On completion of this chapter the reader will be able to: • Recognize common deviations from normal characteristics in the newborn. • Perform a systematic assessment of a high-risk newborn. • Outline a general care plan for a high-risk infant. • Recognize physiologic factors that compromise the preterm infant’s health status. • Discuss the role of the nurse in facilitating positive parent–infant relationships. • Contrast the physical characteristics of preterm and full-term infants. • Discuss the basis for screening newborns for health problems. • Discuss the rationale for performing newborn screening and genetic counseling when a newborn has a hereditary condition. • Modify a general care plan to meet the needs of an infant with specific high-risk health needs. http://evolve.elsevier.com/wong/essentials Animations—Erb Palsy; Paralyzed Diaphragm; Shoulder Dystocia Case Studies—Health Problems of the Newborn; Hyperbilirubinemia Nursing Care Plans—The High-Risk Infant with Respiratory Distress Syndrome; The Infant with Bronchopulmonary Dysplasia; The Newborn with Jaundice Trauma to the head and scalp that occurs during the birth process is usually benign but occasionally results in more serious injury. The injuries that produce serious trauma, such as intracranial hemorrhage and subdural hematoma, are discussed in relation to neurologic disorders in the newborn (see Table 9-9). Skull fractures are discussed in association with other fractures sustained during the birth process. The three most common types of extracranial hemorrhagic injury are caput succedaneum, cephalhematoma, and subgaleal hemorrhage. The most commonly observed scalp lesion is caput succedaneum, a vaguely outlined area of edematous tissue situated over the portion of the scalp that presents in a vertex delivery (Fig. 9-1, A). The swelling consists of serum, blood, or both accumulated in the tissues above the bone, and it often extends beyond the bone margins. The swelling may be associated with overlying petechiae or ecchymoses. No specific treatment is needed, and the swelling subsides within a few days. Infrequently, a cephalhematoma is formed when blood vessels rupture during labor or delivery to produce bleeding into the area between the bone and its periosteum. The injury occurs most often with primiparous delivery and is often associated with forceps delivery and vacuum extraction. Unlike caput succedaneum, the boundaries of the cephalhematoma are sharply demarcated and do not extend beyond the limits of the bone (suture lines) (Fig. 9-1, B). The cephalhematoma may involve one or both parietal bones. The occipital bones are less commonly affected, and the frontal bones are rarely affected. The swelling is usually minimal or absent at birth and increases in size on the second or third day. Blood loss is usually not significant. Subgaleal hemorrhage is bleeding into the subgaleal compartment (Fig. 9-1, C). The subgaleal compartment is a potential space that contains loosely arranged connective tissue; it is located beneath the galea aponeurosis, the tendinous sheath that connects the frontal and occipital muscles and forms the inner surface of the scalp. The injury occurs as a result of forces that compress and then drag the head through the pelvic outlet (Verklan and Lopez, 2011). Instrumented delivery, particularly vacuum extraction and forceps delivery, increase the risk of subgaleal hemorrhage. Additional risk factors include prolonged second stage of labor, fetal distress, macrosomia, failed vacuum extraction, and maternal primiparity (Doumouchtsis and Arulkumaran, 2006). The bleeding extends beyond bone, often posteriorly into the neck, and continues after birth, with the potential for serious complications such as anemia or hypovolemic shock. Early detection of the hemorrhage is vital; serial head circumference measurements and inspection of the back of the neck for increasing edema and a firm mass are essential. A boggy fluctuant mass over the scalp that crosses the suture line and moves as the baby is repositioned is an early sign of subgaleal hemorrhage (Doumouchtsis and Arulkumaran, 2006). Other signs include pallor, tachycardia, and increasing head circumference (Reid, 2007). An early sign of subgaleal hemorrhage is a forward and lateral positioning of the newborn’s ears because the hematoma extends posteriorly. Disseminated intravascular coagulation (DIC) has also been reported in association with subgaleal hemorrhage (Schierholz and Walker, 2010). Computed tomography (CT) or magnetic resonance imaging (MRI) is useful in confirming the diagnosis. Replacement of lost blood and clotting factors is required in acute cases of hemorrhage. Monitoring the infant for changes in level of consciousness and a decrease in the hematocrit are also key to early recognition and management. An increase in serum bilirubin levels may be seen as a result of the degradation of red blood cells (RBCs) within the hematoma. Nursing care is directed toward assessment and observation of the common scalp injuries and vigilance in observing for possible associated complications such as infection or, as in the case of subgaleal hemorrhage, acute blood loss and hypovolemia. Nursing care of a newborn with a subgaleal hemorrhage includes careful monitoring for signs of hemodynamic instability and shock (Schierholz and Walker, 2010). Because caput succedaneum and cephalhematoma usually resolve spontaneously, parents need reassurance of their usual benign nature. Fractures of the neonatal skull are uncommon. The bones, which are less mineralized and more compressible than bones in older infants and children, are separated by membranous seams that allow sufficient alteration in the head contour so that it adjusts to the birth canal during delivery. Skull fractures usually follow a prolonged, difficult delivery or forceps extraction. Most fractures are linear, but some may be visible as depressed indentations that compress or decompress like a ping-pong ball. Management of depressed skull fractures is controversial; many resolve without intervention. Nonsurgical elevation of the indentation using a hand breast pump or vacuum extractor has been reported (Mangurten and Puppala, 2011). Surgery may be required in the presence of bone fragments or signs of increased intracranial pressure (ICP) (Hill, 2008). A similar finding in neonates is craniotabes, which is usually benign or may be associated with prematurity or hydrocephalus (Johnson, 2009). In this condition, the cranial bone(s) move freely on palpation and may easily compress. Pressure on the facial nerve (cranial nerve VII) during delivery may result in injury to that nerve. The primary clinical manifestations are loss of movement on the affected side, such as an inability to completely close the eye, drooping of the corner of the mouth, and absence of wrinkling of the forehead and nasolabial fold (Fig. 9-2). The paralysis is most noticeable when the infant cries. The mouth is drawn to the unaffected side, the wrinkles are deeper on the normal side, and the eye on the involved side remains open. The clinical manifestations of Erb palsy are related to the paralysis of the affected extremity and muscles. The arm hangs limp alongside the body while the shoulder and arm are adducted and internally rotated. The elbow is extended, and the forearm is pronated, with the wrist and fingers flexed; a grasp reflex may be present because finger and wrist movement remain normal (Tappero, 2009) (Fig. 9-3). In lower plexus palsy, the muscles of the hand are paralyzed, with consequent wrist drop and relaxed fingers. In a third and more severe form of brachial palsy, the entire arm is paralyzed and hangs limp and motionless at the side. The Moro reflex is absent on the affected side for all forms of brachial palsy. Treatment of the affected arm is aimed at preventing contractures of the paralyzed muscles and maintaining correct placement of the humeral head within the glenoid fossa of the scapula. Complete recovery from stretched nerves usually takes 3 to 6 months. Full recovery is expected in 88% to 92% of infants (Verklan and Lopez, 2011). However, avulsion of the nerves (complete disconnection of the ganglia from the spinal cord that involves both anterior and posterior roots) results in permanent damage. For injuries that do not improve spontaneously by 3 months, surgical intervention may be needed to relieve pressure on the nerves or to repair the nerves with grafting (Joyner, Soto, and Adam, 2006). In some cases, injection of botulinum toxin A into the pectoralis major muscle may be effective in reducing muscle contractures after birth-related brachial plexus injuries (Price, Ditaranto, Yaylali, and others, 2007). If the eyelid of the eye on the affected side does not close completely, artificial tears can be instilled daily to prevent drying of the conjunctiva, sclera, and cornea. The eyelid is often taped shut to prevent accidental injury. If eye care is needed at home, the parents are taught the procedure for administering eye drops before the infant is discharged from the nursery (see Chapter 22). Nursing care of the newborn with brachial palsy is concerned primarily with proper positioning of the affected arm. The affected arm should be gently immobilized on the upper abdomen; passive range-of-motion exercises of the shoulder, wrist, elbow, and fingers are initiated at 7 to 10 days of age (Joyner, Soto, and Adam, 2006). Wrist flexion contractures may be prevented with the use of supportive splints. In dressing the infant, preference is given to the affected arm. Undressing begins with the unaffected arm, and redressing begins with the affected arm to prevent unnecessary manipulation and stress on the paralyzed muscles. Teach parents to use the “football” position when holding the infant and to avoid picking up the child from under the axillae or by pulling on the arms. Follow-up is also essential because of the extended length of recovery. Parents may wish to contact the Brachial Plexus Palsy Foundation* and visit the website for further information. Candidiasis, also known as moniliasis, is not uncommon in newborns. Candida albicans, the usual organism responsible, may cause disease in any organ system. It is a yeastlike fungus (it produces yeast cells and spores) that can be acquired from a maternal vaginal infection during delivery; from person-to-person transmission (especially from poor hand-washing technique); or from contaminated hands, bottles, nipples, or other articles. Mucocutaneous, cutaneous, and disseminated candidal infections are all observed in this age group. Candidiasis is usually a benign disorder in neonates, often confined to the oral and diaper regions. Diaper dermatitis caused by Candida organisms manifests as a moist, erythematous eruption with small white or yellow pebbly pustules. Small areas of skin erosion may also be seen (see Diaper Dermatitis, Chapter 30). Oral candidiasis (thrush) is characterized by white, adherent patches on the tongue, palate, and inner aspects of the cheeks (Fig. 9-4). It is often difficult to distinguish from coagulated milk. The infant may refuse to suck because of pain in the mouth. Topical application of 1 ml nystatin (Mycostatin) over the surfaces of the oral cavity 4 times a day, or every 6 hours, is usually sufficient to prevent spread of the disease or prolongation of its course. Several other drugs may be used, including amphotericin B (Fungizone), clotrimazole (Lotrimin, Mycelex), fluconazole (Diflucan), or miconazole (Monistat, Micatin) given intravenously, orally, or topically. To prevent relapse, therapy should be continued for at least 2 days after the lesions disappear (Lawrence and Lawrence, 2011). Gentian violet solution may be used in addition to one of the antifungal drugs in chronic cases of oral thrush; however, the former does not treat gastrointestinal Candida infection and may be irritating to the oral mucosa. Some practitioners avoid its use because it is messy, easily stains clothing, and may be irritating to the oral mucosa. Fluconazole is reportedly more effective than nystatin but it does not have Food and Drug Administration approval for use in infants (Su, Gaskie, and Jamieson, 2008). Nursing care is directed toward preventing spread of the infection and correctly applying the prescribed topical medication. For candidiasis in the diaper area, the caregiver is taught to keep the diaper area clean and to apply the medication to affected areas as prescribed (see also Diaper Dermatitis, Chapter 30). Older infants with candidal diaper dermatitis can introduce the yeast into the mouth from contaminated hands. Placing clothes over the diaper can prevent this cycle of self-infection. In addition to good hygienic care, other measures to control thrush include rinsing the infant’s mouth with plain water after each feeding before applying the medication and boiling reusable nipples and bottles for at least 20 minutes after a thorough washing (spores are heat resistant). If used, pacifiers should be boiled for at least 20 minutes once daily. If the mother is breastfeeding, it is recommended that simultaneous treatment of the infant and mother occur if either is infected (Lawrence and Lawrence, 2011). Neonatal herpes is one of the most serious viral infections in newborns, with a mortality rate of up to 60% in infants with disseminated disease. Approximately 86% to 90% of herpes simplex transmission occurs during passage through the birth canal (Shet, 2011). The risk of transmission of genital herpes during vaginal birth is estimated to be between 30% and 50% with active primary infection at term (Gardella and Brown, 2011). However, in up to 80% of cases of neonatal herpes simplex virus (HSV) infection, the mother has no history or symptoms of infection at the time of birth, but serologic testing reveals evidence of the herpes virus (Gardella and Brown, 2011). Neonatal herpes manifests in one of three ways: (1) with skin, eye, and mouth involvement (SEM); (2) as localized central nervous system (CNS) disease; or (3) as disseminated disease involving multiple organs. In skin and eye disease, a rash appears as vesicles or pustules on an erythematous base. Clusters of lesions are common. The lesions ulcerate and crust over rapidly. Most infants with neonatal herpes eventually develop this characteristic rash, but up to 20% of neonates with disseminated disease do not develop a skin rash (Kimberlin, 2007). Ophthalmologic clinical findings include chorioretinitis and microphthalmia; neurologic involvement such as microcephaly and encephalomalacia may also develop (James, Kimberlin, and Whitley, 2009). Disseminated infections may involve virtually every organ system, but the liver, adrenal glands, and lungs are most commonly affected. In HSV meningitis, infants develop multiple lesions of cortical hemorrhagic necrosis. It can occur alone or with oral, eye, or skin lesions. The presenting symptoms, which may occur in the second to fourth weeks of life, include lethargy, poor feeding, irritability, and local or generalized seizures. Neonates with herpesvirus or suspected infection (as a result of exposure) should be carefully evaluated for clinical manifestations. The absence of skin lesions in the neonate exposed to maternal herpesvirus does not indicate absence of disease. Contact precautions (in addition to standard precautions) should be instituted according to the American Academy of Pediatrics (AAP) and American College of Obstetricians and Gynecologists (ACOG) (2007) guidelines or hospital protocol. It is recommended that swabs of the mouth, nasopharynx, conjunctivae, rectum, and any skin vesicles be obtained from the exposed neonate; in addition, urine, stool, blood, and cerebrospinal fluid (CSF) specimens should be obtained for culture. Therapy with acyclovir and vidarabine is initiated if the culture results are positive or if there is strong suspicion of herpesvirus infection (AAP, Committee on Infectious Diseases, 2009; James, Kimberlin, and Whitley, 2009). High-dose acyclovir (60 mg/kg/day) has been shown to decrease mortality rates in infants with disseminated HSV (James, Kimberlin, and Whitley, 2009). Discolorations of the skin are common findings in newborn infants (see discussion on skin assessment of newborns, Chapter 8). Most, such as mongolian spots or telangiectatic nevi, involve no therapy other than reassurance to parents of the benign nature of these discolorations. However, some can be a manifestation of a disease that suggests further examination of the child and other family members (e.g., the multiple light brown café-au-lait spots that often characterize the autosomal dominant hereditary disorder neurofibromatosis and are common findings in Albright syndrome). Vascular birthmarks may be divided into the following categories: vascular malformations, capillary hemangiomas, and mixed hemangiomas. Vascular stains (malformations) are permanent lesions that are present at birth and are initially flat and erythematous. Any vascular structure, capillary, vein, artery, or lymphatic may be involved. The two most common vascular stains are the transient macular stain (stork bite, salmon patch, or angel kiss) and the port-wine stain, or nevus flammeus. The port-wine lesions are pink, red, or, rarely, purple stains of the skin that thicken, darken, and proportionately enlarge as the child grows (Fig. 9-5, A). The macular stain is most often located on the eyelids, glabella, or nape of the neck and usually fades over several months but may be prominent with crying or environmental temperature changes (Morelli, 2011). Capillary hemangiomas, sometimes referred to as strawberry hemangiomas, are benign cutaneous tumors that involve only capillaries. These hemangiomas are bright red, rubbery nodules with a rough surface and a well-defined margin (Fig. 9-5, B). Strawberry hemangiomas may not be apparent at birth but may appear within a few weeks and enlarge considerably during the first year of life and then begin to involute spontaneously. It may take 5 to 12 years for complete resolution. As many as 50% of patients may be left with residual findings such as telangiectasia, redundant fatty tissue, or skin atrophy (Alster and Railan, 2006). Cavernous venous hemangiomas involve deeper vessels in the dermis and have a bluish red color and poorly defined margins. These latter forms may be associated with the trapping of platelets (Kasabach-Merritt syndrome) and subsequent thrombocytopenia (Kelly, 2010; Witt, 2009). Hemangiomas may also occur as part of the PHACE syndrome (Sidbury, 2010): P—Posterior fossa brain malformation H—Hemangiomas (segmental cervicofacial) C—Cardiac defects, including coarctation of the aorta Although most hemangiomas require no treatment because of their high rate of spontaneous involution, some vision and airway obstruction may necessitate therapy. Systemic propranolol or prednisone may deter further growth. Subcutaneous injections of interferon or vincristine may be required if prednisone therapy and the pulsed-dye laser fail to control a problematic hemangioma; however, the associated side effects may outweigh the benefits of therapy in some cases (Holland and Drolet, 2010). Birthmarks, especially those on the face, are upsetting to parents. Families need an explanation of the type of lesion, its significance, and possible treatment.* They can benefit from seeing photographs of other infants before and after treatment for port-wine stains or after the passage of time for hemangiomas. Pictures taken to follow the involution process may further help parents gain confidence that progress is taking place. If laser therapy is performed, the lesion will have a purplish black appearance for 7 to 10 days, after which the blackness fades and gives way to redness with an eventual lightening of the treated area. During the treatment phase, parents are cautioned to avoid any trauma to the lesion or picking at the scab. The child’s fingernails are trimmed as an added precaution. Washing the area gently with water and dabbing it dry is adequate, although in some cases, a topical antibiotic ointment may be used. No salicylates should be taken during the treatment phase because they decrease the effects of the therapy. The child should be kept out of the sun for several weeks and then protected with a sunscreen of at least SPF 25. Complications associated with laser treatment include redness and bruising and, less commonly, hyperpigmentation, hypopigmentation, and atrophic scarring (Alster and Railan, 2006). There has been increased interest in late-preterm infants of 34 to The Association of Women’s Health, Obstetric and Neonatal Nurses has published the Late Preterm Infant Assessment Guide (Askin, Bakewell-Sachs, Medoff-Cooper, and others, 2007) for the education of perinatal nurses regarding the late-preterm infant’s risk factors and appropriate care and follow-up care. High-risk infants are most often classified according to birth weight, gestational age, and predominant pathophysiologic problems. The more common problems related to physiologic status are closely associated with the state of maturity of the infant and usually involve chemical disturbances (e.g., hypoglycemia, hypocalcemia) or consequences of immature organs and systems (e.g., hyperbilirubinemia, respiratory distress, hypothermia). Because high-risk factors are common to several specialty areas—particularly obstetrics, pediatrics, and neonatology—specific terminology is needed to describe the developmental status of the newborn (Box 9-1). Formerly, weight at birth was considered to reflect a reasonably accurate estimation of gestational age; that is, if an infant’s birth weight exceeded 2500 g (5.5 pounds), the infant was considered to be mature. However, accumulated data have shown that intrauterine growth rates are not the same for all infants and that other factors (e.g., heredity, placental insufficiency, maternal disease) influence intrauterine growth and birth weight. From these data, a more definitive and meaningful classification system that encompasses birth weight, gestational age, and neonatal outcome has been developed. (See Fig. 8-2 for size comparison of newborn infants.) Blood pressure (BP) is monitored routinely in sick neonates by either internal or external means. Direct recording with arterial catheters is often used but carries the risks inherent in any procedure in which a catheter is introduced into an artery. BP values gradually increase over the first month of life in preterm and term infants. BP norms vary by gestational age and weight, medications such as corticosteroids, and disease process. One of the primary considerations in the preterm infant is the relationship between systemic BP and the determination of adequate cerebral blood flow. In the neonatal intensive care unit (NICU), frequent laboratory examinations and their interpretation are integral parts of the ongoing assessment of infants’ progress. Accurate intake and output records are kept on all acutely ill infants. An accurate output can be obtained by collecting urine in a plastic urine collection bag specifically made for preterm infants (see Urine Specimens, Chapter 22) or by weighing the diapers, which is the simplest and least traumatic means of measuring urinary output. The preweighed wet diaper is weighed on a gram scale, and the gram weight of the urine is converted directly to milliliters (e.g., 25 g = 25 ml). Blood examinations are a necessary part of the ongoing assessment and monitoring of the high-risk newborn’s progress. The tests most often performed are blood glucose, bilirubin, calcium, hematocrit, serum electrolytes, and blood gases. Samples may be obtained from the heel; by venipuncture; by arterial puncture; or by an indwelling catheter in an umbilical vein, an umbilical artery, or a peripheral artery (see Atraumatic Care box, p. 210, and Collection of Specimens, Chapter 22).
Health Problems of Newborns
Birth Injuries
![]() Several factors predispose an infant to birth injuries (Mangurten and Puppala, 2011; Verklan and Lopez, 2011). Maternal factors include uterine dysfunction that leads to prolonged or precipitous labor, preterm or postterm labor, and cephalopelvic disproportion. Injury may result from dystocia caused by fetal macrosomia, multifetal gestation, abnormal or difficult presentation (not caused by maternal uterine or pelvic conditions), and congenital anomalies. Intrapartum events that can result in scalp injury include the use of intrapartum monitoring of fetal heart rate and collection of fetal scalp blood for acid–base assessment. Obstetric birth techniques can cause injury. Forceps birth, vacuum extraction, version and extraction, and cesarean birth are potential contributory factors. Often more than one factor is present, and multiple predisposing factors may be related to a single maternal condition.
Several factors predispose an infant to birth injuries (Mangurten and Puppala, 2011; Verklan and Lopez, 2011). Maternal factors include uterine dysfunction that leads to prolonged or precipitous labor, preterm or postterm labor, and cephalopelvic disproportion. Injury may result from dystocia caused by fetal macrosomia, multifetal gestation, abnormal or difficult presentation (not caused by maternal uterine or pelvic conditions), and congenital anomalies. Intrapartum events that can result in scalp injury include the use of intrapartum monitoring of fetal heart rate and collection of fetal scalp blood for acid–base assessment. Obstetric birth techniques can cause injury. Forceps birth, vacuum extraction, version and extraction, and cesarean birth are potential contributory factors. Often more than one factor is present, and multiple predisposing factors may be related to a single maternal condition.
Head Trauma
Caput Succedaneum
Cephalhematoma
Subgaleal Hemorrhage
Nursing Care Management
Fractures
Paralysis
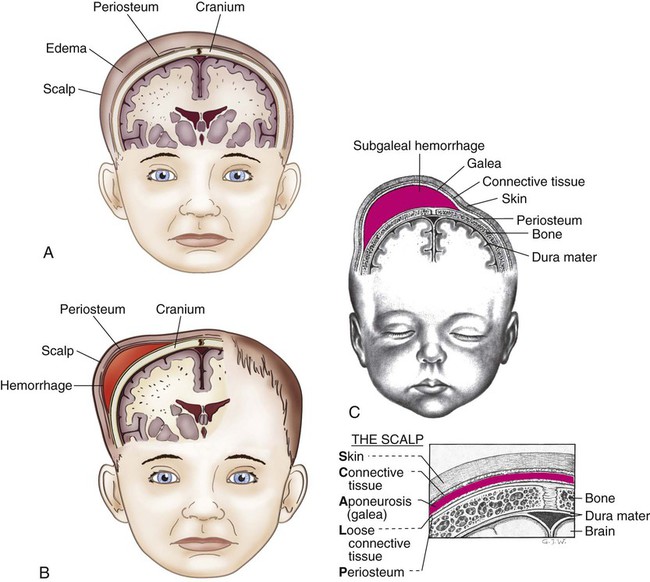
Facial Paralysis
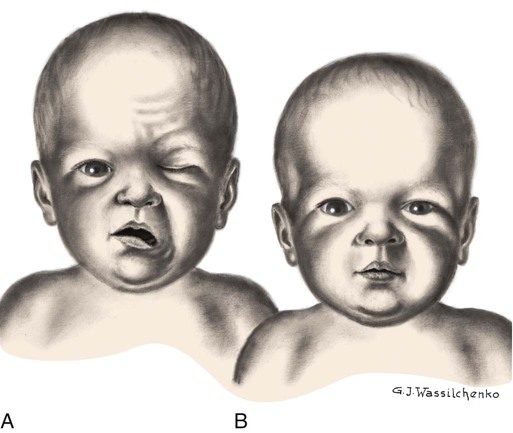
Brachial Palsy
![]() Plexus injury results from forces that alter the normal position and relationship of the arm, shoulder, and neck. Erb palsy (Erb-Duchenne paralysis) is caused by damage to the upper plexus and usually results from stretching or pulling away of the shoulder from the head, as might occur with shoulder dystocia or with a difficult vertex or breech delivery. Other identified risk factors include an infant with birth weight of more than 4000 g (8.8 pounds), a second stage of labor of less than 15 minutes, maternal body mass index greater than 29, a vacuum-assisted extraction, prolonged labor, and a previous history of brachial plexus injury (Hale, Bae, and Waters, 2009; Hudic, Fatusic, Sinanovic, and others, 2006). The less common lower plexus palsy, or Klumpke palsy, results from severe stretching of the upper extremity while the trunk is relatively less mobile.
Plexus injury results from forces that alter the normal position and relationship of the arm, shoulder, and neck. Erb palsy (Erb-Duchenne paralysis) is caused by damage to the upper plexus and usually results from stretching or pulling away of the shoulder from the head, as might occur with shoulder dystocia or with a difficult vertex or breech delivery. Other identified risk factors include an infant with birth weight of more than 4000 g (8.8 pounds), a second stage of labor of less than 15 minutes, maternal body mass index greater than 29, a vacuum-assisted extraction, prolonged labor, and a previous history of brachial plexus injury (Hale, Bae, and Waters, 2009; Hudic, Fatusic, Sinanovic, and others, 2006). The less common lower plexus palsy, or Klumpke palsy, results from severe stretching of the upper extremity while the trunk is relatively less mobile.
Phrenic Nerve Paralysis
![]() Phrenic nerve paralysis results in diaphragmatic paralysis as demonstrated by ultrasonography, which shows paradoxic chest movement and an elevated diaphragm. Initially, radiography may not demonstrate an elevated diaphragm if the neonate is receiving positive-pressure ventilation (Volpe, 2008). The injury sometimes occurs in conjunction with brachial palsy. Respiratory distress is the most common and important sign of injury. Because injury to the phrenic nerve is usually unilateral, the lung on the affected side does not expand, and respiratory efforts are ineffectual. Breathing is primarily thoracic, and cyanosis, tachypnea, or complete respiratory failure may be seen. Pneumonia and atelectasis on the affected side may also occur.
Phrenic nerve paralysis results in diaphragmatic paralysis as demonstrated by ultrasonography, which shows paradoxic chest movement and an elevated diaphragm. Initially, radiography may not demonstrate an elevated diaphragm if the neonate is receiving positive-pressure ventilation (Volpe, 2008). The injury sometimes occurs in conjunction with brachial palsy. Respiratory distress is the most common and important sign of injury. Because injury to the phrenic nerve is usually unilateral, the lung on the affected side does not expand, and respiratory efforts are ineffectual. Breathing is primarily thoracic, and cyanosis, tachypnea, or complete respiratory failure may be seen. Pneumonia and atelectasis on the affected side may also occur.
Nursing Care Management
Common Problems in the Newborn
Erythema Toxicum Neonatorum
![]() Case Study—Health Problems of the Newborn
Case Study—Health Problems of the Newborn
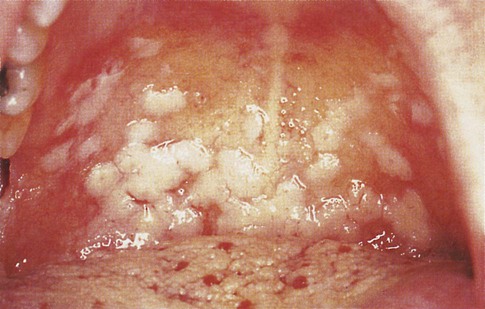
Candidiasis
Oral Candidiasis
Nursing Care Management
Herpes Simplex Virus
Nursing Care Management
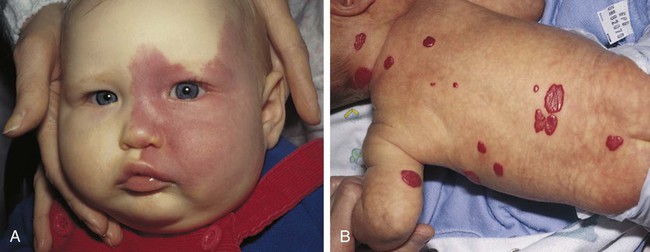
Birthmarks
Nursing Care Management
Nursing Care of the High-Risk Newborn and Family
Identification of High-Risk Newborns
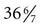 weeks of gestation who may receive the same treatment as term infants. Late-preterm infants often experience similar morbidities to preterm infants, including respiratory distress, hypoglycemia requiring treatment, temperature instability, poor feeding, jaundice, and discharge delays, as a result of illness. Therefore, assessment and prompt intervention in life-threatening perinatal emergencies often make the difference between a favorable outcome and a lifetime of disability. It is estimated that late-preterm infants represent 70% of the total preterm infant population and that the mortality rate for this group is significantly higher than that of term infants (7.9 vs. 2.4 per 1000 live births, respectively) (Tomashek, Shapiro-Mendoza, Davidoff, and others, 2007). Because late-preterm infants’ birth weights often range from 2000 to 2500 g (4.4–5.5 pounds) and they appear relatively mature compared with smaller preterm infants, they may be cared for in the same manner as healthy term infants while risk factors for late-preterm infants are overlooked. Late-preterm infants are often discharged early from the birth institution and have a significantly higher rate of rehospitalization than term infants (Escobar, Clark, and Greene, 2006). Discussions regarding high-risk infants in this chapter also refer to late-preterm infants who are experiencing a delayed transition to extrauterine life. Nurses in newborn nurseries should be familiar with the characteristics of neonates and recognize the significance of serious deviations from expected observations. When providers can anticipate the need for specialized care and plan for it, the probability of successful outcome is increased.
weeks of gestation who may receive the same treatment as term infants. Late-preterm infants often experience similar morbidities to preterm infants, including respiratory distress, hypoglycemia requiring treatment, temperature instability, poor feeding, jaundice, and discharge delays, as a result of illness. Therefore, assessment and prompt intervention in life-threatening perinatal emergencies often make the difference between a favorable outcome and a lifetime of disability. It is estimated that late-preterm infants represent 70% of the total preterm infant population and that the mortality rate for this group is significantly higher than that of term infants (7.9 vs. 2.4 per 1000 live births, respectively) (Tomashek, Shapiro-Mendoza, Davidoff, and others, 2007). Because late-preterm infants’ birth weights often range from 2000 to 2500 g (4.4–5.5 pounds) and they appear relatively mature compared with smaller preterm infants, they may be cared for in the same manner as healthy term infants while risk factors for late-preterm infants are overlooked. Late-preterm infants are often discharged early from the birth institution and have a significantly higher rate of rehospitalization than term infants (Escobar, Clark, and Greene, 2006). Discussions regarding high-risk infants in this chapter also refer to late-preterm infants who are experiencing a delayed transition to extrauterine life. Nurses in newborn nurseries should be familiar with the characteristics of neonates and recognize the significance of serious deviations from expected observations. When providers can anticipate the need for specialized care and plan for it, the probability of successful outcome is increased.
Classification of High-Risk Newborns
Care of High-Risk Newborns
Systematic Assessment
Monitoring Physiologic Data

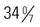 and
and 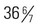 weeks of gestation regardless of birth weight
weeks of gestation regardless of birth weight

