CHAPTER 15. Pharmacology
Michelle S. Turner, PharmD, BCPS and Judy Hankins, BSN, CRNI®∗
Considerations for Intravenous Drug Administration, 263
Drug Administration, 266
Calculations, 270
Drug Classifications, 272
Summary, 298
The intravenous (IV) route is a common route of drug administration in many health care settings. IV drugs were once reserved for emergency situations or critically ill patients. They are now routinely used throughout the hospital, as well as in outpatient, long term care, and home care settings. Many of the quality initiatives as well as quality core measures include medication administration (e.g., pneumonia, acute myocardial infarction [MI], heart failure, and surgical infection prevention). There is a great deal of interest in safe medication administration, including The Joint Commission’s National Patient Safety Goals.
With the dramatic increase in the use of the IV route for drug delivery, nurses have assumed increased responsibility. Nurses administer IV drugs, monitor patients’ responses to pharmacological agents, and instruct patients regarding the prescribed drug therapy. Nurses must therefore have a thorough understanding of the principles of IV drug administration. This knowledge is necessary for the safety of both patients and health care workers to ensure quality patient outcomes.
It is the intent of this chapter to broaden the nurse’s knowledge regarding IV drug therapy. Because of the breadth of the subject, this chapter has been divided into four sections. In the first, the nurse’s role in IV drug administration is reviewed. Specific consideration is given to legal aspects of IV drug administration and application of the nursing process. The second section discusses the various aspects of IV drug delivery. Commonly used equipment is described, as well as the different modes of IV drug administration. The third section discusses drug dosage and flow rate calculations. The greatest emphasis is placed on the fourth section, where the different classifications of IV drugs are presented and the drug most representative of each classification is examined in detail.
CONSIDERATIONS FOR INTRAVENOUS DRUG ADMINISTRATION
The administration of IV medications has increased dramatically, with some medications only being manufactured for IV infusion. The IV route for drug administration offers many advantages including the following: the IV route is ideal for patients who are unable to tolerate, use, or absorb drugs via the gastrointestinal (GI) tract; the IV route may eliminate pain or trauma at the delivery site; IV infusion provides rapid delivery/utilization and better control of administration rates. The increased need for drug-related education goes hand-in-hand with the increased use of IV drugs and the nursing role. This education is provided in schools of nursing; from health facility courses, computer-based learning, webcasts, and manuals/books; as well as through one-on-one information-sharing with pharmacists.
NURSING RESPONSIBILITIES
The determination of who may administer IV drugs is based on the Nurse Practice Act. Each state has such an act, but most only broadly define the scope of professional nursing responsibilities. This differs for a licensed practical nurse (LPN) in that several states have established boundaries in which the LPN may practice.
In addition to the Nurse Practice Act, health care facilities have established policies regarding nursing responsibilities. Although these policies cannot exceed the limits established by the Nurse Practice Act, they may clarify or place further restrictions on nursing functions. For example, institutional guidelines for IV drug administration define who may administer certain drugs and in what areas the drug can be used.
A third set of criteria regarding IV drug administration is contained in the Infusion Nursing Standards of Practice (INS, 2006a). This document provides an emphasis on evidence-based practice and research and includes information to measure the quality of IV nursing care, including IV drug administration. Because the standards are meant to protect the public and define nursing accountability, they are the basis for the development of IV policies in all practice settings in which IV drugs are administered.
LEGAL CONSIDERATIONS
To legally administer an IV drug, or any drug, the medication must be prescribed by a physician or appropriate licensed professional. However, it is the nurse’s responsibility to ensure that the order is complete, correct, appropriate, and valid. The nurse needs to be familiar with information related to the use of investigational drugs and controlled substances. To provide quality, safe medication delivery, basic knowledge related to medications and administration is needed along with knowing how to correctly respond to medication errors.
A complete order must contain the name of the drug (generic or trade), dosage, route of administration, frequency or time of administration, date and time the order was written, and signature as well as the reason for as-needed (PRN) orders. On some occasions, a verbal or telephone order may be needed. For these orders, the signature of the nurse is required and the prescriber should co-sign the order within 24 hours. Drug orders may be standing, PRN, or single dose, which could include a “stat” or “now” request.
A correct and appropriate order is one that is indicated and proper for the patient’s condition. Indications for each drug classification and specific drugs are discussed later in this chapter. Determination of the appropriateness of the order is based on the nursing assessment of the patient and requires knowledge of pharmacokinetics and pharmacodynamics. Pharmacokinetics focuses on the effects of the body on the drug; pharmacodynamics involves the effects of the drug on the body.
A valid medication order requires that the order be written and signed by the physician or other authorized health care professional. Although verbal orders are acceptable in some situations, the order is not legally finalized until it is counter-signed by a licensed physician or appropriate licensed professional.
There are additional legal considerations regarding controlled substances. Controlled substances are prescription drugs that, because of their high potential for drug dependence or abuse, are covered by the Controlled Substances Act of 1970. The Act established five categories of controlled substances, known as schedules, based on potential for abuse and dependence and the medical indications for the drug’s use (Elkin, Perry, and Potter, 2007). Many of the IV drugs administered for pain control are Schedule II drugs, which have specific prescription and record-keeping requirements. The result of failing to follow established policies could be loss of professional license as well as fines and imprisonment.
Another area that has legal implications is the administration of investigational drugs. Informed consent must be obtained from all patients and/or families participating in clinical drug trials. This means that the patient must be supplied information by the principal investigator regarding the risks, benefits, expected effects on the disease process, and alternatives to the investigational therapy before consenting to participate in the trial. In addition, the clinical trial must be approved by the appropriate institutional committee, and drug information must be readily available for the nurse administering the drug. All documentation must meet the guidelines of the study and information returned according to established times.
Legal consideration must also be given to the occurrence of medication errors. Errors may be associated with prescribing and dispensing the drug, but they more commonly occur because of failure to follow safe administration procedures. The traditional five rights of medication administration specifying that the right dose of the right drug must be administered to the right patient at the right time by the right route should now include a sixth right, right documentation. Errors have resulted because of the “look alike” generic names (e.g., the cephalosporin antibiotics) (Box 15-1), failure to identify the patient appropriately, administering the drug by an incorrect route, illegible writing, improper abbreviations (Table 15-1), range orders, and the physician’s failure to specify the route. Errors may be documented by manually completing paper forms or by populating data in computer-based programs. The programs should be nonpunitive and include follow-up, education, trending, reporting to appropriate individuals/committees, and practice change. The person discovering the error is responsible for documentation.
Box 15-1
LOOK-ALIKE/SOUND-ALIKE MEDICATIONS
| acetohexamide | acetazolamide |
| amphotericin lipid | amphotericin conventional |
| Ativan (lorazepam) | Xanax (alprazolam) |
| atracurium | cisatracurium |
| carboplatin | cisplatin |
| Cipro IV | heparin IV |
| dobutamine | dopamine |
| Doxil | doxorubicin |
| doxorubicin conventional | doxorubicin liposomal |
| epinephrine | phenylephrine; ephedrine |
| hydralazine | hydroxyzine |
| hydromorphone | morphine |
| methocarbamol | metronidazole; methyldopa |
| quinidine | quinine |
| Taxol | Taxotere |
| Use | Do not use | Reason |
|---|---|---|
| Units | U or u | Mistaken as a “0,” “4,” or “cc” |
| 0.1 mg | .1 mg | No leading zero results in easily missed decimal point (i.e., 10 times the dose) |
| 0.1 mg or 1 mg | .10 mg or 1.0 mg | Trailing zero results in easily missed decimal point (i.e., 10-100 times the intended dose) |
| mcg or micrograms | μg | Mistaken for mg |
| Morphine sulfate | MS | Mistaken for MSO 4 and MgSO 4 |
| Morphine sulfate | MSO 4 | Mistaken for MS and MgSO 4 |
| Magnesium sulfate | MgSO 4 | Mistaken for MS and MSO 4 |
| International unit | IU | Mistaken for IV and 10 |
| Daily | Q.D. | Mistaken for Q.O.D. The period after the Q can be mistaken for an “I” |
| Every other day | Q.O.D. | Mistaken for Q.D. The period after the Q can be mistaken for an “I” and the “O” can be mistaken for “I” |
| Left ear | AS | Confused with left eye (OS) |
| Right ear | AD | Confused with right eye (OD) |
NURSING PROCESS
The administration of IV drugs requires proper application of the nursing process. The patient must be assessed, the drug therapy planned and implemented, the patient outcomes evaluated, and the plan of care reviewed and revised based on the evaluation. These basic steps are interrelated and are essential to ensure that drug therapy results in the desired patient outcomes.
Assessment
Patient assessment begins with a review of the patient’s health history followed by talking with the patient, family, or significant others (Box 15-2). Where language barriers are present, it is important to have an interpreter. Printed material in various languages is also helpful for understanding and compliance.
Box 15-2
PATIENT ASSESSMENT QUESTIONS
• What is the current problem/disease?
• What other medications are being used?
• What medications were used in the past?
• Did the previous medication(s) have any unexpected effect?
During the questioning, it is particularly important to obtain information about known allergies to drugs, foods, and environmental factors. There are cross-sensitivities between many drugs, so an allergy to one may result in similar effects with another. A primary example is the possible cross-sensitivity between penicillins and cephalosporins.
Assessment should also include the patient’s lifestyle, resources, and knowledge level. This is particularly important in home care and should include the family or other caregiver as well as the patient. Specific factors to consider are the patient’s and family’s daily schedules and the physical and human resources available. The ability to comprehend information may influence adherence to the drug regimen.
The last area to be assessed is patient-related factors that may alter the patient’s response to the drug. These include genetic factors, preexisting conditions, and age. Genetic factors, such as the absence of a specific enzyme, may affect the drug’s action within the body. Preexisting renal, liver, and cardiovascular disease may impair the metabolism or elimination of the drug. Age is also a predictor of drug response. For example, children do not have the mature physiological mechanisms needed for adult dosages, whereas physiological changes in older adults tend to extend the effects of drugs within the body. In addition, drug interactions and incompatibilities with other medications and with body fat may negatively affect the drug response.
Planning
A comprehensive assessment helps develop and follow a plan of care specific to the patient and the drug therapy. Once the plan has been identified, the patient’s plan of care may be established. The care plan should include short- and long-term goals, and it should focus on actions to achieve the desired patient outcomes.
An important feature to consider when planning care for the patient is the time-response nature of drug action. Although the drug may be administered as a single dose, it is more common for it to be administered repeatedly. Repeated administration at regular intervals causes the plasma concentration of most drugs to reach a constant concentration in the blood. More drugs are being developed that require fewer doses over a 24-hour period. If plans are not made to administer the drug at the scheduled times, the plateau may not be reached, seriously affecting the effectiveness of the drug therapy.
Another time-response factor to consider when planning the patient’s care is the therapeutic drug concentration range. Whereas a plateau is achieved with a fixed dosing schedule, there are still peaks and troughs in the drug plasma concentration. The peak concentration occurs immediately after IV administration, and the trough level is the minimum concentration present immediately before administration of the next scheduled dose. Several drugs have a relatively narrow margin of safety, meaning that there is little difference between concentrations that produce therapeutic responses and those that cause serious adverse effects. When such drugs are prescribed, arrangements must be made to determine whether the drug levels are within a therapeutic range. The most important aspect of this planning is ensuring that blood samples are drawn appropriately in conjunction with a scheduled dose. For example, the administration of gentamicin requires that a trough specimen be drawn before the scheduled dose and that a peak specimen be obtained following infusion.
A third time-response aspect to consider is the plasma half-life of the drug. Half-life refers to the amount of time required for the elimination processes to reduce the blood concentration of the drug by 50% (Dipiro et al, 2002). For example, many IV drugs are eliminated by the kidneys. Because renal impairment may increase the half-life of such drugs, planning should include verification that reduced dosages have been prescribed.
Implementation
Important facets of implementing drug therapy are medication reconciliation before beginning treatment and at discharge, patient education before administration and discharge, drug administration, and documentation. Because of differences among health care settings, these facets may not receive equal attention. In home care, greater emphasis is placed on patient education to prepare the patient for the self-administration of medication. This differs from the hospital setting, where IV drugs are usually administered by the nurse. Regardless of the setting, documentation plays a vital role because it validates that the actions have been implemented as well as the outcomes.
Evaluation
Evaluation of the drug therapy determines the drug’s effectiveness and may suggest beneficial modifications of the patient’s plan of care. A therapeutic response is desired because it signifies that the intended effects of the drug have been produced. However, there may be an ineffective or toxic response. An ineffective response may indicate that less than the minimum required dose has been administered or that other factors have interfered with the action of the drug. For example, a patient who experiences only slight relief from pain after the administration of morphine has an ineffective response. In contrast, a toxic response is an exaggeration of the usual pharmacological actions of the drug or the appearance of signs and symptoms related to drug toxicity. For example, the toxic response to digoxin is typically evidenced by the clinical symptoms of anorexia, nausea, and vomiting.
The patient should also be evaluated for unexpected and undesired effects of the drug. Although these responses are often called side effects, this term is misleading. Side effects are therapeutically undesirable, but are an unavoidable secondary effect of the normal action and therapeutic dose of the drug. An example is the loss of potassium from the body after the administration of furosemide. Untoward drug responses, also known as adverse effects, are undesired and unexpected responses. Table 15-2 describes several potential untoward drug responses.
| Response | Effects | Example |
|---|---|---|
| Tolerance | Increasing amounts of drug needed to produce same therapeutic response | Patient receiving morphine infusion over 2 weeks has decreasing relief from pain |
| Tachyphylaxis | A rapidly developing tolerance occurring after very few doses | Has been reported with sodium nitroprusside |
| Accumulation | Amount of drug builds up in body, resulting from input exceeding output | May occur when usual adult dosage of gentamicin is administered to patient with renal impairment |
| Idiosyncrasy | Unpredictable response that differs in quality from expected response but not caused by hypersensitivity | Aplastic anemia develops in 1 in 40,000 patients who receive chloramphenicol |
| Drug allergy | Adverse response to a drug resulting from previous exposure to that or a related drug and mediated by an antigen-antibody reaction; hypersensitivity | Reaction that occurs when penicillin is given to patient with penicillin allergy |
| Dependence | Continued administration of drug required to prevent withdrawal syndrome | Patient receiving hydromorphone for cancer pain has visible tremors, restlessness, and profuse perspiration when a dose is withheld |
Untoward effects may be caused by the interaction of the drug with other drugs within the body. Drug-drug interactions may be potentiative, meaning one drug intensifies the effects of the other drug, or inhibitory, meaning one drug reduces the effects of the other drug; or the drug combination may produce a new response that neither drug used alone would produce. Potentiative and inhibitory interactions may be beneficial or detrimental. Sulbactam and ampicillin illustrate a beneficial potentiative interaction that increases ampicillin’s therapeutic action. A detrimental potentiative interaction occurs with warfarin and aspirin, which increases the risk of bleeding. A beneficial inhibitory interaction occurs between morphine and naloxone when treating overdoses, reversing the toxicity of the opioid analgesic (Gahart and Nazareno, 2008). A detrimental inhibitory interaction occurs between terbutaline and propranolol, which causes a reduction in bronchial dilation (McEvoy, 2007). An example of a drug-drug interaction resulting in a new response is the combination of disulfiram (Antabuse) and alcohol, which causes hazardous effects that do not occur when either drug is used alone (McEvoy, 2007).
DRUG ADMINISTRATION
The nurse’s role in IV drug administration is determined by the health care setting. In the inpatient setting, the nurse may be responsible for drug preparation and administration, whereas home care usually requires the nurse to educate the patient or family in self-administration techniques. Before initiating any infusion, the nurse must identify the patient, order, and drug/IV solution; practice appropriate hand hygiene; and select correct supplies/equipment. The nurse should also understand the drug’s therapeutic effects and be able to recognize side effects/adverse reactions. Use of aseptic technique throughout the process as well as appropriate documentation is paramount. The first dose of medication is preferably administered in the hospital or in the presence of a physician (INS, 2006b). Barcoding of solutions/medications, patient identification bands, and caregiver identification are making drug administration safer for the patient and provide improved processes for the health care employee.
DRUG PREPARATION
Generally, IV drugs and admixtures are prepared in a centralized pharmacy, where there is greater assurance of accuracy and sterility. No matter where the drug is prepared, appropriate manufacturing practices, such as those described in the USP <797> Guidelines, must be met. These guidelines include directions related to the areas of facility, preparation, personnel, and products. Cleaning, environmental testing, and expiration/end-of-use information have been defined in these guidelines. The admixture process may be performed by technicians employed by the pharmacy, working under the direction of the pharmacist. In emergencies or when medication must be prepared immediately before administration, nurses may also prepare IV drugs.
Laminar flow hood
When IV drugs are prepared in the pharmacy, they are usually prepared under a laminar flow hood. The design of the laminar flow hood decreases the possibility of airborne contaminants entering the IV solution. Air enters the back of the hood, and is circulated through filters before it is directed into the work area in uniform, parallel streams. Either a horizontal or a vertical laminar flow hood may be used; however, vertical models are recommended for preparing cytotoxic agents.
Drug containers
IV drugs are available in various containers, including ampules, vials, partially filled solution containers, additive piggyback vials, and premixed admixtures. Ampules pose the greatest risk because of the possibility of particulate contamination. Glass fragments may enter the ampule when it is broken open; the related risks are reduced when the drug is drawn up with a filter needle and infused through a standard needle. Vials pose another risk of particulate contamination because of the potential risk of coring; when the needle is introduced through the stopper of the vial, the needle bevel may cut away fragments of the seal.
Partially filled containers and additive piggyback vials, often referred to as minibags or minibottles, are potential sources of particulate matter. This may be the result of incomplete reconstitution of the powdered drug or precipitates formed by the physical incompatibility of the admixture. Partially filled containers of 50 to 150 mL of 0.9% sodium chloride or 5% dextrose in water (D 5W) require that a liquid form of the drug be added to the container. In an additive piggyback vial, the drug is in powdered form, which must be reconstituted with a small volume of diluent.
Premixed admixtures are prepared by the manufacturer and require no further preparation other than providing proper labeling. However, they are sometimes available in frozen form, which must be restored to room temperature before administration. Cefazolin is an example of an admixture available in frozen form. It is generally recommended that frozen admixtures not be warmed by placing them in a water bath or exposing them to microwave radiation. Special units are available for rapidly thawing premixed frozen minibags, but the preferred method is to allow the admixture to thaw at room temperature.
Diluents
Another consideration for the preparation of IV drugs is the diluent used to reconstitute the drug. Diluents with bacteriostatic properties, such as bacteriostatic sodium chloride, contain benzyl alcohol as a preservative. Although this bacteriostatic agent is desirable in most situations, it is contraindicated for neonates and for the administration of intraspinal or epidural drugs (McEvoy, 2007). In addition, certain drugs, such as amphotericin B, are incompatible with preservatives and must be reconstituted with sterile water for injection (McEvoy, 2007).
DRUG COMPATIBILITY AND STABILITY
Drug incompatibility
Incompatibility is an undesirable reaction that occurs between the drug and the solution, the container, or another drug. The three types of incompatibilities associated with IV drugs are physical, chemical, and therapeutic. A physical incompatibility refers to a visible reaction, such as a color change, haze, turbidity, precipitate, or gas formation, occurring within the drug. The largest number of physical incompatibilities involve precipitate formation, such as that seen when diazepam is added to D 5W.
Chemical incompatibility involves the chemical degradation of the drug, and is the result of hydrolysis, reduction, oxidation, or decomposition. It differs from physical incompatibility in that the reaction may not be visible (Weinstein, 2007). Such a reaction may occur when penicillin is added to a very acidic or alkaline solution.
Therapeutic incompatibility, which occurs within the patient, is the result of the overlapping effects of two drugs administered concurrently. Although the effects may not be evident until the patient’s response to the drug therapy has been evaluated, knowledge of potential incompatibilities may prevent their occurrence. Examples of therapeutic incompatibility were previously discussed as part of the evaluation of drug interactions.
Drug stability
Stability, on the other hand, refers to the length of time the drug retains its original properties and characteristics. One of the most important factors in drug stability is the solution’s hydrogen ion concentration, or pH. Most drugs are stable over a narrow range of pH values. This means that they are unstable in either very acidic (pH below 4) or alkaline (pH above 8) solutions. The concept of stability is best characterized by penicillin. This drug is most stable in a slightly acidic environment (pH of 6.5) but deteriorates if added to a very acidic or alkaline solution. Additional factors that affect drug stability are listed in Table 15-3.
| D 5W, 5% dextrose in water; PN, parenteral nutrition. | ||
| Factor | Effect | Example |
|---|---|---|
| Number of additives | The greater the number of drugs contained in the admixture, the greater the chance of one of the drugs becoming unstable | Multiple additives in PN solution |
| Dilution | Limited amounts of a drug are stable in the solution, whereas large doses may be unstable | Only limited amounts of heparin and hydrocortisone are stable in amphotericin solution |
| Time | The length of time the drug is in solution may affect stability | Ampicillin is stable for only 4 hr when added to D 5W |
| Light | Some drugs are sensitive to light, and exposure may result in degradation of the drug | Exposure of levarerenol may result in degradation of drug |
| Temperature | Lower temperatures usually extend the stability of the drug | Cephalothin is stable for only 6 hr at room temperature, but up to 48 hr when refrigerated |
| Order of additives | The order in which drugs are added to a solution affects compatibility and stability | Addition of lipids to PN solution |
| Container | The composition of the container may affect the stability of the drug | Potency of insulin reduced by at least 20% when added to a plastic container |
METHODS OF IV DRUG ADMINISTRATION
The mode of IV drug administration depends on the drug used, the patient’s condition, and the desired effects of the drug. The mode of administration is specified by the physician or other qualified provider. Each of the four primary modes of administration—continuous infusion, intermittent infusion, direct injection, and patient-controlled analgesia—has advantages and disadvantages.
Continuous infusion
Continuous infusion refers to the admixture of the drug in a large volume of solution that is infused continuously over several hours to several days. The solution container is connected to an administration set, and the drug (in solution) is infused through the venous access device. Depending on the potency, an electronic infusion control device may be used to deliver the drug accurately at the prescribed rate of flow.
Continuous infusion is used when the drug must be highly diluted, constant plasma concentrations of the drug must be maintained, or large volumes of fluids and electrolytes must be replaced. Examples are infusions of nitroprusside or potassium chloride. Disadvantages associated with continuous infusion are possible fluid overload and potential incompatibilities between the infusion and other IV drugs administered through the same venous access device. Patient comfort issues and mobility should also be considered.
Intermittent infusion
For an intermittent infusion, the drug is added to a small volume of fluid (25 to 250 mL) and infused over 15 to 90 minutes at prescribed intervals. Advantages of the intermittent mode are the ability of the drug to produce peak blood concentrations at periodic intervals, decreased risk of fluid overload, and greater convenience for the patient. However, there are disadvantages. The increased concentration of the drug in the intermittent solution may cause venous irritation, the drug may be less effective than if administered by continuous infusion, and additional equipment is required. IV antibiotics are generally administered using this mode.
Intermittent infusions may be administered in various ways. One of the most common methods is for the drug to be given as a piggyback infusion through the established pathway of the primary solution (Figure 15-1). Although the primary infusion is interrupted during the piggyback infusion, the drug from the intermittent infusion container mixes with the primary solution below the piggyback injection port. Hence, if this method is used, the drug and the primary solution should be compatible.
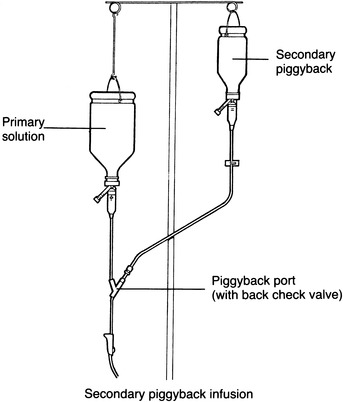 |
| FIGURE 15-1 Secondary piggyback. |
A second way to administer intermittent infusions is simultaneous infusion. With this method, the drug is administered as a secondary infusion concurrently with the primary infusion (Figure 15-2). Rather than connecting the intermittent infusion at the piggyback port, it is attached to the lower secondary port. One of the major disadvantages of this method is the tendency for blood to back up into the tubing once the secondary infusion has been completed, possibly occluding the venous access device. This does not occur with the piggyback method because hydrostatic pressure closes the back check valve (incorporated into the administration set) once the intermittent infusion is completed. Although drug incompatibility is a possibility with both methods, it is a greater risk with a simultaneous infusion.
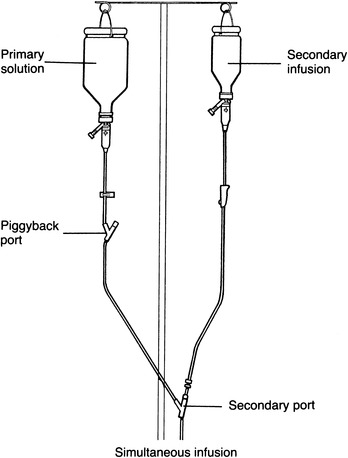 |
| FIGURE 15-2 Simultaneous infusion. |
A third method is the use of a volume control set. Although it was originally designed to control the fluid volume delivered to the patient, a drug may be added to a small amount of solution in the volume control set and infused at the desired rate (Figure 15-3). This method shares many of the disadvantages previously discussed. However, it is still used in some pediatric settings because it limits the amount of fluid the child receives.
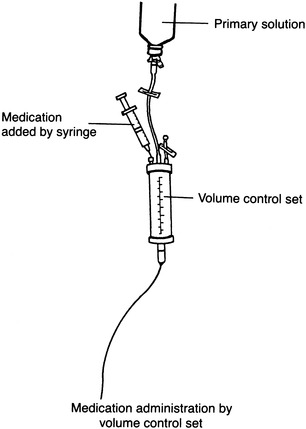 |
| FIGURE 15-3 Volume control set. |
The fourth method for administering intermittent infusions is directly into the venous access device. The device must be one that is intended for intermittent administration, such as a peripheral heparin or saline lock or a long-term, centrally placed catheter. The drug is added to a minibag or minibottle and infused intermittently. Between doses, the drug container and administration set are eliminated. This method is generally preferred because it decreases the risk of fluid overload, and affords greater freedom of movement for the ambulatory patient. However, failure to promptly remove the empty drug container and administration set and flush the venous access device may result in occlusion of the venous access device.
Technological developments have produced alternatives for the administration of intermittent doses. One manufacturer has introduced a system whereby the drug is supplied in a powdered form that is attached between the primary solution and the infusion set. Once the drug vial is connected, the solution flows from the primary container through the drug vial and to the patient. Although the use of this system eliminates the costs associated with preparing and administering the drug by the traditional piggyback method, it is applicable only to situations in which the drug and primary solution are compatible.
A second innovation has been the introduction of intermittent doses of drugs that are activated at the time of use. Rather than preparing and refrigerating the drug before administration, the pharmacy simply dispenses the drug vial attached to a small container of solution. Immediately before administering the drug, the nurse activates the system by removing the barrier between the drug and the solution. Although this has proven cost-effective, errors have been reported because of failure to remove the barrier and activate the system.
The third major innovation is the result of the space program and is based on elastomeric technology. The system consists of an elastomeric drug container that is specially designed to establish a set delivery rate. Once the pharmacist fills the system with the drug, it may be infused at the preset rate by opening the slide clamp on the tubing attached to the container. An advantage of this system is that neither gravity nor electronic assistance is required for precise delivery of the drug. However, not all drugs may be administered with an elastomeric container. Because of the expense, it is usually reserved for the home setting.
Finally, drug delivery systems containing dual compartments, one for fluid and one for powdered medication, are available. To reconstitute, at the time of administration the “hanger tab end” is rolled toward the port end, which is pointing downward, until the seal between the diluent and powder opens, releasing the diluent into the drug chamber. The container is agitated until the drug is completely dissolved. This system also offers the advantages of longer end-of-use (expirational) datings and eliminates need for refrigeration. With the advantages, comes the disadvantage of an increased cost (Figure 15-4).
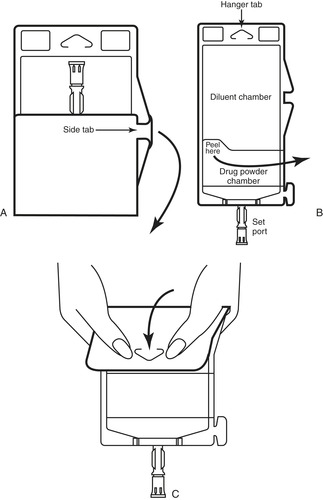 |
| FIGURE 15-4 Duplex drug delivery system. A, Unfold duplex container. B, Inspect diluent and powder chambers. C, Activate: fold container just below the diluent meniscus and squeeze folded diluent’s chamber to release diluent into powder chamber. Agitate mixture until completely dissolved. (Used with permission from B. Braun Medical, Inc., Bethlehem, Pa.) |
Systems that offer premixed drugs that are enclosed in a single container offer some advantages. The diluent and drug are measured and verified in a single sterile container by the manufacturer. These systems decrease the risks of incorrect drugs/diluents, volume of drug/diluent, and labeling, and of contamination during processing.
Direct injection
Direct injection, also known as IV push or bolus, is the administration of a drug directly into the venous access device or through the proximal injection port on a continuous infusion set. The purpose is to achieve rapid serum concentrations, but this may be accompanied by a greater risk of adverse effects. Instead of regulating the rate of administration by the infusion rate, direct injection requires only the time it takes to push the plunger of the syringe. Many drugs have maximum rates at which they may be administered, so the rate of the injection must be timed and the drug injected in increments. Because the drug may be incompatible with the infusing solution or heparin may be present in the intermittent device, the venous access device should be flushed with 0.9% sodium chloride before and after injecting the drug.
Direct injections may require that the drug be drawn into a syringe before administration or that the drug be available in a prefilled syringe. There are a variety of systems available to allow for direct injections. Some include prefilled syringes that are added to syringe holders, which may or may not be included in the packaging. Others require removing an affixed plunger from the outside of the syringe and attaching it at the plunger end of the prefilled syringe.
A needleless system is mandated to prevent needlestick injuries and inadvertent puncture of the tubing or device.
Patient-controlled analgesia
A fourth mode of administration is patient-controlled analgesia (PCA), which promotes patient comfort through the self-administration of analgesic agents. With this method, an electronic pump (PCA pump) is programmed to administer a small bolus of the drug when activated by the patient. The bolus amount and the time between doses (lock-out interval) are predetermined by the physician and programmed into the pump by the nurse. At the end of a shift, the nurse can review the infusion history to determine the amount of drug received as well as the number of unsuccessful attempts, possibly indicating inadequate pain control. Technological advances have added features to the pump, permitting both continuous infusion and patient-controlled doses.
CALCULATIONS
A physician or authorized prescriber is responsible for prescribing patient medications, but the nurse must ensure that the medication order is accurate and will be safe for the patient. This section primarily focuses on a variety of calculations that will help ensure the ordered medication can be administered correctly.
METRIC SYSTEM
The metric system is universally accepted by the medical profession. It provides the most accurate means for calculating drug dosages. The effects of a drug administered directly into the vascular system may be immediate, so accuracy is very important. The precise measurements attainable by using the metric system make it preferable to the apothecary and household measuring systems. Box 15-3 lists common abbreviations used in the metric system, and Box 15-4 provides conversions among metric units.
Box 15-3



 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

METRIC SYSTEM ABBREVIATIONS
WEIGHT
Kilogram = kg
Gram = g
Milligram = mg
Microgram = mcg
VOLUME
Liter = L
Milliliter = mL
Cubic centimeter = cc
LENGTH
Centimeter = cm
Millimeter = mm
Box 15-4
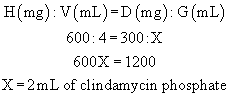
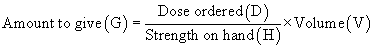 Example: A vial contains clindamycin phosphate, 600 mg/4 mL. What volume must be given to administer 300 mg?
Example: A vial contains clindamycin phosphate, 600 mg/4 mL. What volume must be given to administer 300 mg?
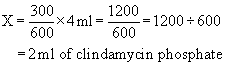
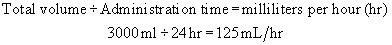 Step 2:Determine the rate per minute.
Step 2:Determine the rate per minute.
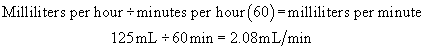 Step 3: The administration set drop factor is 15 drops (gtt)/mL. Determine the number of drops per minute.
Step 3: The administration set drop factor is 15 drops (gtt)/mL. Determine the number of drops per minute.
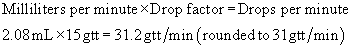
PATIENT OUTCOMES
An infusion at a rate of 31 gtt/min administers the 3000 mL over 24 hours.
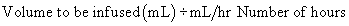
METRIC CONVERSION FACTORS
WEIGHT
| Kilogram | × | 1000 | = | 1000 grams |
| 1 gram | × | 1000 | = | 1000 milligrams |
| 1 milligram | × | 1000 | = | 1000 micrograms |
| 1 microgram | ÷ | 1000 | = | 0.001milligram |
| 1 milligram | ÷ | 1000 | = | 0.001gram |
| 1 gram | ÷ | 1000 | = | 0.001kilogram |
VOLUME
| 1 liter | × | 1000 | = | 1000 milliliters |
| 1 milliliter | ÷ | 1000 | = | 0.001liter |
DRUG DOSAGE DETERMINATION
In determining dosages, most nurses are familiar with either the ratio-proportion or the formula method. Each method is reviewed here; however, readers should use their preferred method.
Ratio-proportion method
To use this method effectively, it must be remembered that a ratio is a comparison between two related items, and a proportion is the equality of two ratios.
When setting up a proportion, start with what you know about the drug from the label: the strength of the drug on hand (H) and volume of the drug on hand (V). Place this information in the form of a ratio (H:V) on the left of the equal sign (=). The ratio for the dose desired is the relationship of the dose ordered (D) and the amount to give (G), and this is placed on the right of the equal sign:
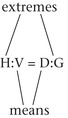 |
Thus the strength on hand (H) is related (:) to the volume (V) as (=) the dose ordered (D) is related (:) to the amount to give (G).
For an answer to be correct, the product of the means must equal the product of the extremes. The extremes are always the two outside numbers.
Example:
A vial contains clindamycin phosphate, 600 mg/4 mL. What volume must be given to administer 300 mg?
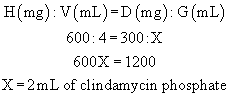
Formula method
The terminology applied to the ratio-proportion method remains the same for the formula method; the equation, however, is changed to the following:
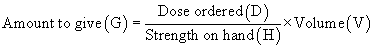
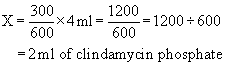
FLOW RATE DETERMINATION
To administer an IV solution accurately, the flow rate must be regulated carefully. Flow rates may be expressed in the form of an infusion over a period of hours, milliliters per hour or milliliters per minute, or number of drops per minute. The responsibility for administering the solution rests on the nurse, who should evaluate the available administration sets and select the one most appropriate for administering the solution. Criteria for administration set selection include its ability to deliver the required flow rate. When calculating the flow rate, it is important to know that the exact amount of solution in a manufacturer’s container is unknown as there is an acceptable range of overfill allowed.
Milliliters per hour
Several formulas provide the information necessary for the delivery of a precise flow rate; however, the three-step method is the most complete.
Three-step method
The three-step method is a comprehensive flow rate calculation because it determines the rate in milliliters per hour and milliliters per minute, as well as the number of drops per minute for solution administration.
Example: A physician has ordered 3000 mL of solution to be administered over 24 hours.
Step 1:Determine the flow rate per hour.
Formula Method
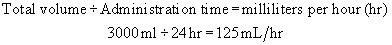
Formula Method
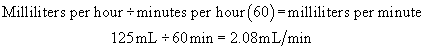
Formula Method
PATIENT OUTCOMES
Expected outcomes with medication administration include:
• The patient will experience the desired effect of the medication.
• The patient will not experience adverse effects or reactions from medication administration.
• The patient will be able to explain medication information.
Length of administration and flow rate
The hours for administration are based on milliliters per hour. On occasion, it may be necessary to determine the hours required for an infusion. The calculation can be completed through the use of simple arithmetic.
Formula Method
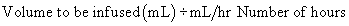
DRUG CLASSIFICATIONS
Drug reference books generally list medications alphabetically, even when a classification system is used. Such a system aids the nurse in clinical practice, but it is not conducive to learning. To help the nurse understand the wide array of IV drugs discussed in this chapter, a prototype approach is used. The drugs are divided into different categories: antibiotics, other anti-infective drugs, central nervous system (CNS) drugs, cardiovascular drugs, hematological agents, agents for electrolyte and water balance, gastrointestinal (GI) drugs, hormones and synthetic substitutes, immune modulators, respiratory smooth muscle relaxants, and vitamins. The drug most representative of each classification is examined in detail. Additional drugs related to the prototype, including antidotes, are then discussed in terms of their differences and similarities or their relationship to the prototype.
Drug dosages vary widely, even among the prototype and its related drugs. Inclusion of dosing information on all the drugs discussed would require more pages than are allotted for this chapter. Therefore drug dosing is discussed only when pertinent to understanding administration of the drugs and their potential side effects. Information on dosing schedules for each drug is available in American Hospital Formulary Service Drug Information (McEvoy, 2007) and in Drug Facts and Comparisons (Wickersham, 2007). These were the primary references consulted for specific drug information, although additional references may be cited to explain the various drug classifications.
ANTIBIOTICS
Antibiotics are used to treat infection and represent the largest category of commonly used IV medications. Antibiotics may be categorized as either bactericidal or bacteriostatic. Bactericidal agents can destroy the organism by lysis of the cell wall or by prevention of its intact formation. Bacteriostatic agents inhibit growth of the organism by inhibiting protein synthesis. Although bacteriostatics may eliminate the organism in high concentrations, they usually depend on the patient’s immune system to eradicate the organism once its growth has been inhibited. Sometimes combinations of antibiotics with differing mechanisms of actions are used for synergistic effects.
Antibiotics also have differing pharmacokinetic and pharmacodynamic profiles. Pharmacokinetics is the effects the body has on the drug, and pharmacodynamics refers to the effects the drug has on the body. Pharmacokinetic parameters such as volume of distribution and elimination half-life influence the dose of a drug and how often it must be administered. Pharmacodynamics examines different drug exposure measurements to determine which measurement is most closely tied to efficacy. Examples include time above minimum inhibitory concentration (MIC) and peak to MIC ratio, with minimum inhibitory concentration being the lowest concentration of antibiotic that will inhibit growth of the organism. Time and peak are specific to the concentrations of antibiotic achieved. Knowledge of which pharmacodynamic parameter is most optimal for the selected antibiotic combined with its pharmacokinetic profile often yields a customized regimen for the patient.
The primary contraindication for antibiotics is a known sensitivity to the drug. A patient with a history of hypersensitivity reactions to a certain antibiotic should be considered allergic to all antibiotics within the same class. For example, a patient who has a known sensitivity to penicillin is also considered allergic to aminopenicillins. In addition, there is evidence of a cross-sensitivity among antibiotics that are structurally similar, such as penicillins and cephalosporins. Therefore cephalosporins are used cautiously in patients who are sensitive to penicillin.
One of the major disadvantages of antibiotics is that their prolonged use may result in a bacterial or fungal superinfection. A superinfection occurs when the normal flora of the body is altered by an antibiotic, allowing the proliferation of bacteria or fungi that are resistant to the antibiotic.
Since antibiotics have been overused for decades, some are proving ineffective against resistant strains of bacteria. Whenever available, the clinician should check sensitivity data prepared by the clinical laboratory before selecting a specific antibiotic.
Penicillins
The penicillins include natural and semisynthetic antibiotics produced or derived by the fermentation of certain strains of the fungus Penicillium. Penicillins are still some of the most important antibiotics because of their relatively low cost, low toxicity, and good clinical efficacy in the treatment of many infections.
Natural penicillins
The mechanism of action of the natural penicillins is bactericidal. These agents disrupt the synthesis of the bacterial cell wall, making the organism osmotically unstable. Instability of the cell wall causes it to lyse, and the organism is destroyed. Since the cell walls of gram-positive bacteria are relatively permeable to most penicillins, these drugs are generally effective against such organisms. However, gram-negative bacteria have an outer membrane around the cell wall that decreases accessibility to the natural penicillins.
Prototype: penicillin G potassium
Penicillin G is indicated for the treatment of severe infections caused by some gram-positive and anaerobic organisms. Since it exerts specific antibiotic action against these organisms and is relatively nontoxic to the host, penicillin G is generally considered the drug of choice for many streptococcal, pneumococcal, and spirochetal infections.
Penicillin G may be administered as an intermittent infusion, although because of its short half-life it may also be administered continuously. Adverse reactions are rare and are usually limited to hypersensitivity reactions. The manifestations of a hypersensitivity reaction may be varied and can be divided into four basic types (Goldman and Ausiello, 2008).
The most common hypersensitivity reactions are dermatological reactions. Symptoms include urticarial, erythematous, or maculopapular rash accompanied by pruritus. These reactions are delayed, occurring 48 hours or more after the administration of penicillin G.
The second type of hypersensitivity reaction is serum sickness–like reactions. These reactions are usually evident 6 to 10 days after the initiation of therapy, and are characterized by fever, malaise, urticaria, arthralgia, myalgia, lymphadenopathy, and splenomegaly. Although the reaction may be severe, it is usually short-lived and disappears within days or weeks after discontinuing the drug.
The third type of reaction includes hematological reactions, such as hemolytic anemia, agranulocytosis, and leukopenia. Typically, this type of reaction is associated with large doses of penicillin G. A positive direct antiglobulin (Coombs’) test occurs in up to 3% of patients (McEvoy, 2007) receiving large doses, and a small number of these patients develop hemolytic anemia during or after penicillin therapy. Once the drug is discontinued, the hemoglobin concentration and reticulocyte count return to pretherapy levels, although the Coombs’ test may not revert to negative for 3 months or longer.
The fourth and most serious type of hypersensitivity reaction is anaphylaxis. Although anaphylaxis is reported in less than 0.05% of patients receiving penicillin, it has been fatal in 5% to 10% of reported cases (McEvoy, 2007). Anaphylactic reactions to penicillin typically occur within 30 minutes of administration. These reactions are characterized by laryngeal edema, bronchospasm, stridor, cyanosis, and circulatory collapse. Treatment includes immediate discontinuation of the drug and emergency measures such as maintaining a patent airway and administering epinephrine, oxygen therapy, and corticosteroids.
Penicillin interacts with several other antibiotics. Aminoglycosides are physically and chemically incompatible with penicillin, and are inactivated if administered in the same IV container or administration set. However, it is not uncommon for aminoglycosides and penicillins to be used concomitantly because of their differing mechanisms of action. Penicillins and cephalosporins have similar mechanisms of action and are customarily not used together.
Related drugs
Penicillin G is commercially available as a potassium or sodium salt. Both are readily soluble in water and are considered aqueous, crystalline penicillins, but penicillin G potassium is usually preferred. The administration of either form requires special consideration because every 1 million units of penicillin G potassium contains 1.7 mEq of potassium and every 1 million units of penicillin G sodium contains 2 mEq of sodium.
Penicillinase-resistant penicillins
The critical component of the natural penicillins is the β-lactam ring incorporated into the penicillin molecule. Some strains of bacteria produce an enzyme known as penicillinase, which destroys the ring. Thus the organism is considered penicillin resistant. To overcome this resistance, the penicillinase-resistant penicillins have been developed. These antibiotics are used exclusively to treat penicillinase-resistant organisms such as Staphylococcus aureus and Staphylococcus epidermidis. Their mechanism of action, contraindications, and precautions are similar to those of the natural penicillins.
Prototype: nafcillin sodium
Nafcillin sodium (Unipen) is mainly indicated for the treatment of infections caused by penicillinase-producing staphylococci. It is also used preoperatively to reduce the incidence of staphylococcal infections associated with certain surgical procedures. Nafcillin is commonly given as an intermittent infusion, although it may also be administered as a continuous infusion.
Certain strains of staphylococci are resistant to all penicillinase-resistant penicillins and are often termed “methicillin-resistant” because of the historical fact that methicillin previously was the penicillinase-resistant penicillin used for resistance testing. These resistant strains are prevalent in both the hospital and the community and are being reported with increasing frequency.
The side effects of nafcillin are similar to those associated with the use of penicillin G, with hypersensitivity reactions being the most common adverse effects reported. One of the more common hypersensitivity reactions linked to nafcillin is acute interstitial nephritis that manifests both systemically (fever, rash) and locally in the kidneys (hematuria, proteinuria). In rare instances, transient neutropenia, leukopenia, granulocytopenia, and thrombocytopenia have been reported. Generally, these hematological effects are not evident until more than 10 days after the initiation of therapy and resolve within 2 to 7 days once the drug is discontinued. Rarely, nafcillin causes transient asymptomatic elevations of serum alkaline phosphatase, alanine aminotransferase, and aspartate aminotransferase levels.
Related drugs
An additional penicillinase-resistant penicillin is oxacillin sodium (Bactocill). Oxacillin is similar to nafcillin, but nafcillin is more likely to cause phlebitis than other penicillinase-resistant penicillins. However, oxacillin may cause more adverse hepatic effects than nafcillin.
Aminopenicillins
Aminopenicillins have a free amino group added to the penicillin nucleus, which increases their bactericidal effectiveness against gram-negative bacteria. However, they are deactivated by penicillinase-producing organisms.
Prototype: ampicillin sodium
Ampicillin sodium (Omnipen-N) is highly effective in the treatment of severe infections caused by Salmonella, Shigella, Proteus mirabilis, and Escherichia coli. Ampicillin is also used for treatment of susceptible enterococcal and streptococcal infections. Although ampicillin may be administered by direct injection, it is generally administered as an intermittent infusion. Concentrated doses of ampicillin are stable for 4 hours in 5% dextrose in water and 5% dextrose in 0.45% sodium chloride, but potency is extended to 8 hours when added to 0.9% sodium chloride without dextrose. When the drug is further diluted in a minibag or minibottle, stability may be extended up to 48 hours.
Adverse reactions to ampicillin are similar to those of penicillin G. Ampicillin sodium may also produce a distinct, nonimmunological reaction characterized by a generalized erythematous, maculopapular rash similar to measles. The rash typically occurs 3 to 14 days after the initiation of therapy and generally disappears despite the continuation of therapy. It is more common in patients with mononucleosis and is not indicative of a hypersensitivity to penicillin. Hematological effects such as neutropenia, agranulocytosis, and thrombocytopenia have also been reported, but these are usually reversible once the drug is discontinued.
Because of the probability of patients with infectious mononucleosis developing a rash during therapy, ampicillin is generally not prescribed to patients with this disease. In addition, the potential for ampicillin rash is increased for patients receiving allopurinol and ampicillin concomitantly.
Related drugs
Ampicillin sodium-sulbactam sodium (Unasyn) is a combination of ampicillin and sulbactam that prevents inactivation of the drug by β-lactamase-producing organisms. It is primarily indicated in the treatment of skin and skin structure, intra-abdominal, and gynecological infections.
Extended-spectrum penicillins
As a result of the structural differences in the side chains of the extended-spectrum penicillins, they have a wider spectrum of activity than the other penicillins. Specifically, the extended-spectrum penicillins are effective against gram-negative organisms, including Pseudomonas. Like the natural penicillins, the extended-spectrum penicillins are ineffective against penicillinase-producing organisms.
Prototype: piperacillin sodium
Piperacillin sodium (Pipracil) is bactericidal against several gram-negative, gram-positive, and anaerobic organisms. It is used for treatment of bacterial septicemia, febrile neutropenia, and infections of the respiratory, genital, and urinary tracts.
Piperacillin may be administered by direct injection, intermittent infusion, or continuous infusion. However, administration should not exceed the recommended rates. Too rapid infusions of extended-spectrum penicillins and higher-than-normal dosages have resulted in seizures. Other side effects of piperacillin are similar to those of penicillin G and include dermatological and hematological reactions, thrombophlebitis, and anaphylaxis.
Drug interactions associated with piperacillin are similar to those of the other penicillins. There may also be an increased risk of bleeding in patients who are receiving anticoagulants.
Related drugs
Another extended-spectrum penicillin includes ticarcillin disodium (Ticar). Piperacillin sodium-tazobactam sodium (Zosyn) and ticarcillin disodium-clavulanate potassium (Timentin) are co-formulated with a β-lactamase inhibitor that expands the activity of the extended-spectrum penicillin. These drugs are usually selected based on laboratory sensitivity data and availability.
Cephalosporins
Cephalosporins are bactericidal antibiotics that share a close structural similarity with the penicillins. Therefore they have similar mechanisms of action, side effects, and contraindications. Cephalosporins are classified by their spectrum of activity, or generation.
First-generation cephalosporins
First-generation cephalosporins are active against susceptible gram-positive bacteria, such as S. aureus and S. epidermidis, and some gram-negative organisms. As a result of their low cost, they are the preferred drugs for most gram-positive infections.
Prototype: cefazolin sodium
Cefazolin sodium (Kefzol) is effective in the treatment of serious infections of the soft tissue, respiratory tract, genitourinary tract, and cardiovascular system. It may be administered as a direct injection or as an intermittent infusion. The primary route of elimination for cefazolin is the kidney; therefore patients with renal impairment require a dosage reduction.
Although cefazolin is considered relatively nontoxic, allergic reactions have been reported in up to 5% of patients receiving the drug. These reactions are similar to those associated with penicillin G and range from a mild rash to anaphylaxis. Nephrotoxicity has been reported, but it is rare and more likely to occur in geriatric patients or in those with renal impairment.
There are few reported drug interactions involving cefazolin. However, the concurrent use of nephrotoxic drugs such as aminoglycosides and cefazolin may increase the risk of renal toxicity.
Second-generation cephalosporins
The second-generation cephalosporins have greater gram-negative activity than the first-generation drugs, but they are less effective against gram-positive organisms. Their mechanism of action, side effects, contraindications, and precautions are similar to those of cefazolin.
Prototype: cefoxitin sodium
Cefoxitin sodium (Mefoxin) is indicated for the treatment of serious infections of the respiratory and genitourinary tracts, gynecological infections, and septicemia. Cefoxitin also has increased activity against anaerobic bacteria, making it an option for treatment of intra-abdominal infections. Since it is excreted by the kidneys, a reduced dosage of cefoxitin is indicated in patients with renal impairment.
Related drugs
Additional second-generation cephalosporins are cefotetan disodium (Cefotan) and cefuroxime sodium (Zinacef). Second-generation cephalosporins are often used for surgical prophylaxis.
Third-generation cephalosporins
Third-generation cephalosporins are more active against gram-negative organisms, but are less effective against gram-positive organisms. An important characteristic of this category of drugs is their ability to cross the blood-brain barrier when the meninges are inflamed. Third-generation cephalosporins share many of the characteristics of the first- and second-generation drugs.
Prototype: cefotaxime sodium
Cefotaxime sodium (Claforan) is indicated for the treatment of bacteremia, meningitis, and serious infections of the respiratory and genitourinary tracts. It is usually administered by intermittent infusion but may also be given by direct injection.
Related drugs
Related third-generation drugs include ceftizoxime sodium (Cefizox) and ceftriaxone sodium (Rocephin). Ceftriaxone is generally preferred for home care because it requires only a single daily dose. Ceftazidime (Fortaz) also has activity against Pseudomonas aeruginosa.
Fourth-generation cephalosporins
Fourth-generation cephalosporins, like the third generations, have an expanded spectrum of activity against gram-negative bacteria compared with the first- and second-generation drugs. Activity is improved in vitro against Pseudomonas aeruginosa and certain Enterobacteriaceae, as well as gram-positive bacteria.
Prototype: cefepime
Cefepime (Maxipime) is used for the treatment of uncomplicated and complicated urinary tract infections, uncomplicated skin and skin structure infections, moderate to severe pneumonia caused by susceptible organisms, and febrile neutropenia.
Carbapenems
Carbapenems are β-lactam derivatives that inhibit cell wall synthesis, resulting in bactericidal activity. The carbapenems are resistant to β-lactamases and therefore have one of the broadest spectra of activity. Carbapenems are effective against many gram-positive, gram-negative, and anaerobic organisms and are therefore useful for treatment of polymicrobial infections.
Prototype: imipenem-cilastatin sodium
Imipenem-cilastatin sodium (Primaxin) is used for treatment of lower respiratory tract, urinary tract, intra-abdominal, gynecological, and skin and soft tissue infections. Doses are diluted in 100 mL of IV solution and administered over 30 to 60 minutes. Imipenem-cilastatin requires dose adjustment for renal insufficiency.
Adverse effects include gastrointestinal effects such as nausea, vomiting, and diarrhea. Hypersensitivity reactions have been reported, and since imipenem is a β-lactam derivative there is a slight potential for cross-reactivity in patients allergic to penicillins or cephalosporins. Adverse CNS effects including seizures may occur with imipenem therapy. Imipenem is nephrotoxic when administered alone and therefore it is co-formulated with cilastatin, a substance with no antimicrobial activity that prevents the metabolism of imipenem in the renal tubules.
Related drugs
Meropenem (Merrem) is a synthetic carbapenem with a similar spectrum of activity as imipenem. It is used for treatment of intra-abdominal infections, bacterial meningitis, respiratory tract infections, and febrile neutropenia. Doripenem (Doribax) is the newest carbapenem and is similar to meropenem. Both meropenem and doripenem report seizures as an adverse effect, but the risk of seizures with these two agents is felt to be less than that with imipenem. Ertapenem (Invanz) has a slightly narrower spectrum of activity than the other carbapenems, lacking activity against Pseudomonas aeruginosa and Acinetobacter. It is often used in the outpatient setting since its long half-life allows for once-daily administration.
Aminoglycosides
Aminoglycosides are bactericidal antibiotics that are effective against several gram-negative organisms. Their name is derived from the amino sugars contained within their chemical structure.
Prototype: gentamicin sulfate
Gentamicin sulfate (Garamycin) is well distributed throughout all body fluids, achieves peak plasma concentrations within 30 minutes to 2 hours of administration, and maintains serum levels for up to 12 hours. It is often administered concurrently with a penicillin because the two drugs have synergistic bactericidal effects. Gentamicin is effective in the treatment of serious infections of the respiratory, urinary, and GI tracts; septicemia; and infections of the skin and soft tissue.
The most significant side effects associated with gentamicin are ototoxicity (associated with high peak plasma levels) and nephrotoxicity (associated with high trough levels). It has been suggested that this is the result of accumulation of the drug intracellularly in the inner ear and kidneys. Otic effects are manifested by vestibular symptoms (such as dizziness, nystagmus, vertigo, and ataxia) or auditory symptoms (such as tinnitus and hearing impairment). Nephrotoxicity is evidenced by increased blood urea nitrogen and serum creatinine levels and decreased creatinine clearance and urine specific gravity. Both ototoxicity and nephrotoxicity are more likely to occur in geriatric patients, patients with renal impairment, patients receiving high dosages or extended length of therapy, and patients receiving other ototoxic or nephrotoxic drugs. However, symptoms are usually reversible once the drug is discontinued.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access
