Chapter 7 Wound healing
Skin anatomy
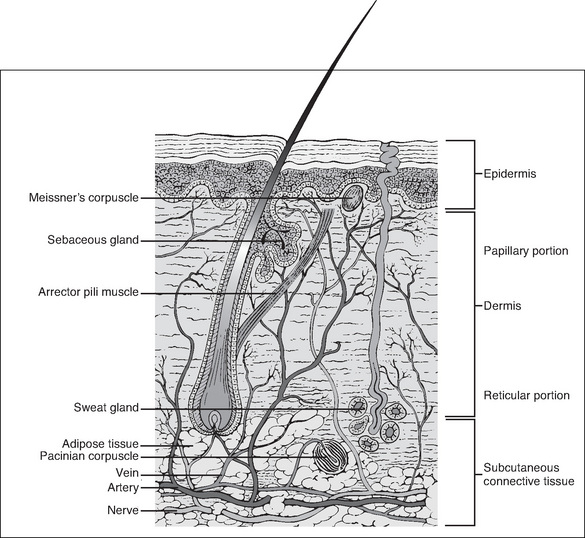
Knowledge of the anatomy of the skin and its associated structures is important in order to understand the physiology of wound healing. The skin consists of the dermis (a type I collagen) and the epidermis, which together compose the outermost layer (Wysocki, 2007). The epidermis is made up of many overlapping layers of epidermal cells and has no blood vessels, so this layer is avascular, receiving its nutrients from blood vessels in the underlying dermis (Wysocki, 2007). The structures associated with the skin, including the capillaries, lymph channels, hair follicles, sebaceous glands, sweat glands and nerve endings, are located in the dermis. Other individual cells, such as mast cells, melanocytes and fibroblasts, are also found in the dermis (Fig 7-1).
The next layer of tissue is the subcutaneous layer. This layer contains adipose or fatty tissue, which is yellow, and greasy or slippery to touch, with globules of fat that frequently dislodge. Blood vessels, lymphatics and nerves are found within the subcutaneous layer. The fascia, the next layer, is a thin membrane that fully encapsulates muscle. It is often glossy in appearance, transparent and separates the subcutaneous layer from muscles, tendons and bones (Wysocki, 2007).
Wound healing
A wound is an injury that disrupts the continuity of body tissue, with or without tissue loss, and which may be intentional or unintentional. Wounds may be surgical, traumatic or chronic (McEwen, 2007; Phillips, 2007).
Unintentional wounds (or traumatic injuries)
Unintentional wounds can be classified by cause—mechanical, thermal or chemical destruction. For example, wounds can occur following trauma (mechanical), as a result of being burnt (thermal) or from contact with chemicals such as acid (chemical).
Chronic wounds
A chronic wound has not completed the usual wound healing process in the expected time frame (Ramundo, 2007). These wounds are caused by an underlying pathophysiological process. For example, a decubitus ulcer, which may be caused by compromised circulation over bony prominences or venous ulcers, develops due to venous stasis or arterial insufficiency. On assessment, a wound that does not appear to be healing by approximately 14–21 days is at risk of becoming a chronic wound.
The phases of wound healing
Wound healing depends on many local and systemic factors and is a complex process that involves a series of cellular processes and biochemical events. There is disagreement among researchers about the exact number of phases of wound healing but all agree that it is complex and that there is some overlapping of these phases because they occur almost simultaneously (Benbow, 2007; Doughty & Sparks-Defries, 2007; Karukonda et al., 2000; Schultz, 2007; Schultz et al., 2003). Following haemostasis, healing progresses through three phases:
The same basic biochemical and cellular processes are involved in the healing of all soft tissue injuries, whether they are acute or chronic.
Haemostasis and the inflammatory phase
The inflammatory response is immediate, non-specific, self-limiting and lasts for 3–4 days. It is also referred to as the defensive phase of healing because it is essential to enable healing to occur (Trask et al., 2006). It is induced by:
Haemostasis
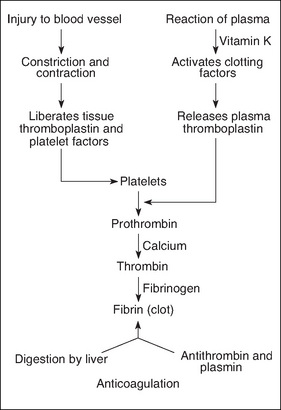
Following injury, bleeding in large vessels must be artificially stopped or the patient will suffer from hypovolaemia and, without treatment, death will occur. Following injury, blood vessels briefly constrict. Platelets accumulate at the damaged site, adhere to one another and form a platelet plug (Benbow, 2007). When damage occurs to endothelial cells, which line the blood vessel walls, collagen fibres are exposed. When collagen fibres contact the platelets, an important release of adenosine phosphate (ADP), histamine and serotonin occurs. The coagulation cascade is also triggered (Karukonda et al., 2000; Trask et al., 2006). The mechanism of haemostasis is shown in Figure 7-2.
Biochemical mediator release
Mast cells are found in the extracellular spaces close to blood vessels. These are the most important activators of the inflammatory response. Activation occurs in two ways. Firstly, as a result of degranulation, preformed granular contents are released into the extracellular matrix (e.g. histamine release). Secondly, certain pain producing mediators (e.g. prostaglandins) are synthesised in response to the injury stimulus; leukotrines, which cause increased vascular permeability and exudation, are also produced. Mast cell degranulation also attracts leucocytes to the site of injury; these cells phagocytose damaged tissue and fight bacteria (Trask et al., 2006).
Reconstructive phase (proliferative)
Fibroblasts
Fibroblasts synthesise collagen and other connective tissue proteins. They multiply rapidly and enter the wound, forming fibres that bridge the wound edges and restore tissue continuity (Trask et al., 2006). Collagen is the most abundant protein in the body and is the material of tissue repair. It cannot be produced without iron, vitamin C or oxygen. Collagen is produced within 6 days of fibroblasts entering a wound.
Granulation
Repair continues as granulation tissue grows inwards from the surrounding healthy tissue. Granulation tissue is filled with new capillaries, giving it a red, granular appearance. It is surrounded by fibroblasts and macrophages. Capillary buds sprout out of vascular endothelial cells and extend into the debrided areas, eventually forming capillaries. Loops form when these capillaries anastomose and leak neutrophils and erythrocytes, causing further debridement of the wound. Capillaries differentiate into arterioles and venules as repair continues; lymphatics are formed in the same way.
Epithelialisation
As a clot is being dissolved and granulation tissue formed, the healing wound must be protected. This occurs during a process by which epithelial cells grow into the wound from surrounding healthy tissue. Macrophages secrete a factor that attracts epithelial cells, which migrate under a clot or seal. Eventually these epithelial cells contact other migrating cells and seal the wound; migration/proliferation then ceases. However, the epithelial cells remain active, undergoing differentiation and giving rise to various epidermal layers (Trask et al., 2006). This process is hastened when the wound is moist.
Wound contraction
Wound contraction occurs over 6–12 days and is the final reconstructive phase necessary to close all wounds, especially those that heal by secondary intention. Granulation tissue contains specialised cells called myofibroblasts, which cause wound contraction (Trask et al., 2006). The scar will form at this point and may appear red to pink in colour (Myers, 2004). (Note: Wounds heal side to side and not end to end.)
Types of wound closure
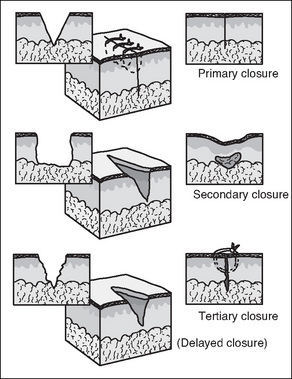
The three mechanisms by which surgical wounds may be closed and subsequently heal are: primary intention, secondary intention and delayed primary closure or tertiary intention (Fig 7-3).
Primary intention
Surgical (and other) clean wounds heal by a process of collagen synthesis, which seals the wound. This is facilitated by minimal tissue loss and approximation of wound edges, with sutures, clips or tapes. There is no dead space on closure and contamination is held to a minimum by adherence to aseptic technique. Very little epithelialisation is required for healing and most wounds are sealed with fibrin several hours after closure (McEwen, 2007; Phillips, 2007).
Secondary intention
Secondary intention (granulation) healing occurs when there is loss of tissue and the wound cannot be closed; consequently, the wound edges are not approximated. Healing occurs by granulation, eventual re-epithelialisation and wound contraction (Harvey, 2005). The wound will heal spontaneously as long as the dermal base is preserved. Secondary intention healing takes longer than primary intention healing and produces extensive scarring. However, it is often the best option for large open wounds (e.g. decubitus ulcers), traumatic wounds or in wounds where infection is present (McEwen, 2007;Myers, 2004).
Delayed primary closure
When approximation and suturing is delayed intentionally by 3 or more days, or is secondary for the purpose of walling off an area of gross infection or where extensive tissue has been removed, then healing by tertiary intention/delayed primary closure occurs. This method may also be used for haemodynamically unstable trauma patients (McEwen, 2007). The wound edges are closed 4–6 days postoperatively/post-trauma after meticulous debridement (Benbow, 2007).
Box 7-1 Subcutaneous management of vertical incisions with 3 cm or more of subcutaneous fat
Surgical wound infections have been shown to be multifactorial. Obesity or an increased depth of subcutaneous fat have been regarded as a significant risk factor for developing a surgical site infection. The multifactorial nature of wound infection was explored in a study by Cardosi et al. (2006), with no definitive conclusion as to the causative factors in any given patient. The researchers found no significant correlation between the depth of subcutaneous fat and the incidence of wound complications or wound disruption.
Wound classification
Wounds are classified into the following four types (Box 7-2):
Box 7-2 Classification of surgical wounds from the Centers for Disease Control
Class I Clean wounds
Clean wounds are defined as uninfected operative wounds in which no inflammation is encountered and the respiratory, alimentary, genital or uninfected urinary tract are not entered. Additionally, clean wounds are a result of elective surgery with primary closure and, if necessary, drained with a closed drainage device. Examples include eye surgery, hernia repair, breast surgery, neurosurgery (non-traumatic), cardiac or peripheral vascular surgery (Phillips, 2007).
Class II Clean–contaminated wounds
Clean–contaminated wounds are operative wounds in which the respiratory, alimentary, genital or uninfected urinary tract is entered under controlled conditions and without unusual contamination. Specifically, operations involving the biliary tract, appendix, vagina and oropharynx are included, providing there is no evidence of infection or no major break in aseptic technique. Examples include gastrectomy, cholecystectomy (without spillage), elective appendicectomy, cystoscopy and/or cystoscopy/transurethral resection (negative urine cultures), total abdominal hysterectomy, dilation and curettage of the uterus, Caesarean section and tonsillectomy (not infected at time of surgery) (Phillips, 2007).
Class III Contaminated wounds
Contaminated wounds are classified as those contaminated in surgery that involves open, fresh, traumatic wounds, or with major breaks in sterile technique, or with gross spillage from the gastrointestinal tract, and incisions in which acute non-purulent inflammation is encountered. Examples include rectal surgery, laparotomy (with significant spillage), traumatic wounds (e.g. gunshot, stab wounds—non-perforation of viscera) or acute inflammation of any organ without frank pus present (e.g. acute appendicitis or cholecystitis) (Phillips, 2007).
Debridement
Debridement involves the removal of dead or necrotic tissue and the manual removal of microorganisms, often by irrigation. The aim of debridement is to provide a clean surface with a minimum of microorganisms and dead tissue that provides a focus for infection and physically obstructs contraction of the wound and closure of the wound edges. Different types of debridement materials are available (Ramundo, 2007). Sharp debridement has been described as what usually happens in the operating room (Ramundo, 2007).
Surgical haemostasis
Surgical haemostasis is the deliberate halting of blood flow. It is essential to wound management and is a necessary and ongoing process during surgery. It is necessary to prevent the patient experiencing the physiological effects of excessive blood loss, which would lead to an increased length of stay in hospital. Additionally, bleeding from the operative site reduces visibility for the surgeon, and is a risk factor for developing a surgical site infection (Rubin, 2006). The mechanisms used encourage the formation of a blood clot, thereby stopping the flow of blood into the surgical site. Surgical haemostasis is achieved by several means, which are classed as mechanical, chemical or electrosurgical; several other methods are unclassified.