The Newborn at Risk
Conditions Associated with Gestational Age and Development
Objectives
2. Demonstrate how gestational age is determined.
3. Review the causes of intrauterine growth restriction.
4. Compare and contrast the preterm newborn, the term newborn, and the postterm newborn.
5. Outline the care of the preterm newborn.
6. Explain the factors that predispose the newborn to necrotizing enterocolitis.
7. Describe developmentally supportive care of preterm newborns.
Key Terms
appropriate for gestational age (AGA) (p. 303)
bronchopulmonary dysplasia (BPD) (brŏng-kō-PŬL-mă-nār-ē dĭs-PLĀ-zhă, p. 314)
intrauterine growth restriction (IUGR) (p. 303)
intraventricular hemorrhage (IVH) (ĭn-tră-vĕn-TRĬK-ū-lăr HĔM-ŏr-ĭj, p. 315)
kangaroo care (p. 316)
large for gestational age (LGA) (p. 303)
late preterm (p. 303)
necrotizing enterocolitis (NEC) (NĔK-rō-TĪZ-ĭng ĕn-tă-rō-kō-LĪ-tĭs, p. 314)
patent ductus arteriosus (PDA) (PĂ-tĕnt DŬK-tŭs ăr-tē-rē-Ō-sŭs, p. 314)
postterm (p. 303)
preterm (p. 303)
pulse oximeter (ŏk-SĬM-ĕ-tĕr, p. 314)
respiratory distress syndrome (RDS) (p. 310)
retinopathy of prematurity (ROP) (rĕ-tĭ-NŎP-ă-thē, p. 313)
small for gestational age (SGA) (p. 303)
 http://evolve.elsevier.com/Leifer/maternity
http://evolve.elsevier.com/Leifer/maternity
An “at-risk” newborn is one who is susceptible to illness that may be mild to severe resulting from immaturity, physical disorders, or complications during or after birth. By appropriately assessing the gestational age and size, health care providers can make important clinical judgments about the functioning of the body’s systems and risk factors facing the newborn. Clinical problems may be quite different in a newborn who is born at 33 weeks’ gestation and weighs 1.4 kg (3 lbs) compared with a newborn born at 35 weeks’ gestation and weighing the same. Because of these discrepancies, a more precise system of terminology has evolved. The classification of newborns at birth, based on gestational ages and birth weights, is as follows:
Preterm or premature: An infant born before the end of 38 weeks’ gestation, regardless of weight.
Late preterm: An infant born between 34 weeks’ gestation and 37 weeks, regardless of weight.
Term or full term: An infant born between 38 and 42 weeks’ gestation, regardless of weight.
Low birth weight: Any newborn who weighs less than 2500 g (5.5 lbs) at birth.
Small for gestational age (SGA): Any newborn whose weight is below the 10th percentile (according to intrauterine growth curve), regardless of gestational age.
Appropriate for gestational age (AGA): Any newborn whose intrauterine growth has been normal (according to intrauterine growth curve) for that length of gestation.
Large for gestational age (LGA): Any newborn whose weight is above the 90th percentile (according to intrauterine growth curve), regardless of gestational age.
Intrauterine growth restriction (IUGR): Failure of the fetus to grow as expected during gestation.
Postterm: A newborn born after 42 weeks’ gestation, regardless of weight.
The gestational age can help identify problems to be anticipated. Specific physiologic problems have been linked with specific gestational ages. For example, the preterm infant born before 34 weeks’ gestation will potentially face respiratory distress because of immature lungs and lack of surfactant. When the newborn is identified as postterm (postmature), the potential problem is meconium aspiration syndrome as a result of failing placental function.
Improvement in ultrasound and fetal Doppler technology enhances the ability to detect fetal growth restriction, expands the understanding of the pathophysiologic condition, improves the ability to predict certain neonatal outcomes, and enables preparation for the birth of the newborn who may be physiologically compromised. The rate of preterm births increased from 10.2 in 1987 to 12.3 live births in 2003 and accounts for 70% of neonatal morbidity (Giavratano, 2006). The cause of preterm births is thought to be an interaction of genetic factors with environmental factors, such as maternal illness, that increase risk, and therefore prenatal nurses should take a careful family history prenatally and teach the woman risk reduction strategies such as proper nutrition, prevention of infection, and management of fatigue (Giavratano, 2006).
Gestational Age and Birth Weight
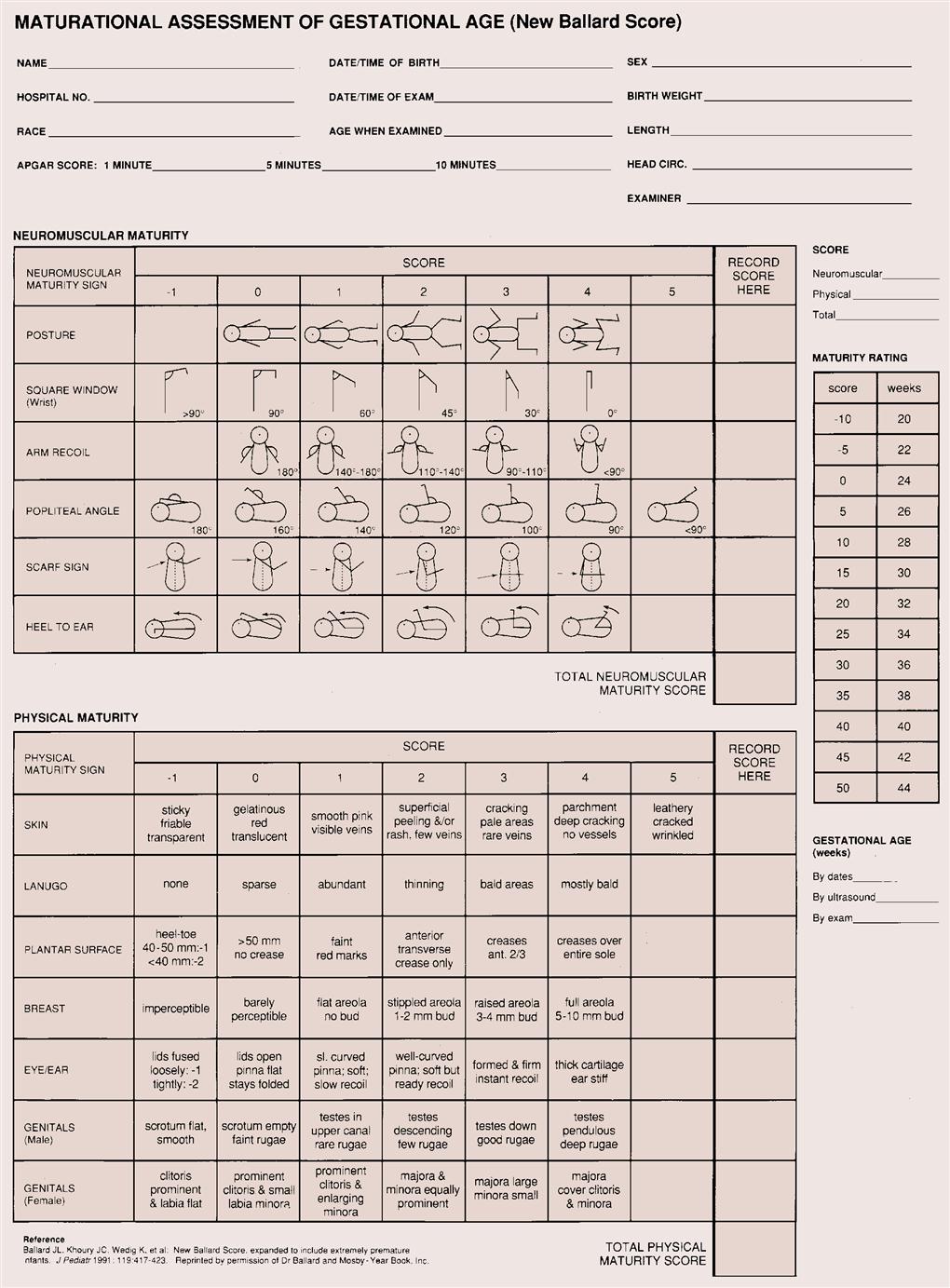
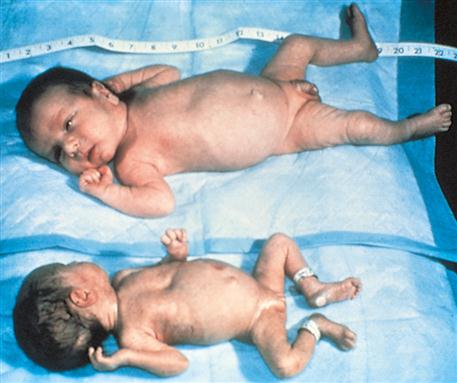
The Ballard scoring system helps determine the newborn’s gestational age (Figure 15-1). This system uses growth and maturity criteria, as indicated, by physical and neuromuscular signs. The examiner assigns a score for each characteristic, such as genital development. It is important to remember that newborns can vary in size and weight yet be the same gestational age (Figure 15-2). The clinical course and strategy for management depend on early identification of gestational age.
Small-for-Gestational-Age (SGA) Newborn
Factors Contributing to the SGA or IUGR Newborn
Factors that contribute to impaired growth may be caused by maternal, placental, or fetal conditions. The most common factors affecting growth restriction are presented in Table 15-1. SGA newborns may be preterm, term, or postterm.
Table 15-1
Factors Contributing to Growth Restriction
| Factor | Examples |
| Genetics | Small parents more likely to have small newborn |
| Maternal disease | Complications related to vascular problems, decreasing blood flow to uterus (advanced diabetes, gestational hypertension, kidney disease) |
| Maternal factors | Smoking, substance abuse |
| Environmental factors | High altitude, radiographs |
| Malnutrition | Maternal starvation |
| Placental factors | Small placenta, placenta previa, decreased placental perfusion |
| Fetal factors | Congenital infections (rubella, toxoplasmosis, syphilis), multifetal pregnancy, chromosomal abnormalities |
Two Types of Growth Restriction
Fetal cellular growth occurs by an increase in cell number and an increase in cell size. If some interference with growth occurs early during the critical period of organ development (organogenesis) of the embryo, fewer cells are formed, organs are small, and the organ weight is subnormal. This type of growth impairment is called symmetric growth restriction because all parts of the body are small (including the brain). Causes of symmetric IUGR include chronic maternal hypertension, severe malnutrition, intrauterine infection, substance abuse, and anemia. Symmetric IUGR can be noted on ultrasound in the first half of the second trimester. Growth interference that begins later in pregnancy does not affect the total number of cells but their size only. This type of growth restriction is asymmetric IUGR. It is most often associated with compromise of uteroplacental blood flow. Causes of asymmetric IUGR include gestational hypertension, smoking (causing vasoconstriction), maternal drug use (such as cocaine), uncontrolled maternal diabetes, and placental infarcts. The newborn weight is below normal, but length and head circumference are normal. Growth-restricted newborns are at risk for hypoxia, acidosis, and death. Table 15-2 lists problems and risk factors for SGA newborns.
Table 15-2
Problems and Risk Factors in Small-for-Gestational-Age Newborns
| Problem | Risk Factor |
| Hypoxia | Lack of adequate oxygen |
| Meconium aspiration | Relaxation of anal sphincter with passage of meconium in utero resulting from hypoxia |
| Hypoglycemia | Poor liver glycogen stores |
| Hypothermia | Diminished subcutaneous fat and large body surface area |
| Intraventricular hemorrhage | Fragile blood vessels |
| Polycythemia | Physiologic response to hypoxia in utero |
| Hypocalcemia | Calcium depletion caused by birth asphyxia |
| Maternal factors that cause low birth weight from hypoxia stress | Vascular complications caused by gestational hypertension, advanced diabetes, smoking, poor nutrition, use of drugs |
SGA and IUGR newborns are those who are at or below the 10th percentile of weight for the gestational age (Gabbe, Niebyl, & Simpson, 2007). The incidence of SGA newborns is 3% to 10% of all pregnancies. The mortality rate of SGA newborns is higher than that of normal newborns. Prenatal Doppler studies to assess flow abnormalities of fetal or placental vessels and an antenatal biophysical profile should be performed weekly, especially if oligohydramnios is present, because elective delivery may be necessary.
Physical Appearance
On first inspection, many of the SGA newborns show physical characteristics that suggest impaired intrauterine growth. The head might seem large, but its circumference is normal or near normal. The chest and abdominal circumferences are reduced because of decreased subcutaneous fat. The adipose tissue and muscle mass over the cheeks, buttocks, and thighs are diminished. Because the body length is usually normal, the newborns appear thin and long. They often have thin faces, resembling little old people. The skull’s sutures are often wide apart as a result of impaired bone growth; thus, the newborn’s fontanelles are often large and somewhat sunken.
Behavior
Many SGA newborns are more active than expected for their size. This observation is especially true when comparing them with preterm (AGA) newborns. The cry of an SGA newborn is vigorous and may be impressive in relation to size. The SGA newborn usually has a strong sucking response compared with the weaker sucking response in the preterm newborn. The SGA newborn often eats well and gains weight more quickly than the preterm newborn. The overall impression of vigor and well-being may be misleading. The newborn’s wide-eyed, alert facial expression may be caused by chronic hypoxia (lack of oxygen). The SGA newborn is also prone to hypoglycemia because the glycogen stores are often inadequate or depleted. Because of such potential complications, the health care provider needs to observe the SGA newborn closely and intervene as indicated.
Assessment and Management
Because the causes of IUGR are so varied, the care of the SGA newborn must be adapted to meet the specific problems that the newborn demonstrates. These newborns need to be carefully examined for congenital anomalies and monitored for hypoglycemia. Calorie needs are higher for these newborns than for an AGA newborn. Often their care is similar to that of the preterm newborn (Nursing Care Plan 15-1).
Simple measures during prenatal care, such as elimination of cigarette smoking, alcohol, and illicit drugs; improved nutrition; and eradication of genitourinary tract infection would lower the number of infants born with IUGR.
Large-for-Gestational-Age (LGA) Newborn
The newborn whose birth weight is at or above the 90th percentile typically weighs 4000 g (8 lbs, 13 oz) or more at birth and is referred to as LGA. The LGA infant may be term infants, postmature, or may be born to a mother of normal weight and health. The diabetic woman who does not have advanced vascular changes is likely to have a large newborn (macrosomia) because of high glucose levels that cross the placenta. The large newborn often poses a mechanical problem during delivery and, after a prolonged labor, is usually delivered by cesarean birth. If delivered vaginally, the newborn may incur an injury (e.g., fractured clavicle or central nervous system trauma) during the birth process. LGA newborns are often sluggish, hypotonic, and hypoactive at birth; they may have hypoglycemia or polycythemia. If the newborns have diabetic mothers, they are prone to hypoglycemia; therefore, they need frequent blood glucose level assessments. The newborn of a diabetic mother is discussed in Chapter 16.
Postterm Newborn
The postterm newborn (postmature) is a newborn born after 42 weeks’ gestation. Postterm births occur in about 12% of all pregnancies (Lee, 2008). Many are large. The placenta may not function as well after 40 weeks’ gestation because placental insufficiency develops. Because of the progressive degeneration of the placenta, the fetus does not receive adequate oxygen and nutrients. During labor, poor oxygen reserves and limited placental function place the newborn at risk for meconium aspiration. The fetus may use up some of the subcutaneous fat and, when born, will look thin with loose skin. The skin is often cracked and dry, with a parchment-like texture from the decrease in
protective vernix caseosa. The postterm newborn is at risk for hypoxia, meconium aspiration, hypoglycemia, polycythemia, cold stress, and asphyxia. The infant may appear wide-eyed and alert because of chronic intrauterine hypoxia. There is little lanugo or vernix, and the infant has long fingernails. Health care providers should closely monitor all postterm newborns for hypoglycemia, cold stress, and airway obstruction caused by meconium aspiration, regardless of size (Table 15-3).
Table 15-3
Problems Related to Large-for-Gestational-Age and Postterm Newborns
| Cause | Problem | Neonatal Response | Nursing Interventions |
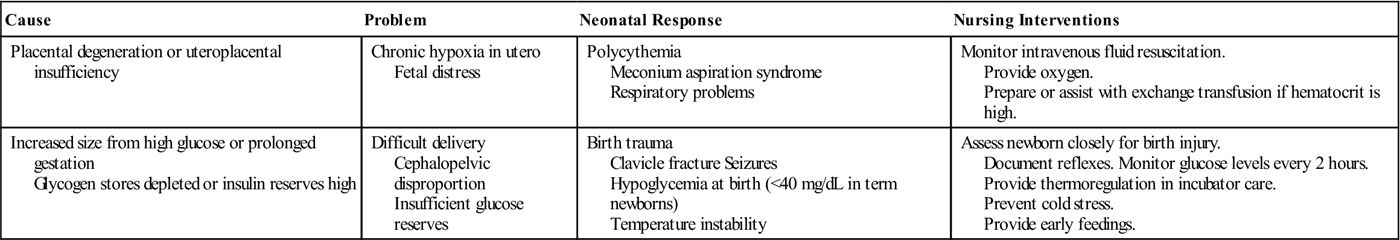
| Placental degeneration or uteroplacental insufficiency | Chronic hypoxia in utero Fetal distress | Polycythemia Meconium aspiration syndrome Respiratory problems | Monitor intravenous fluid resuscitation. Provide oxygen. Prepare or assist with exchange transfusion if hematocrit is high. |
| Increased size from high glucose or prolonged gestation Glycogen stores depleted or insulin reserves high | Difficult delivery Cephalopelvic disproportion Insufficient glucose reserves | Birth trauma Clavicle fracture Seizures Hypoglycemia at birth (<40 mg/dL in term newborns) Temperature instability | Assess newborn closely for birth injury. Document reflexes. Monitor glucose levels every 2 hours. Provide thermoregulation in incubator care. Prevent cold stress. Provide early feedings. |
Preterm Newborn
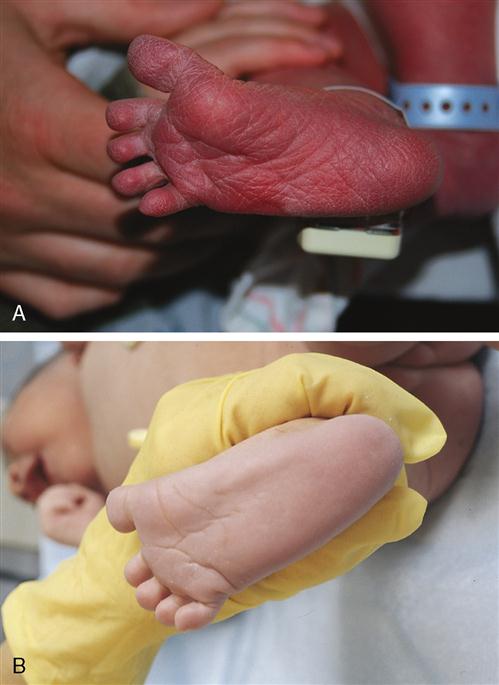
The most common factor associated with neonatal death is prematurity. The preterm newborn is defined as one who is born at less than 38 weeks’ gestation. Preterm births have risen from 9.4% of live births in the United States in 1981 to 12.3% in 2008 (NCHS, 2010). The majority of the increase was in late preterm births, formerly known as “near-term births” (Engel, 2007). Late preterm infants may look more like full-term infants, but they are metabolically and physiologically immature and have needs similar to the more preterm infant. However, a newborn may be born alive much earlier than 34 weeks’ gestation and still live, but he or she may be poorly equipped to survive outside of the uterus. Specific organ systems may not be developed enough to function at a level necessary to maintain life and promote growth. The preterm newborn’s skin is often wrinkled and delicate and is usually covered with lanugo. The plantar creases on the soles of the feet begin to develop from the toes toward the heel during fetal development, and the term infant has deep creases on the soles of the feet extending from the toes to the heel. In contrast, the preterm has few creases on the sole of the foot (Figure 15-3). The preterm newborn is thin, has little subcutaneous fat, and has prominent fontanelles and suture lines of the skull. Depending on the gestational age, the cry can be very weak, matching the frail appearance, and the body appears limp, with poor muscle tone. Extremities are in extension rather than flexion, exposing a larger body surface area and increasing the risk of hypothermia (Figure 15-4).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Nursing Care Plan 15-1
Nursing Care Plan 15-1