5. Wound healing in the surgical patient
Rosie Pudner
CHAPTER CONTENTS
The structure and function of the skin52
Mechanisms of wound closure54
The process of wound healing54
Factors affecting the healing process56
Methods of skin closure58
Use of wound drains62
Principles of care for a patient with a surgical wound65
Discharge advice72
Conclusion73
At the end of the chapter the reader should be able to:
• describe the structure and function of the skin
• discuss the different mechanisms of wound closure
• discuss the normal physiological process of wound healing and factors which may affect healing
• state the various methods used in wound closure
• discuss the use and care of surgical wound drains
• discuss the principles of caring for a patient with a surgical wound
• discuss potential postoperative complications of wound healing.
Introduction
Surgical wounds are formed from an incision in the skin and underlying structures, which is usually performed in a clean environment where asepsis is maintained at all times. The majority of surgical wounds heal by primary (first) intention, with minimal intervention from the nurse. The type of incision, manner and type of materials used in skin closure, and length of time spent in hospital have changed over the years, which has influenced the delivery of wound management.
The main principles of surgical wound management are:
• to achieve healing of the wound
• to avoid complications, e.g. infection
• to achieve good pain control
• to ensure a cosmetically acceptable scar
• to allow the individual to return to a normal lifestyle as soon as possible.
A holistic approach to the care of a surgical patient will assist in achieving the above. Postoperative complications of the wound can be minimized by the preoperative care given to the patient, patient education and an evaluation of healing of the wound. A sound knowledge of the structure and functions of the skin, the physiology of wound healing and factors which may interfere with this process are essential if a nurse is to deliver an optimum standard of wound care. This knowledge is fundamental to the assessment of an individual with a wound and future management, with regard to cleansing and the application of an appropriate wound dressing.
The structure and function of the skin
The skin is the largest organ (i.e. related to surface area) of approximately 2 square metres. The skin covers the body and gives protection to underlying structures. It varies in thickness in different parts of the body and has variations in its pigmentation too (Tortora and Derrickson, 2006). The skin performs five main functions:
1. Protection: the skin acts as a physical barrier, protecting underlying structures from minor mechanical trauma, chemicals and gases, bacterial invasion, dehydration, cold, heat and ultraviolet (UV) radiation.
2. Sensation: the skin is the largest sensory organ of the body as it contains numerous nerve endings which are sensitive to temperature, pain, touch, pressure and vibration, giving information about the external environment which can then be acted upon.
3. Temperature regulation: the skin plays a vital role in maintaining a constant core temperature. Homeostasis is achieved by conduction, convection or radiation of heat from the surface of the skin. Secretion and evaporation of sweat assists in cooling the body. The circulatory mechanisms of vasodilatation and vasoconstriction also help to control the temperature of the body.
4. Excretion: water, salts and other organic materials are excreted through the skin.
5. Synthesis of vitamin D: the effect of ultraviolet rays falling on the skin brings about the synthesis within it of vitamin D from 7-dehydrocholecalciferol, which indirectly promotes the absorption of calcium from the gut.
The skin also has an absorptive capability, as it can absorb various substances, e.g. oestrogens, glyceryl trinitrate, etc. These substances can be applied as a slow-release skin patch, allowing the substance to be slowly absorbed through the skin (Hinchliff et al, 1996). Certain cells within the epidermis, i.e. Langerhans cells, also undertake a role in bolstering immunity.
The skin is a complex and multilayered structure, comprising the epidermis, dermis and subcutaneous tissue. These layers and the structures within each layer will now be discussed.
The epidermis
The epidermis is the most superficial layer and is connected to the dermis. It is avascular, receiving nutrients from the dermis below, and is composed of keratinized stratified squamous epithelial cells. Four principal types of cells are found in this layer: keratinocytes, melanocytes, Langerhans cells and Merkel cells. The epithelial cells are produced in the basal layer and gradually migrate upwards over a period of 40–56 days.
The epidermis is made up of four to five layers of cells, depending on the location in the body. The first layer, next to the dermis, is the stratum basale. Cell division occurs here, with the cells dipping down into the dermis to surround sweat glands and hair follicles. Keratin is manufactured by the keratinocytes found in this layer. Keratin is an insoluble protein that is resistant to changes in temperature and pH, and helps waterproof and protect the skin. Also found in this layer are Merkel cells, which are in contact with the flattened end of sensory neurons, and are involved in the sensation of touch. The second layer is the stratum spinosum, which contains prickle cells and Langerhans cells (Tortora and Derrickson, 2006). The prickle cells prevent cell separation by their intercellular bridges. Langerhans cells participate in immune responses and are thought to have a role in allergic or immunological skin disorders (Bennett and Moody, 1995).
The third layer is the stratum granulosum, where the keratinocytes flatten and accumulate lamellar granules. Secretions from the lamellar granules slow the loss of body fluids and entry of foreign materials (Tortora and Derrickson, 2006). The fourth layer is the stratum lucidum, which is only found in the thick skin on the palms of the hands and soles of the feet, i.e. areas of excessive wear and tear. The cells in this layer start to undergo nuclear degeneration and contain large amounts of keratin. The fifth and final layer is the stratum corneum, consisting of many layers of dead cells that are completely filled with keratin. These cells are constantly shed from the body surface, i.e. desquamation, as a result of friction and washing. They also have the ability to soak up extra moisture (Hinchliff et al, 1996).
The dermis
The dermis lies beneath the epidermis and forms the main part of the skin, providing the skin with its strength and elasticity. It is formed of connective tissue containing collagen and elastic fibres, and contains blood and lymph vessels, sensory nerve endings, hair follicles, and sweat and sebaceous glands. In the dermo–epidermal junction, melanin is produced by melanocytes, under the influence of sunlight. Melanin gives colour to various body structures, e.g. hair, iris and skin, but the main function of melanin is to protect the body from ultraviolet light (Tortora and Derrickson, 2006).
The upper region of the dermis is the papillary region, which consists of a series of undulations called dermal papillae. This cellular arrangement prevents the epidermis shearing off from the dermis when shearing forces are applied to the skin. The reticular layer is the remaining portion of the dermis, and consists of dense, irregularly arranged connective tissue containing interlacing bundles of collagenous, reticular and coarse elastic fibres. It is within the spaces between these fibres that hair follicles, sensory nerves and sweat glands are located (Hinchliff et al, 1996).
The dermis is constructed of ground substance or matrix, various fibres and cells. Ground substance is an amorphous matrix, resembling a gel, which provides connective tissue with its bulk. It is permeated by strands of collagen, elastin and fibronectin and other cells. The gelatinous material is composed of water, electrolytes, glycoproteins and proteoglycans, and is synthesized by fibroblasts (Hinchliff et al, 1996).
Collagen, reticular fibres and elastin fibres are all produced by mesodermal fibroblasts, situated in the dermis. Collagen is a family of connective tissue proteins with immense tensile strength, due to their rigid and durable structure. The fibres come together to form thick bundles in which numerous cross-links form, so increasing the strength of the fibres. Collagens are important as a structural support, but also control many cellular functions, including cell shape and differentiation. Ascorbic acid is necessary for the formation of collagen. There are many types of collagen which have been identified, with Types I and III being found to be important in wound healing (Fletcher, 2000). Type I is usually physically allied with Type III collagen, whereby Type III collagen synthesis is dominant in the early stage of wound healing, but with Type I synthesis being more predominant in the later stages of healing. The reticular fibres form a loose framework in the dermis and envelop the collagen bundles. The elastin fibres (elastin) are branching yellow fibres which provide elasticity and resilience to the skin (Hopkinson, 1992).
Four types of cell are found in the dermis: fibroblasts, tissue macrophages, tissue mast cells and white blood cells. The fibroblasts lie between the collagen bundles and are concerned with collagen and elastin synthesis. The tissue macrophages or histiocytes are wandering phagocytic cells. Tissue mast cells produce histamine and heparin, and are found near blood vessels and hair follicles. Neutrophils, lymphocytes and monocytes are transient cells, and are constantly moving between blood vessels (Hinchliff et al, 1996).
The cutaneous blood vessels lie entirely within the dermis and have a rich sympathetic nerve supply, so allowing vasoconstriction or vasodilatation, depending on the local environment. The lymphatic vessels are found throughout the dermis and are responsible for draining excess tissue fluid and plasma proteins which may have leaked into the tissues. The dermis contains sensory nerves which have three types of nerve ending, each responding to a specific stimulus. The hair follicles lie in the dermis, each surrounded by its own blood and nerve supply. The basal layer of the epidermis dips down to surround the hair follicle, so the process of epithelialization can occur from this area. The sweat glands – eccrine and apocrine – are formed as coiled tubular downgrowths from the epidermis. The sebaceous glands are formed as outgrowths of the developing hair follicles, producing sebum which waterproofs the skin and has some action against fungal and bacterial infections (Hinchliff et al, 1996).
Subcutaneous fat
Adipose tissue lies beneath the dermis and is a valuable store of triglycerides, which provide a potential source of energy. The adipose tissue insulates the body, so preventing heat loss, and acts as a shock absorber, so preventing trauma to the underlying structures. It has very little ground substance, and the tissue is divided into lobes by septa which carry blood vessels and nerves. The cells which make up adipose tissue consist of a flat nucleus surrounded by a large single fat globule.
Mechanisms of wound closure
There are three mechanisms of wound closure: primary intention, secondary intention and tertiary intention (delayed primary closure).
• Primary intention. The skin edges are pulled together and held in apposition by a mechanical means, e.g. sutures, staples, adhesive strips or tissue adhesive. This method of closure is adopted in most surgical incisional wounds.
• Secondary intention. The wound is left open to allow granulation, contraction and epithelialization to occur. This method is adopted if there is extensive tissue loss, a large superficial surface area, or presence of infection.
• Tertiary intention (delayed primary closure). The wound is initially left open to allow granulation to begin, and after approximately 3–5 days wound closure is achieved by either approximation of the skin edges or by the application of a skin graft. This method is adopted in wounds where there is a high risk of contamination and possible infection; a poor blood supply which may lead to non-viable tissue; or excessive swelling in the area, as in orthopaedic trauma. The wound is managed in this manner in order to ensure that the wound is not infected, has a good blood supply and swelling has reduced before skin closure is attempted (Westaby, 1985).
The process of wound healing
Wound healing is a complex systematic process comprising a complex interaction of cellular, chemical and physical events. This process is divided into several stages or phases of wound healing. As wound healing is a continuous process, there will be some overlap between the phases. The phases of wound healing are: haemostasis, early inflammatory phase, late inflammatory (destructive) phase, proliferative phase (including epithelialization and contraction) and the maturation phase.
The extent and timing of these phases are controlled by a variety of mediators, e.g. growth factors. Growth factors are proteins which are secreted from a variety of cells, acting as soluble mediators in cutaneous repair. Their effects are exerted locally via specific receptors on the surface membrane of the targeted cells within the wound. They create a vital signalling network for the regulation, coordination and control of cellular interactions during wound healing (Schultz, 2007).
Haemostasis and the early inflammatory phase
The early inflammatory phase occurs from the time of injury to approximately 3 days. The body’s immediate response to wounding is to stop bleeding and prevent the entry of microorganisms. Vasoconstriction occurs in the immediate area, due to the release of serotonin and other chemical mediators from the platelets, which helps to reduce the flow of blood to the wound. Damage to the blood vessels in the wound causes the platelets to become sticky and clump together to form a platelet thrombus, so further reducing blood loss. The clotting cascade is also initiated following injury to the vascular endothelium, resulting in the formation of a fibrin thrombus in the wound (Silver, 1994).
Inflammation is a natural response to trauma. A number of mediators (chemical substances), including prostaglandins, are released into the wound, resulting in vasodilatation, increased capillary permeability and the stimulation of pain fibres. The effect of increased capillary permeability is that mediators, plasma proteins, antibodies, neutrophils and monocytes migrate into the wound and surrounding tissue. The neutrophils and macrophages phagocytose any microorganisms or dead tissue present in the wound space. The wound and surrounding area will therefore appear red, swollen, hot and painful, with possible loss of function.
The late inflammatory phase
The late inflammatory phase occurs approximately 2–5 days after injury. The polymorphonuclear leucocytes (polymorphs) and macrophages continue the process of phagocytosis, so cleaning the wound of any debris or microorganisms. The tissue macrophages also control wound healing through the production of a variety of growth factors, e.g. platelet-derived growth factor (PDGF), transforming growth factor (TGF), interleukin (IL) and tumour necrosis factor (TNF) (Schultz, 2007). These growth factors stimulate the production of various cells, e.g. PDGF stimulates the growth of blood vessels (angiogenesis). This process demands substantial resources and energy, and considerable amounts of heat and fluid can be lost, especially in an open wound.
The proliferative phase
Following the initial inflammatory response, the phase of tissue repair takes place. This occurs from approximately 4–28 days, but may be longer in some wounds. The macrophages continue phagocytosis of cell debris and microorganisms. Through monocyte-derived growth factor (MDGF), the macrophages attract fibroblasts to the area. The fibroblasts, in the presence of vitamin C, ferrous iron, nutrients, oxygen and a slightly acidic environment, produce collagen fibrils, which are laid down in a haphazard fashion. Vitamin C is vital in this phase of healing, as it is involved in the hydroxylation of proline in collagen to hydroxyproline, which aids the cross-linkage of the collagen fibres. Endothelial cells respond to the secretion of various growth factors and form new capillaries, which grow into the wound. This process is known as angiogenesis and is stimulated by the hypoxic environment. The matrix of the collagen fibres forms scaffolding for the new capillaries, while the new capillaries provide nutrients and oxygen for continued growth (Doughty and Sparks-Defriese, 2007). This process is often called granulation because in a wound healing by secondary intention the wound bed appears red and granular. As the wound defect is filled with the newly formed tissue, the numbers of macrophages and fibroblasts diminish.
Contraction of the wound can also occur in wounds healing by secondary intention during this phase of healing, and is thought to be due to the presence of myofibroblasts within the wound. Myofibroblasts are cells containing the features of a fibroblast and a smooth muscle cell, which appear to have contractile qualities, so reducing the surface area of the wound (Monaco and Lawrence, 2003).
Epithelialization is the last stage in this phase of wound healing. Epithelial cells at the wound edges divide and migrate across the surface of the wound until they meet other epithelial cells. When this occurs, migration ceases, a process called contact inhibition. If the remnants of hair follicles are still present in the wound bed, epithelial cells will migrate from these areas and traverse the wound bed until they meet other epithelial cells. The rate of epithelialization is enhanced by maintaining a moist environment, as it allows the epithelial cells to migrate across the surface of the wound more easily (Winter, 1962) (Fig. 5.1). However, in surgical wounds it is important that the incisional wound is not allowed to become too wet as maceration can occur, so affecting the cosmetic result of the scar.
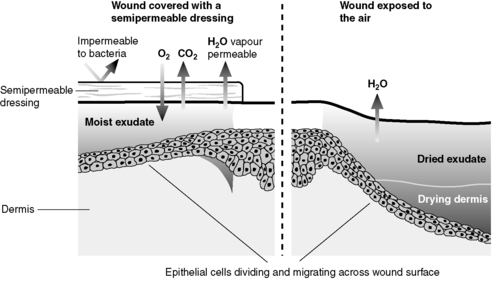 |
| Figure 5.1 • Epithelialization in wound healing in a moist and in a dry environment. |
The maturation phase
This is the final phase of wound healing and occurs from 15 days to 365 days approximately. The original Type III collagen laid down in the wound bed is converted to Type I collagen, which is laid down following the tension lines within the wound, and is also cross-linked so as to give strength to the scar tissue. As the remodelling process continues, cellular activity reduces and there is a gradual closure of the nutrient blood vessels in the wound. The scar should become paler and flatter in appearance. However, for some people this process may lead to hypertrophic scars (thickened appearance of the scar), whereas keloid scarring is due to a local disturbance during the healing process where the scar tissue extends outside the wound margins (Doughty and Sparks-Defriese, 2007).
Factors affecting the healing process
A variety of factors can affect the healing process, slowing down the rate of healing or impairing healing altogether. These can be divided into intrinsic factors, extrinsic factors, and social and psychological factors (Box 5.1).
 Box 5.1
Box 5.1 Factors affecting wound healing
Intrinsic
• Advanced age
• Dehydration
• Disease processes
• Impaired blood supply
• Poor nutritional state
• Reduced supply of oxygen
Social factors
• Poverty
• Poor housing
• Cultural/religious beliefs
• Patient’s lifestyle
Extrinsic
• Drug therapy
• Infection
• Inappropriate wound management
• Obesity
• Poor surgical technique
• Smoking
• Stress
• Wound temperature
Psychological factors
• Motivation of the patient
• Concordance with treatment
• Knowledge and understanding of patient/carer
• Altered body image
Intrinsic factors
Advanced age
The inflammatory response is reduced, so increasing the likelihood of invasion by microorganisms and infection. Increasing age reduces fibroblastic activity and migration. Collagen metabolism is reduced, with the collagen being weaker and thinner and so not able to support the blood vessels in the dermis, causing the blood vessels to be easily damaged. Angiogenesis is delayed and epithelialization is hindered (Desai, 1997). Advanced age is also often accompanied by multiple medical problems which may affect wound healing, e.g. cardiac and respiratory problems.
Dehydration
A person who is dehydrated is not able to metabolize efficiently, and subsequent electrolyte imbalance can impair cellular function. An adequate intake of 2–2.5 litres of fluid a day is required for efficient metabolism.
Disease processes
Cancer, diabetes, inflammatory diseases, jaundice and diseases affecting the immune response all have an influence on wound healing (Moncada, 1992). Patients suffering from cancer often receive chemotherapy in order to destroy the malignant cells in the body, or undergo radiotherapy. Radiotherapy has a fibrosing effect on the local blood vessels, so impairing the blood supply to that area. At the end stage of the disease the patient may suffer from cachexia, i.e. a chronic state of malnutrition produced by the absorption of toxins.
Diabetes can delay healing for a variety of reasons, due to the altered metabolism associated with diabetes (Silhi, 1998). Hyperglycaemia has a deleterious effect on phagocytosis, so increasing the risk of infection. Decreased tensile strength is due to a decreased collagen synthesis and retarded capillary ingrowth (Greenhalgh, 2003). Jaundice appears to affect the tensile strength of the wound, and is sometimes associated with abdominal wound dehiscence (Carlson, 1997). Uraemia causes a delay in the proliferative stage in healing, i.e. laying down of granulation tissue (Orgill and Demling, 1988).
Impaired oxygen delivery to the area
Reduced supply of oxygen can be caused by prolonged hypoxia due to shock, anaemia, impaired arterial blood supply, or in patients with chronic obstructive airways disease. Inflammation is delayed, as the neutrophils are not able to reach the wound, and collagen synthesis and epithelial growth are impaired. Without oxygen, ischaemia results and the newly formed tissue is compromised (Cooper, 1990). Excessive caffeine intake, through drinking large amounts of coffee or cola drinks, can lead to vasoconstriction (Bonavita, 1985), which will lead to impaired tissue perfusion.
Impaired nutritional status
Adequate supplies of protein, calories, vitamins C and K, zinc and copper are required for wound healing. If supplies are inadequate due to poor intake, abnormal absorption or greatly increased demands, this can result in poor wound healing, reduced tensile strength of the scar, increased risk of wound dehiscence, increased susceptibility to infection and poor-quality scars (Haridas and Malangoni, 2008 and McLaren, 1992). (See Ch. 6 for further information on nutrition and wound healing.)
Extrinsic factors
Drug therapy
Steroids and non-steroidal anti-inflammatory drugs (NSAIDs) reduce the normal inflammatory response. Corticosteroids also suppress the synthesis of fibroblasts and collagen, with long-term usage leading to ‘tissue paper’ skin, which is easily damaged. Cytotoxic drugs delay the inflammatory response, suppress protein synthesis and inhibit the replication of cells. Immunosuppressive drugs reduce white blood cell activity, delaying the inflammatory response and increasing the risk of infection. Anticoagulant therapy, if not given in the correct dosage, can cause excessive bleeding and the potential formation of a haematoma.
Infection
Healing is delayed as invading bacteria compete with the macrophages and fibroblasts for oxygen and nutrients at the wound surface. The inflammatory phase is prolonged, collagen synthesis is delayed, and epithelialization may be prevented. Infection can lead to further local tissue destruction as a result of inflammatory cytokines being produced, and can cause the formation of an abscess and breakdown of the wound (Doughty and Sparks-Defriese, 2007).
Inappropriate wound management
The inappropriate application of a dressing which causes maceration of the surrounding skin or adheres to the wound bed, inaccurate assessment of the patient and their wound, or failure to evaluate care can all lead to inappropriate management of the patient’s wound.
Obesity
It has been shown, especially in abdominal surgery, that obesity can lead to an increased risk of infection in clean wounds (Cruse and Foord, 1973 and Wilson and Clark, 2003). Obesity decreases perfusion to the wounded tissues, and, as a consequence, wound infection and wound dehiscence can occur. Contraction is reduced and the risk of dehiscence is also increased, because of the amount of tension exerted on the wound in an obese patient.
Smoking
Smoking has a vasoconstricting effect, inhibits epithelialization, can affect the immune response and can cause problems with scarring. Siana et al (1992) found that the width of scars in smokers was twice that in non-smokers, and the scars of smokers tended to be lighter, so giving an overall poorer cosmetic result. Smoking can also lead to a deficiency in vitamin C, an essential factor needed for tissue repair.
Poor surgical technique
If any type of tissue is handled roughly during surgery, then it can become devitalized and so provide a suitable site for infection. If haemostasis is not achieved or a drain is not inserted in a dead space, then a haematoma can form. This can cause tissue damage through the pressure exerted at the edges of the wound, and is also an ideal environment in which microorganisms can grow. The inappropriate use of diathermy can also cause problems with healing, and if sutures or staples are applied too tightly, then the result is damaged tissue and tissue death, as well as a poor cosmetic result (Singer et al, 2002).
Stress
Psychological problems may well affect the health of a patient, and it is known that stress has an effect on the immune system (Maier and Laudenslager, 1985) and on wound healing (Broadbent et al., 2003 and Kielcolt-Glaser et al., 1995). The stress of surgery is known to stimulate the sympathetic nervous system, and continues into the postoperative period. Stress caused by hypoxia, hypothermia, pain and hypovolaemia stimulates the sympathetic nervous system, whereby excess levels of noradrenaline (norepinephrine) cause vasoconstriction and altered peripheral perfusion, so decreasing the oxygen available for healing (West, 1990). The release of glucocorticoids may also impair the inflammatory response.
Temperature
Frequent dressing changes and the use of cleansing solutions at room temperature significantly reduce intrawound temperature (McGuiness et al, 2004). Cell division takes place at normal body temperature, and a drop of 1°C takes up to 3 hours for mitotic cell division to recommence (Lock, 1980), so delaying the healing process.
Social and psychological factors
Social factors
Poverty can lead to a poor nutritional intake. It can also affect the patient’s ability to afford to have sufficient heating during the cold weather, so causing peripheral vasoconstriction and a decreased blood supply to the wound. Poor housing can mean a lack of cleanliness, so increasing the risk of wound infection. Cultural and religious beliefs can have an influence on the patient’s diet, hygiene and acceptance of medical interventions. The lifestyle of the patient can influence healing, especially if the person smokes, drinks excessive amounts of alcohol or abuses drugs.
Psychological factors
Poor motivation of the patient and/or carers can affect concordance with treatment, as they may lack the capability to continue with the recommended treatment of the wound. This may also be linked to a lack of knowledge or understanding of the wound and how it is to be managed. The effect of surgery on the body and the resulting scar can alter the patient’s body image. (See Ch. 7 for further information on altered body image and the surgical patient.)
Methods of skin closure
The purpose of wound closure is to achieve approximation of the wound edges to produce a strong scar, with minimal disturbance of function and a good cosmetic result. In wounds healing by primary intention, various types of suture material, staples, adhesive strips and tissue adhesive can be used to bring the skin edges together and hold the edges in apposition until healing has occurred. Choice of wound closure and technique depends upon the type of tissue, the position of the wound and the surgeon’s preference.
Sutures
Sutures are used to promote healing by eliminating dead space in a wound, realigning tissue planes and holding the skin edges in apposition until healing has taken place and the wound no longer needs the support of the suture material. They can be used to aid haemostasis, but if applied too tightly can cause necrosis of the surrounding tissue. The type of suturing technique, technique of knot tying and the width of the tissue bites all affect wound strength and healing.
Suture materials are chosen for their strength, handling characteristics and absorptive properties. Different types of suture material are required in a variety of circumstances, and for different types of tissue and parts in the body. The choice of suture material depends on the rate at which the tissue is likely to heal, the amount of strain or stress to which the wound site will be subjected, the likely growth of the wound and whether the suture is to give temporary or permanent support to the wound (Ethicon, 2005). The surgeon will select a suture material which loses its strength relative to the gain of strength in the wound itself as it heals.
Types of suture material
Suture materials are either absorbable or non-absorbable (Table 5.1). Absorbable sutures are made out of material which is digested either by proteolytic enzymes released from the polymorphonuclear cells, or by hydrolysis whereby the action of water on the suture causes the breakdown of the material. The action of hydrolysis is increased by a rise in temperature or a change in the pH. Non-absorbable sutures are made out of materials which resist enzymatic digestion, and therefore need removal when applied to the skin, e.g. Prolene™.
| Suture | Type | Absorption | Area of use |
|---|---|---|---|
| Absorbable suture materials | |||
| Coated VICRYL™ RAPIDE | Braided | 42 days | Skin; perineum; scalp; oral |
| MONOCRYL™ | Monofilament | 90–120 days | Subcuticular; muscle |
| Coated VICRYL™ | Braided Monofilament | 56–70 days | Ligating. Suturing all tissues except where extended approximation is required Ophthalmic surgery only |
| Coated VICRYL™ Plus | Braided | 56–70 days | Ligating. Suturing all tissues except where extended approximation is required |
| PDS™ II | Monofilament | 180–210 days | All tissues except where approximation is required indefinitely |
| Non-absorbable suture materials | |||
| PROLENE™ | Monofilament | Non-absorbable. Remains encapsulated in tissue | Cardiac; fascia; skin; blood vessels |
| ETHILON™ | Monofilament | Fascia; skin; nerves; blood vessels | |
| ETHIBOND™ EXCEL | Braided | Vascular; cardiac | |
| NUROLON™ | Braided | Most body tissues; skin | |
| MERSILENE™ | Monofilament | Most body tissues | |
| MERSILK™ | Braided | Ligating most tissues (buried); skin | |
| Virgin silk | Twisted | Ophthalmic | |
| Stainless steel | Monofilament/ multifilament | Cardiac; thoracic | |
| PRONOVA™ | Monofilament | Cardiac; vascular | |
Non-absorbable skin sutures are left in place for different periods of time, depending on the site of the wound and the amount of tension the wound is under (Box 5.2). Any non-absorbable suture material that is left in place for too long can cause excessive scarring, and is a focus for infection, leading to the formation of a stitch abscess or sinus (Harding and Jones, 1996). However, it is frequently used in hernia repairs in a form of mesh, and Prolene™ is often used for suturing blood vessels and grafts in place.
 Box 5.2
Box 5.2 Suggested times for removal of sutures
• Skin on head or neck: 2–5 days
• Upper limbs: 7 days
• Trunk or abdomen: 10 days
• Lower limbs: 14 days
• Retention sutures: 2–6 weeks
Source: Ethicon (2005).
It is important to remember that no one suture material is suitable for all purposes, and that the choice of material depends upon the individual wound. It is also important to be aware that all suture materials within the wound stimulate their own inflammatory response, which lasts for about 7 days.
Suturing techniques
The choice of suturing technique relates to the type of tissue, and site and size of the wound. Westaby (1985) states that there are three vital factors in the technique of inserting sutures:
• the tightness of the suture tie
• the size of tissue bite, i.e. amount of tissue taken up
• the distance between the sutures.
If these are not addressed, then wound healing may be impaired as well as giving poor cosmetic results. Sutures which are pulled too tight do not allow for swelling, and may induce vascular compromise at the wound edges, which leads to necrosis, delayed healing and a poor cosmetic result. The marks left by the sutures may also be very prominent, affecting the cosmetic result. A poor cosmetic scar can also be the result of sutures inserted too loosely, causing the wound to gape, as the wound edges have not been brought together; overlapping of the wound edges may lead to dehiscence or cause a ridge effect. Sutures placed too near to the edge of the wound can result in the sutures pulling apart from the wound edges and causing further trauma to the wound.
Sutures can be inserted in a continuous or an interrupted manner. The continuous method of insertion is used to close an incision with one running stitch, which is tied to the skin at each end, ensuring the tension is the same along the incision, e.g. subcuticular, continuous over-and-over stitch, blanket stitch and mattress stitch (Fig. 5.2). When a Prolene™ subcuticular suture is inserted, it is held in position by means of a bead at each end of the incision (see Fig. 5.2). One disadvantage of using a continuous suture is that if it breaks, the wound edges are not held together and so the suture has to be reinserted. Interrupted sutures are where a suture is knotted and cut individually along the incision, e.g. interrupted over-and-over stitch, vertical or horizontal mattress stitch (Fig. 5.3). It produces a stronger incisional line and avoids devascularization of the skin edges. Abdominal incisions are generally closed using a layered approach, where each layer is closed separately with either continuous or interrupted sutures.
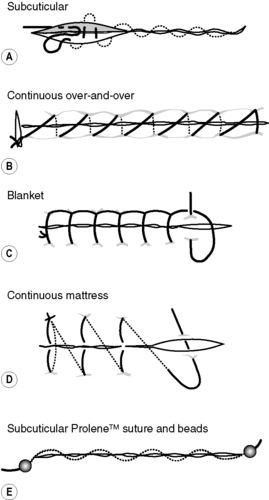 |
| Figure 5.2 • Types of continuous suturing techniques. |
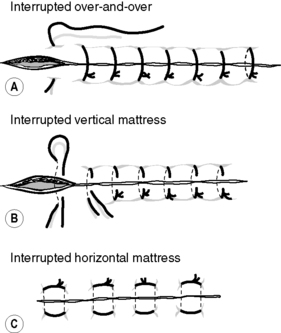 |
| Figure 5.3 • Types of interrupted suturing techniques. |
Sometimes a purse string suture is inserted around a drain. This is a continuous suture which is placed around an opening, so that once the drain is removed, the edges can be pulled together, e.g. removal of a chest drain.
Removal of sutures
The time period for removal of sutures depends upon the position of the wound (see Box 5.2), condition of the skin and any underlying pathologies which may delay the healing process, e.g. steroid therapy. When removing sutures it is important to ensure that the suture material which has been above the skin is not pulled through under the skin edges, as microorganisms may be dragged through into the underlying tissue and so cause infection. In interrupted sutures, each suture is lifted by the knot and cut below the knot, as near to the skin as is possible, at the point where it has been withdrawn from the skin. The suture is then pulled out towards the side on which it has been cut, to avoid the risk of dragging the skin edges apart. It is important to ensure that no portion of the suture has been left behind, as it will act as a foreign body and cause a local inflammatory response.
In continuous subcuticular sutures, one end of the suture is cut, and the suture is then gently pulled away from the incision, ensuring the wound is supported during this procedure, as it can be very uncomfortable for the patient. In a subcuticular Prolene™ suture with beads, one end of the suture is cut and the bead removed, while the opposing beaded end is pulled in order to remove the suture. In other types of continuous sutures, several cuts in the suture are required to ensure that all the suture material is removed, without causing contamination to the underlying tissues.
Staples
The use of staples for skin closure is often preferred if the cosmetic appearance of the scar is a concern, as well as for their time-saving nature in application and painless removal. As the stapler is squeezed, the open staple is forced against an anvil located in the nose of the stapler. This action bends the staple legs, causing them to penetrate the everted skin edges, and results in the staple’s final rectangular shape (Fig. 5.4). They will also allow haemostasis without causing necrosis to the tissue.
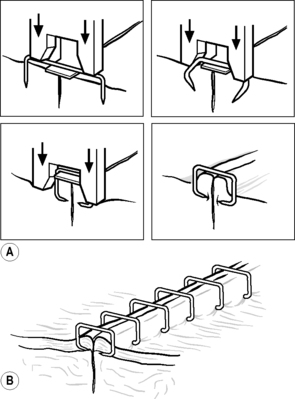 |
| Figure 5.4 • (A) Formation of skin staples. (B) Skin staples in situ. (Reproduced with permission from Ethicon Ltd.) |
Removal of staples is achieved by inserting the lower jaw of the staple remover under the staple and closing together the two edges of the staple remover. The staple needs to be placed in the V-shaped retaining slot situated in the bottom jaw of the staple remover, in order to ensure it is removed correctly. Squeezing the handles of the staple remover reforms the staple, so that it can be lifted from the skin.
Adhesive skin tapes
Adhesive skin tapes are used for some types of skin closure, especially if the cosmetic appearance of the scar is a concern of the patient. Westaby (1985) states that the lax skin of the face and abdomen makes them amenable sites for wound closure by tapes, whereas the skin over joints is subjected to frequent movement, and so limits the adherence of the tapes and success of wound closure. Westaby (1985) also claims that the use of adhesive skin tapes keeps inflammation at the skin edges to a minimum, and results in a good cosmetic outcome with only minimal scarring.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access

