicin over 550 mg/m2 body surface area (Shanholtz, 2001). However, there does not appear to be any absolutely safe dose of anthracycline (Lipshultz et al., 2005)
• Infusion rates: Continuous infusion over 48 to 96 hours reduces the incidence over standard rapid infusion (Singal & Iliskovic, 1998)
• Earlier age at onset.
PROGNOSIS
In the past, the prognosis for heart failure was linked to the severity of functional limitations. The New York Heart Association (NYHA) Classification has been used to predict survival, tailor therapy, and determine readiness for cardiac transplantation. Class I patients are those with left ventricular dysfunction but no symptoms. Class II patients become symptomatic with moderate exertion. Class III patients are symptomatic with minimal exertion, and Class IV patients are symptomatic at rest. This classification system has been very useful to clinicians; however, it does not emphasize the progressive nature of the disease, because a patient’s classification can vary depending on how well controlled the symptoms are. In their most recent guidelines, the American Heart Association and the American College of Cardiology published a staging system for heart failure (HF), which can be summarized as follows (Hunt et al., 2005):
Stage A: High risk for HF without structural heart disease or symptoms
Stage B: Heart disease with asymptomatic left ventricular dysfunction
Stage C: Prior or current symptoms of HF
Stage D: Advanced heart disease and severely symptomatic or refractory HF
In chemotherapy-induced heart failure, three recognized syndromes can occur. The first, acute toxicity causing CHF during the course of chemotherapy, is the rarest. Patients become acutely short of breath with onset of pulmonary edema. This condition is easily reversed with discontinuation of therapy and management of volume overload. However, permanent myocardial injury may have occurred that could lead to late toxicity. (Wouters et al., 2005) The second syndrome, early-chronic progressive heart failure, can occur during therapy or within the first year after completion of therapy. The onset of symptoms is a little more gradual and not as acute. The third syndrome, late-onset chronic progressive heart failure remote, can occur at least 1 year to several years after therapy. Symptoms generally appear gradually. This form has the worst prognosis, because LV dysfunction usually is irreversible and progresses; the 5-year mortality rate is as high as 70%. In women receiving trastuzumab who develop heart failure, the survival rate is 33% (Kannel, 2000), a rate far worse than the survival rate for women with breast cancer and either regional or no metastases (Reis et al., 2000).
PROFESSIONAL ASSESSMENT CRITERIA (PAC)
Signs and Symptoms
1. Dyspnea on exertion (DOE) and fatigue with exertion are typical symptoms (Coates 1997). Other hallmark symptoms include paroxysmal nocturnal dyspnea, edema of the lower extremities, orthopnea, cough, abdominal fullness or swelling, nausea, anorexia, polyuria at night, weight gain, dizziness, and mental status changes.
2. History of chemotherapy (see Table 19-1) with or without mediastinal radiation
3. Hypoxemia: PaO2 less than 92%
4. Tachypnea
5. Tachycardia
6. Signs of elevated cardiac filling pressures, as evidenced by elevated jugular venous pressure, presence of S3 gallop, rales on auscultation, hepatojugular reflex, ascites, and edema.
7. Signs of cardiac enlargement, including laterally displaced or prominent apical pulse and/or murmurs indicative of valvular dysfunction.
Diagnostic Testing
1. Chest x-ray (CXR) films show prominent pulmonary vasculature, cephalization of flow, Kerley’s B lines, pleural effusions, and cardiomegaly (Fig. 19.2).
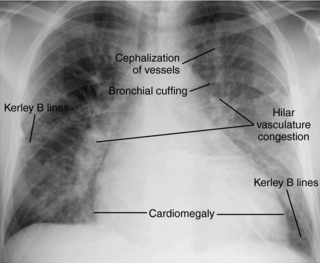 |
| Fig. 19.2Chest x-ray film of congestive heart failure, showing characteristic cardiomegaly, cephalization of pulmonary vessels, hilar congestion, and Kerley’s B lines.(From www.med.yale.edu/intmed/cardio/imaging/findings/pulmonary_edema/index.html. Yale University School of Medicine. Cardiothoracic imaging. [Accessed July 1, 2007.] Copyright 2004, Yale University School of Medicine. All rights reserved.) |
3. An echocardiogram may show increased left atrial size, LV dilation, reduced LV ejection fraction, presence and degree of valvular lesions, or presence of pericardial effusions (Fig. 19.3). Serial evaluation of echocardiograms has been shown to be useful.
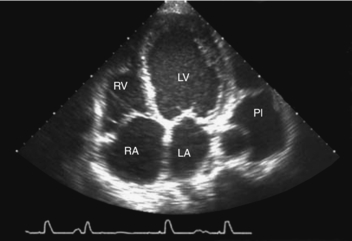 |
| Fig. 19.3Echocardiogram image of dilated cardiomyopathy.(From Zipes, D. P., Libby, P., Bonow, R. O., et al. [2005]. Braunwald’s heart disease:A textbook of cardiovascular medicine, [7th ed.], Philadelphia, W.B. Saunders.) |
4. Hemodynamic monitoring indicates increased pulmonary hypertension. Increased PA pressures with increased pulmonary artery occlusion pressure (PAOP). Depending on the presence of cardiogenic shock, MAP < 65 mm Hg.
5. Although an electrocardiogram (ECG) provides no specific indicators of CHF, the ECG may show atrial and ventricular arrhythmias. Atrial fibrillation is common. The ECG may also show evidence of CAD, MI, or left ventricular hypertrophy. QRS voltage may be low as a result of loss of functional myocardium (Young & Mills, 2004).
6. Multigated blood pool scintigraphy (MUGA) is useful in the evaluation of both right and left ventricular size and function (Dae & Botninick, 1997). It has been used for serial evaluation of ventricular function during and after anthracycline therapy (Lu, 2005).
NURSING CARE AND TREATMENT
Acute Therapy (Hobbs, 2004)
1. Hold chemotherapy, radiation therapy if acute onset.
2. Elevate head of bed to high Fowler’s position.
3. Vital signs—assess for hypotension, tachycardia, and tachypnea.
4. Obtain and interpret O2 saturation values (signs of hypoxia include restlessness, dyspnea, anxiety, and cyanosis).
5. Administer oxygen to treat hypoxia.
6. Maintain venous access via peripheral IV (18 gauge in an adult) or with a venous access device (VAD).
7. Auscultate lung fields for crackles and adventitious breath sounds and assess for decreased chest expansion.
Get Clinical Tree app for offline access



