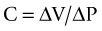15 Ventilation and Oxygenation Management
After reading this chapter, you should be able to:
• describe oxygen therapy, including low-flow and high flow devices, complications associated with oxygen therapy, and management priorities
• state nursing priorities for airway management strategies including laryngeal masks, endotracheal tubes and tracheostomy tubes
• summarise current knowledge on the physiological benefits, indications for use, associated monitoring priorities, complications, modes, settings and interfaces for non-invasive ventilation
• state the indications for use, associated monitoring priorities, complications, classification framework, modes and settings for invasive mechanical ventilation
• outline the weaning continuum and current evidence for optimising safe and efficient weaning from mechanical ventilation
• discuss ventilation management strategies for refractory hypoxaemia
• discuss ventilation management strategies for severe airflow limitation
Introduction
Supporting oxygenation and ventilation are two of the most common interventions in intensive care; in 2007–2008, approximately 41% of patients in Australian and New Zealand ICUs received invasive mechanical ventilation and 8% received non-invasive ventilation (NIV).1 The technology available for supporting oxygenation and ventilation is complex, ranging from simple interventions, such as nasal cannulae through to invasive mechanical ventilation and extracorporeal support. Additionally, the meaning of ventilator terminology is often unclear and terms may be used interchangeably. Critical care nurses must have a strong knowledge of the underlying principles of oxygenation and ventilation that will facilitate an understanding of respiratory support devices, associated monitoring priorities and risks.
Oxygen Therapy
Oxygen is required for aerobic cellular metabolism and ultimately for human survival, with some cells, such as those in the brain, being more sensitive to hypoxia than others. Refer to Chapter 13 for discussion of oxygen delivery and consumption, the oxyhaemoglobin dissociation curve, hypoxaemia and tissue hypoxia; this material provides rationales for clinical decisions regarding the administration of oxygen therapy or ventilation strategies. Oxygen therapy should be considered for patients with a significant reduction in arterial oxygen levels, irrespective of diagnosis and especially if the patient is drowsy or unconscious.
Complications
Hypoventilation and CO2 Narcosis
High-dose oxygen therapy may lead to hypoventilation, worsening hypercapnia and CO2 narcosis due to inhibition of the hypoxic drive in a small proportion of patients with chronic obstructive pulmonary disease (COPD). These patients require close monitoring of PaCO2 levels when oxygen therapy is instituted or increased. Although COPD patients frequently may have a lower baseline SpO2 (88–94% compared to 96–100% in patients with no lung pathology), treatment of hypoxia is still essential, and oxygen should not be withheld or withdrawn while hypoxia remains, even if hypercapnia worsens.2,3
Oxygen Toxicity
Administration of high concentrations of oxygen may lead to oxygen toxicity; symptoms include non-productive cough, substernal pain, reduced lung compliance, interstitial oedema, and pulmonary capillary haemorrhage. These symptoms may be mistakenly attributed to the underlying illness, especially in a sedated and ventilated patient. Many of the symptoms abate once the percentage or fraction of inspired oxygen (FiO2) is reduced, although irreversible pulmonary fibrosis may occur (see Box 15.1). The concentration and duration of oxygen exposure that induces oxygen toxicity varies between patients;4 the lowest possible FiO2 should therefore be used to achieve the target PaO2 or SpO2.
Oxygen Administration Devices
• patient factors: inspiratory flow rate, respiratory rate, tidal volume, respiratory pause
• oxygen device factors: oxygen flow rate, volume of mask/reservoir, air vent size, tightness of fit
Normal inspiratory flow in a healthy adult ranges between 25 and 35 L/min. Patients with respiratory failure tend to increase their flow demand from 50 up to 300 L/min. Patients in respiratory distress are characterised by high respiratory rates and low tidal volumes4,5 that can significantly decrease the FiO2 available via an oxygen delivery device, depending on the type in use.
Variable Flow Devices
High-flow Nasal Cannulae
High-flow nasal cannulae (HFNC) have slightly larger prongs that facilitate oxygen flow of up to 60 L/min leading to less air entrainment effect than with other oxygen delivery systems.5,6 HFNC generate low levels of end-expiratory pressure and can therefore reduce tachypnoea and work of breathing.7,8 The high gas flow may flush CO2 from the anatomical dead space preventing CO2 rebreathing and thereby decreasing PaCO2, although this is not well supported by the literature.9,10 These systems are also generally well-tolerated by the patient, but must be used with heated humidification to avoid drying the respiratory mucosa.8 HFNC are now used more frequently in clinical practice to avoid more invasive therapies but there is limited high-quality evidence on their use in adults.
Oxygen Masks
Loose-fitting oxygen masks include simple (Hudson) face masks, aerosol masks used in combination with heated humidification and nebuliser treatments, tracheostomy masks and face tents. All are considered low-flow or variable-flow devices, with the delivered FiO2 varying with patient demand. Flow rates ≥5 L/min minimise CO2 rebreathing. The addition of ‘tusks’ to a Hudson mask may increase the oxygen reservoir,11 but does not guarantee a consistent FiO2 and has probably been superseded by high-flow systems.12
Partial rebreather and non-rebreather masks have an attached reservoir bag that enables delivery of higher levels of FiO2. Both mask types have a one-way valve precluding expired gas entering the reservoir bag. A non-rebreather mask has two one-way valves on the mask preventing air entrainment.13 The maximum FiO2 delivery with non-rebreather masks is 0.85 with low flow demand, with a steep decline in FiO2 concentration at the alveoli level as the patient’s minute volume increases. Non-rebreather masks may perform worse than a Hudson mask without a reservoir bag.5
Venturi Systems
Venturi systems use the Bernoulli Effect to entrain gas via a side port; gas flow through a narrowing increases speed and gains kinetic energy, resulting in an area of low pressure that entrains room air through the side port. An FiO2 concentration can be selected by widening or narrowing the aperture in the Venturi device to a maximum FiO2 of 0.6. The FiO2 concentration using a Venturi system is less affected by changes in respiratory pattern and demand compared to other low-flow oxygen devices.5
Bag–Mask Ventilation
Bag–mask ventilation (BMV) with a self-inflating bag (and reservoir), non-return valve and mask delivers assisted ventilation at an FiO2 of 1. Addition of a positive end-expiratory pressure (PEEP) valve will improve oxygenation. Manual ventilation requires a good seal between the patient’s face and the mask; this may be difficult to achieve as a single operator. One person may be required to hold the mask and lift the patient’s chin, while another squeezes the bag. Effective bag–mask ventilation is confirmed when the chest visibly rises as the bag is squeezed as well as improved oxygen saturations.14 BMV may cause gastric insufflation, increasing the risk of vomiting and subsequent aspiration.
Airway Support
The most common cause of partial airway obstruction in an unconscious patient is loss of oropharyngeal muscle tone, particularly of the tongue. This may be alleviated by tilting their head slightly back and lifting the chin, or thrusting the jaw forward. The head-tilt/chin-lift manoeuvre is not used if cervical spine injury is suspected.15 The jaw-thrust manoeuvre may require two hands to maintain.16 If more prolonged support is required, an oro- or nasopharyngeal airway can be used that may also facilitate bag–mask ventilation.
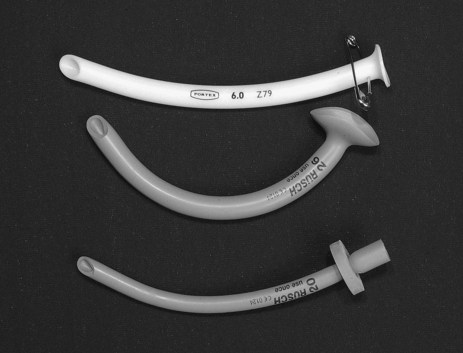
Oro- and Nasopharyngeal Airways
The Guedel oropharyngeal airway is available in various sizes (a medium-sized adult requires a size 4). The airway is inserted into the patient’s mouth past the teeth, with the end facing up into the hard palate, then rotated 180 degrees, taking care to bring the tongue forward and not push it back. Oropharyngeal airways are poorly tolerated in conscious patients and may cause gagging and vomiting.14
A nasopharyngeal airway (see Figure 15.1) is inserted through the nares into the oropharynx; it can be difficult to insert and require generous lubrication to minimise trauma. This type of airway should not be used for patients with a suspected head injury. As well as opening the airway, suction catheters can be passed to facilitate secretion clearance. Once inserted these airways are better tolerated than an oropharyngeal airway.
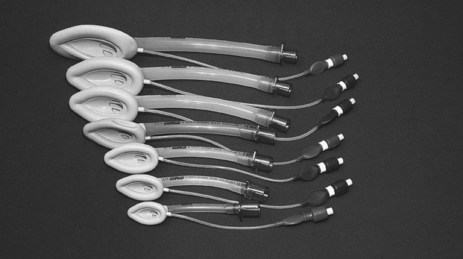
Laryngeal Mask Airway and Its Intubation
The classic laryngeal mask airway (cLMA) (see Figure 15.2) is positioned blindly into the pharynx to form a low-pressure seal against the laryngeal inlet. It is easier and quicker to insert than an endotracheal tube, and is particularly useful for operators with limited airway skills; the cLMA does not carry the same potentially fatal complications such as oesophageal intubation although the risk of aspiration remains.17
Mechanical ventilation can be delivered with low-airway pressures (less than 20 cmH2O) via a cLMA. This device is widely used in elective general anaesthesia,15 and can be used in critical care as an alternative to bag–mask ventilation17 or endotracheal intubation when initial attempts at intubation have failed.18 The ‘intubating’ LMA is most commonly used when a difficult intubation is anticipated or encountered. This device has a handle and is more rigid, wider and curved than the cLMA, enabling passage of a purpose-made endotracheal tube.17
Combitube
The combitube is more widely used in North America for emergency situations than in Australia and the UK.15 It is a dual-lumen, dual-cuff oesophageal-tracheal airway that enables ventilation if inserted into either the oesophagus or trachea. Inexperienced operators may find a combitube more difficult to insert correctly than a cLMA.19 Complications may occur in up to 40% of patients and include aspiration pneumonitis, pneumothorax, airway injuries and bleeding, oesophageal laceration and perforation and mediastinitis.20
Intubation
Endotracheal intubation is the ‘gold standard’ for airway support, providing airway protection in the presence of an airway oedema, absent gag, cough or swallow reflex. Intubation facilitates delivery of mechanical ventilation and pulmonary secretion clearance.16
Endotracheal Tubes
Endotracheal tubes (ETT) are available with internal diameters ranging from 2–10 mm (common adult sizes are 7–9 mm), and are up to 30 cm long. A longitudinal radio-opaque line allows visualisation of tube placement on a chest X-Ray. Markings at 1 cm intervals indicate the length from the distal end. Tubes are available with and without a distal cuff. Adults typically require a cuffed ETT to seal their trachea, facilitating positive pressure ventilation and preventing aspiration of oropharyngeal contents. Cuffs come in a range of profiles and volumes, but are commonly high-volume, low-pressure (see Figure 15.3).
Endotracheal tubes may be reinforced with a wire coil embedded within the plastic for the entire length of the tube to prevent kinking and occlusion. These tubes are more commonly used in the operating room.21 The wire coils can however be irreversibly compressed by a strong bite that occludes the airway. Reinforced tubes also increase the risk of tracheal damage and therefore should be replaced with a standard endotracheal tube on arrival in the ICU. Most endotracheal tubes have a ‘Murphy eye’, an oval-shaped hole in the side of the tube between the cuff and the end of the tube that provides a patent aperture if the distal opening is occluded.22
Preparation for Intubation
Adequate preparation of the patient, equipment and environment, as well as strong knowledge of emergency procedures is important to ensure safe and efficient intubation. Up to 50% of patients undergoing endotracheal intubation in ICU will experience a complication; 28% will have a serious complication, including hypoxaemia, circulatory collapse, cardiac arrhythmia, cardiac arrest, oesophageal intubation, aspiration and death.23
Patient Preparation
Equipment and Drugs
All equipment should be checked immediately prior to intubation, including
• suction supply, with a range of Yankaeur and y-suction catheters
• laryngoscope blades and holder are compatible, with a functioning light
• appropriately-sized face mask
• manual ventilation (ambubag™) available and attached to oxygen supply
• ETT cuff inflated in sterile water to ensure no leaks and even inflation
• water-based lubricant of tube and cuff (while maintaining sterility)
• capnography (chemical CO2 detectors are often used in emergency situations)
• emergency/resuscitation trolley at bedside
Procedure
The patient is preoxygenated to minimise desaturation during apnoea and laryngoscopy, commonly via bag and mask, although other methods such as non-invasive ventilation have been suggested.24 Intubation in ICU is usually performed via laryngoscopy with insertion of an oral ETT. Intubation may be performed using a fibreoptic bronchoscope when difficulty is encountered, or for nasal intubation.
Oral vs Nasal Intubation
Oral intubation is preferred unless there are specific indications for nasal intubation. Oral intubation is easier to perform and allows use of a larger diameter tube. While nasal intubation provides better splinting for the ETT and facilitates oral hygiene, it can damage nasal structures, is contraindicated in skull fractures and increases the risk of maxillary sinusitis and ventilator-associated pneumonia.25
Cricoid Pressure
Cricoid pressure (Sellick manoeuvre) was introduced in the 1960s to prevent aspiration of gastric contents during intubation. The oesophagus lies behind and in line with the trachea. The cricoid cartilage, situated below the thyroid prominence, is a closed tracheal ring which, when compressed, closes the oesophagus while the trachea remains open. Cricoid pressure is performed by placing your thumb on one side of the patient’s trachea, middle finger on the other side and index finger directly on the cricoid.26 Although widely used over the last 50 years, its efficacy is being questioned as technique is frequently poor,27 and there is wide anatomical variation in the exact orientation of the oesophagus in relation to the trachea.28
Backwards, Upwards, Rightward Pressure Manoeuvre
The backwards, upwards, rightward pressure (BURP) manoeuvre on the thyroid cartilage was introduced in the mid-1990s to improve visualisation during difficult laryngoscopy. The patient’s jaw is thrust forward, so their head is in the ‘sniffing’ position. Place your thumb and third finger on either side of the thyroid cartilage and index finger on top. Pressure is applied in the sequence backwards (towards the spine), upwards (towards the head), rightward (towards the patient’s right side). This is easier to perform following administration of muscle relaxants.29,30
Cuff Management
Endotracheal and tracheostomy tube cuffs prevent airway contamination by pharyngeal secretions and gastric contents and loss of tidal volume during mechanical ventilation. The cuff does not secure the tube in the trachea. Cuff inflation pressures should be maintained at 20–30 cmH2O.31,32 Cuff inflation pressures ≤20 cmH2O (15 mmHg) are associated with an increased risk of aspiration and a 2.5-fold increase in ventilator-associated pneumonia (VAP).33 Conversely, tracheal wall damage may occur if cuff pressure exceeds the capillary perfusion pressure in the trachea (27–40 cmH2O/20–30 mmHg).
There are four methods described for assessing cuff inflation:
In Australia and New Zealand, CPM is the most common form of cuff pressure assessment,34 in contrast to the UK35 and North America36 where CPM is used infrequently. Cuff pressure varies with head and body position, tube position and airway pressures.37 The optimum frequency of cuff pressure monitoring is unclear; at a minimum it should be done post-intubation, on arrival in ICU and once per nursing shift. A persistent cuff leak or pressures of ≥30 cmH2O (22 mmHg) to generate a seal should be reviewed and referred to medical staff.
Endotracheal Tube Fixation
The purpose of ETT fixation is to maintain the tube in the correct position, prevent unintended extubation and facilitate mechanical ventilation while maintaining skin integrity and oral hygiene.38 ETT fixation methods include:
• tying cotton tape around the tube, then around the patient’s neck
• taping the tube to the patient’s face using medical adhesive tape
There is no evidence supporting a preferred method39 with each having specific strengths and weaknesses. Two nurses are required to prevent ETT dislodgement during fixation. Although there is also no evidence to recommend a preferred frequency, ETT fixation is generally changed at least daily, to allow assessment of the underlying skin with particular attention to the tops of the ears and corners of the mouth and to facilitate oral hygiene.38 The ETT position in the mouth is alternated at this time.
Confirmation of Tube Position
The correct position of the ETT distal end is 3–5 cm above the carina. A lip level of 20 cm for women and 22 cm for men should prevent endobronchial intubation, with the proximal end fixed at either the centre or the side of the mouth.40 Confirmation of the ETT position is required immediately following intubation and at regular intervals thereafter as movement of the tube can occur.
Chest auscultation is the traditional method to confirm ETT position. Observation of chest expansion is, however, unreliable, as the chest may appear to rise with oesophageal intubation. Conversely the chest may not rise with a correctly positioned tube if the patient is obese or has a rigid chest wall. Patients with left main bronchus intubation may exhibit bilateral breath sounds.41 End-tidal CO2 monitoring is the ‘gold standard’ method for confirming ETT placement. Disposable devices that change colour in the presence of CO2 are inexpensive and easy to use, but may be inaccurate during cardiopulmonary resuscitation, or if contaminated. Capnography is the most reliable technique to identify ETT placement in both arrest and non-arrest situations.18 Continuous end-tidal CO2 monitoring during intubation is recommended as a minimum standard by the College of Intensive Care Medicine of Australia and New Zealand.42
Tracheostomy
Tracheostomy may be required for upper airway obstruction, although it is most commonly performed for ICU patients who require prolonged mechanical ventilation. The advantages of tracheostomy over endotracheal intubation include decreased risk of laryngeal damage and subglottic stenosis, reduced airway resistance and deadspace which decreases the work of breathing and therefore supports weaning,43 and improved patient tolerance enabling reduction of sedation. The optimum time to perform tracheostomy remains contentious, and is often influenced by a patient’s diagnosis.44
Procedure
Tracheostomy can be performed using a surgical technique (ST) or percutaneous dilatational technique (PDT). PDT is contraindicated in patients with anatomical anomalies of the neck and serious bleeding disorders, and should be undertaken with caution in patients who are obese, have a cervical spine injury, coagulopathy, difficult airway or require high levels of ventilatory support.45 PDT is more commonly performed than ST in Australian and New Zealand ICUs.45
Tracheostomy Care
The aim of tracheostomy care is to keep the site free of infection, and prevent tube blockage or dislodgement. The site is cleaned with normal saline and fixation devices changed at least 12-hourly with two nurses to safely perform tape changes.46 Velcro tapes are easier to change and more comfortable than cotton tape.47 Lint-free or superabsorbent foam dressings may be placed under the flange to absorb secretions. Adequate humidification and suctioning will usually prevent tube obstruction (see later in this chapter). The use of inner cannulae has obviated the need for frequent tracheostomy tube changes. Single lumen (no inner cannula) tracheostomy tubes should be changed every 7–10 days.46
Complications of Endotracheal Intubation and Tracheostomy
Tube blockage, tube dislodgement and aspiration are major complications. Partial ETT or tracheostomy tube dislodgement can cause greater harm than complete removal because of delays in diagnosis and resultant aspiration or worsening gas exchange. Tube dislodgement is most likely to occur when turning the patient, if the patient is agitated or when nursing staff are distracted or on breaks.48 While physical restraint may be considered to prevent tube dislodgement, multiple studies noted patients were restrained at the time of self-extubation or device removal.49–54 Effective levels of analgesia and sedation is therefore most appropriate in minimising the risk of self-extubation.
Complications during and immediately after endotracheal intubation and tracheostomy include cardiovascular compromise, bleeding, injury to the tracheal wall, damage to the vocal cords, pneumothorax, pneumomediastinum and subcutaneous emphysema. Late complications of tracheostomy include tracheal stenosis, tracheomalacia and tracheo-oesophageal fistula and infection. As noted earlier, damage to the trachea is exacerbated by high cuff pressures.55 PDT results in fewer wound infections, decreased incidence of bleeding and reduced mortality compared to ST.56
Tracheal Suction
Patients with an ETT or tracheostomy tube require tracheal suction to remove pulmonary secretions that can lead to atelectasis or airway obstruction and impair gas exchange.57 Suction should be performed as clinically indicated, with assessment of visible or audible secretions, rising inspiratory pressure, decreasing VT or increased work of breathing.58 A sawtooth pattern on the flow-volume waveform may also indicate the need for suction (discussed later in this chapter).59
Preoxygenation using a FiO2 of 1 for 60 seconds prior to performing suction minimises hypoxia and the potential for cardiac arrhythmias. Manual hyperinflation is discouraged due to the risk of barotrauma and lack of benefit. Similarly, installation of saline is not supported due to increased risk of flushing pathogens into distal lung regions.60
Methods
The three methods of suctioning are:
• open suction: a suction catheter is passed under aseptic technique directly into the ETT/tracheostomy after disconnection from the ventilator circuit. Disadvantages include loss of PEEP resulting in loss of alveolar recruitment and increased risk of transmission of infective organisms. A surgical mask and protective eyewear should be worn.61
• semi-closed suction: a suction catheter is passed through a swivel connector with a self-sealing rubber flange.
• closed suction: in-line system is attached between the ETT/tracheostomy tube and the ventilator circuit where the suction catheter is contained in an integrated plastic sleeve. Alveolar derecruitment occurs to a lesser degree than with open suction.
Adverse Effects
Adverse effects of suction can include hypoxaemia, introduction of infective organisms, tracheal trauma, bradycardia, hypertension and increased intracranial pressure. Tracheal suctioning causes discomfort, and should therefore be performed only when clinically indicated, such as audible presence of secretions and desaturation.58
Extubation
Following successful weaning from mechanical ventilation (see later in this chapter), assessment of the patient prior to extubation should include adequate gas exchange, respiratory rate and work of breathing on minimal support for prolonged periods, respiratory muscle strength, the ability to cough and clear secretions spontaneously and a stable haemodynamic status and mental status.62 Serious post-extubation complications of laryngospasm and stridor cannot be reliably predicted,63 so the ease/grade of intubation should be considered prior to extubation and provision made for immediate re-intubation.64
Mechanical Ventilation
As stated in the introduction, 41% of patients in Australian and New Zealand ICUs received invasive mechanical ventilation and 8% received non-invasive ventilation (NIV) in 2007–08.1 The median duration of invasive mechanical ventilation for these patients was 2.5 days. In the most recent international study of mechanical-ventilation practices, reporting data from 4968 patients in 349 ICUs and 23 countries found the median duration of ventilation to be 4 days (interquartile range 2–8 days).65 In this patient cohort the three most common reasons for mechanical ventilation were postoperative respiratory failure, coma and pneumonia. This international report did not include data from Australia and New Zealand. A study describing ventilation and weaning practices of 55 ICUs in Australia and New Zealand in 2005 reported a similar profile for the most common indications for mechanical ventilation.66
Principles of Mechanical Ventilation
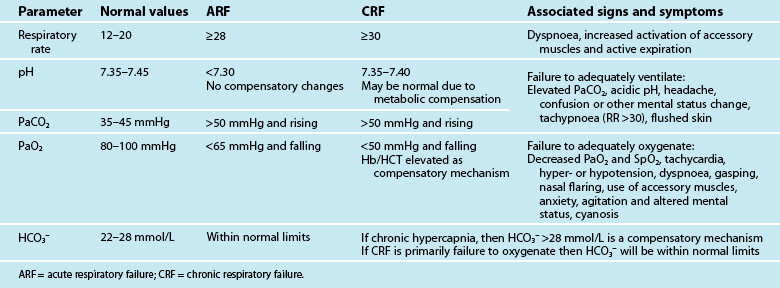
Mechanical ventilation describes the application of positive or negative pressure breaths using non-invasive or invasive techniques. Indications for initiation of mechanical ventilation are discussed below. Table 15.1 lists the patient parameters typically observed in acute and chronic respiratory failure that may be influential in the decision to ventilate. During positive pressure ventilation, the type of ventilation used most commonly in critical care, the ventilator delivers a flow of gas into the lungs during inspiration using a pneumatic system. Expiration is passive.
The Equation of Motion
The equation of motion for the respiratory system is a mathematical model that relates pressure volume and flow during the delivery of a breath, with the pressure required to deliver a volume of gas to the lungs determined by the elastic and resistive properties of the respiratory system67 (see Table 15.2).
| Equation: PT (Pairway + Pmuscle) = VT/Cr + VT/TI × R + PEEPT | |
|---|---|
| Abbreviations: | |
| Notes: | |
Compliance and Elastance
Lung tissue and the surrounding thoracic structures contribute to respiratory compliance. Normal compliance for a mechanically ventilated patient ranges from 35–50 mL/cmH2O.68
Resistance
Resistance refers to the forces that oppose airflow. Resistance in the airways is affected by the diameter and length of the airways, including the artificial airway, the gas flow rate and the density and viscosity of the inspired gas. During mechanical ventilation, bronchospasm, airway oedema, endotracheal tube lumen size, increased secretions, and inappropriate setting of flow rates can influence airway resistance. Normal resistance for intubated patients is 6 cmH2O/(L/sec).68
Ventilator Circuits
Delivery of mechanical ventilation requires a ventilator circuit to transport gas flow to the patient. To prevent condensation from cooling of warm humidified gas, inspired gas is heated via a wire inside the wall of the circuit in either the inspiratory limb alone or both the inspiratory and expiratory limbs.69 Historically ventilator circuits were changed frequently (48–72 hours) to decrease the risk of VAP.70 Current guidelines for prevention of VAP found evidence that the frequency of ventilator circuit changes had no relationship to the incidence of VAP and therefore recommended routine circuit changes were not necessary and circuits should only be changed when soiled or damaged.71
Humidification
Humidification techniques warm and moisten gas to facilitate cilia action and mucus removal as well as to prevent drying and irritation of respiratory mucosa and solidification of secretions. During endotracheal intubation and mechanical ventilation, the normal humidification processes of the nasopharynx are bypassed. This, in combination with the use of dry medical gas at high flow rates, means alternative methods of humidification are required. The best conditions for mucosal health and function over prolonged periods are when inspired gas is warmed to core body temperature and is fully saturated with water.72
Absolute and Relative Humidity
1. the inspired gas delivered into the trachea is at 37°C with a water content of 30–43 g/m3 (relative humidity is 100% at 37°C in the bronchi)
2. the set temperature remains constant without fluctuation
3. humidification and temperature are unaffected by a large or differing types of gas flow
4. the device is simple to use
5. the humidifier can be used with spontaneously breathing and ventilated patients
6. safety alarms prevent overheating, overhydration and electrocution
7. the resistance, compliance and dead space characteristics do not adversely affect spontaneous breathing modes
Heat–moisture Exchanger
Heat–moisture exchangers conserve heat and moisture during expiration, and enable inspired gas to be heated and humidified. Two types of HMEs exist: hygroscopic and hydrophobic. Hygroscopic HMEs absorb moisture onto a chemically impregnated foam or paper material and have been shown to be more effective than hydrophobic HMEs.74 HMEs are placed distally to the circuit Y-piece in line with the endotracheal tube and increase dead space by an amount equal to their internal volume.75 HMEs should be changed every 24 hours or when soiled with secretions and are usually reserved for short term humidification.
Heated Humidification
Generally, heated humidification (HH) is used for patients requiring greater than 24 hours of mechanical ventilation. Various models of heater bases and circuits are on the market and we recommend their use in accordance with manufacturer instructions. A recent systematic review and meta-analysis reported no overall effect on artificial airway occlusion, mortality, pneumonia, or respiratory complications when HMEs were compared to HHs, although it noted that PaCO2 and minute ventilation were increased and body temperature was lower with the use of HMEs.76
Non-Invasive Ventilation
Terminology
Positive pressure NIV can be further categorised as non-invasive positive pressure ventilation (NIPPV) or continuous positive airway pressure (CPAP). NIPPV is the provision of inspiratory pressure support (also referred to as inspiratory positive airway pressure [IPAP]) usually in combination with positive end expiratory pressure (PEEP) (also referred to as expiratory positive airway pressure [EPAP]). CPAP does not actively assist inspiration but provides a constant positive airway pressure throughout inspiration and expiration.77
The terms Biphasic (or bilevel) positive airway pressure (BiPAP®) and non-invasive pressure support ventilation (NIPSV) are also used to refer to NIPPV.78 The acronym BiPAP® is registered to Respironics (Murrayville, PA), a company that produces a number of non-invasive ventilators including the BIPAP Vision, a NIV ventilator commonly used in the ICU. The acronym NIPSV is primarily used in European descriptions of NIPPV.
Physiological Benefits
The efficacy of NIV in patients with acute respiratory failure is, at least in part, related to avoidance of inspiratory muscle fatigue through the addition of inspiratory positive pressure thus reducing inspiratory muscle work.79 Application of positive pressure during inspiration increases transpulmonary pressure, inflates the lungs, augments alveolar ventilation and unloads the inspiratory muscles.80 Augmentation of alveolar ventilation, demonstrated by an increase in tidal volume, increases CO2 elimination and reverses acidaemia. High levels of inspiratory pressure may also relieve dyspnoea.81
The main physiological benefit in patients with congestive heart failure (CHF) is attributed to the increase in functional residual capacity associated with the use of PEEP that reopens collapsed alveoli and improves oxygenation.82 Increased intrathoracic pressure associated with the application of positive pressure also may improve cardiac performance by reducing myocardial work and oxygen consumption through reductions to ventricular preload and left ventricular afterload.82–84 NIV also preserves the ability to speak, swallow, cough and clear secretions, and decreases risks associated with endotracheal intubation.85
Indications for NIV
The success of NIV treatment is dependent on appropriate patient selection.86 Table 15.3 outlines indications and contraindications to NIV.
TABLE 15.3 Indications and contraindications for non-invasive ventilation77
| Indications | |
| Bedside observations | Increased dyspnoea: moderate to severeTachypnoea: >24 breaths per min [obstructive] >30 breaths per min [restrictive] Signs of increased work of breathing, accessory muscle use and abdominal paradox |
| Gas exchange | Acute or acute-on-chronic ventilatory failure (best indication), PaCO2 >45 mm Hg, pH <7.35 Hypoxaemia (use with caution), PaO2/FIO2 ratio <200 |
| Contraindications | |
| Absolute | Respiratory arrest Unable to fit mask |
| Relative | Medically unstable: hypotensive shock, uncontrolled cardiac ischaemia or arrhythmia, uncontrolled upper gastrointestinal bleeding Agitated, uncooperative Unable to protect airway Swallowing impairment Excessive secretions not managed by secretion clearance techniques Multiple (i.e. two or more) organ failure Recent upper airway or upper gastrointestinal surgery |
PaCO2: partial pressure of carbon dioxide in arterial blood; PaO2: partial pressure of oxygen in arterial blood; PaO2/FIO2: ratio of partial pressure of oxygen in arterial blood to fraction of inspired oxygen.
Acute Respiratory Failure
Evidence supporting the role of NIV in patients with hypoxaemic respiratory failure is limited and conflicting.82 For patients with community-acquired pneumonia, NIV has been shown to reduce intubation rates, ICU length of stay and 2-month mortality but only in the subgroup of patients with COPD.87 Pneumonia also has been identified as a risk factor for NIV failure.88
Acute Exacerbation of COPD and CHF
Strong evidence exists to support the use of NIV for patients with acute exacerbation of chronic obstructive pulmonary disease (COPD) and congestive heart failure (CHF). Three meta-analyses have shown a reduction in intubation rates, hospital length of stay and mortality for COPD patients managed with NIPPV compared to standard medical treatment.89–91 COPD patients most likely to respond favourably to NIPPV include those with an unimpaired level of consciousness, moderate acidaemia, a respiratory rate of <30 breaths/minute and who demonstrate an improvement in respiratory parameters within two hours of commencing NIV.79,92
Early use of NIV in combination with standard therapy for patients with CHF has also been shown to reduce intubation rates and mortality when compared to standard therapy alone.93–95 A recent meta-analysis found CPAP reduced hospital mortality whereas NIPPV did not have an effect on mortality.94 Both NIV modes were shown in this meta-analysis to reduce the need for intubation. An early study comparing NIPPV to CPAP in patients with CHF reported a higher incidence of myocardial infarction.96 Based on this finding, practice guidelines from the British Thoracic Society recommend NIPPV should only be used for patients with CHF when CPAP has been unsuccessful.97 More recently several studies have found no difference in myocardial infarction rates when comparing the two modes.98–101 A recent large multicentre randomised controlled trial found NIV delivered by either CPAP or NIPPV resulted in symptomatic improvements, but failed to demonstrate a mortality benefit.102 Practice surveys indicate CPAP may be the preferred method of NIV for patients with CHF in Australia and internationally.103,104
NIV in Weaning
NIV may be used as an adjunct to weaning to reduce the duration of invasive ventilation and associated complications.105 Patients are extubated directly to NIV and then weaned to standard oxygen therapy. This use of NIV differs from its role in preventing reintubation in patients that develop, or who are at high risk of, postextubation respiratory failure.106 A recent systematic review and meta-analysis of 12 trials of NIV as a weaning adjunct found reductions in mortality, ICU and hospital lengths of stay, duration of ventilation and rates of VAP.107 Conversely the largest study of NIV use in postextubation respiratory failure reported worsened survival rates hypothesised as a result of delayed reintubation.108 A subsequent meta-analysis suggested NIV may have a role in preventing the development of respiratory failure postextubation for those at risk, but should be used with caution once respiratory failure has developed and should not delay the decision to reintubate.106
Interfaces and Settings
NIV requires an interface that connects the patient to either a ventilator, portable compressor or flow generator with a CPAP valve. The selection of an appropriate interface can influence NIV success or failure. Oronasal masks cover both the mouth and nose and are the preferred mask type for the management of acute respiratory failure.110 Nasal masks enable speech, eating and drinking, and therefore are used more frequently for long-term NIV use. An oronasal mask enables delivery of higher ventilation pressures with less leak and greater comfort for the patient.111 Other interfaces include full-face masks111 that seal around the perimeter of the face and cover the eyes as well as the nose and mouth, nasal pillows, mouthpieces that are placed between the patient’s lips, and helmets that cover the whole head and consist of a transparent plastic hood attached to a soft neck collar.112,113 These alternative interfaces may increase patient tolerance by reducing pressure ulceration, air leaks and patient discomfort.114
Initiation and Monitoring Priorities
Successful initiation of NIV is dependent on patient acceptance and tolerance. Patient acceptance of NIV may be aided by a brief explanation of the procedure and its benefits. Strategies to enhance patient tolerance include: use of an interface that fits the patient’s facial features, commencing with low pressure levels, holding the mask gently in position prior to securing with the straps/headgear, and ensuring straps prevent major leaks but are not so tight they increase discomfort. Once NIV is commenced, the patient should be monitored for respiratory and haemodynamic stability, response to NIV treatment, ongoing tolerance, and presence of air leaks (Table 15.4). Arterial blood gas analysis should be performed at baseline and within the first one to two hours of commencement.97 During the initiation and stabilisation period, patients should be monitored using a nurse-to-patient ratio of 1 : 1 with ongoing coaching to promote NIV tolerance throughout the early stabilisation period.
TABLE 15.4 Monitoring priorities for non-invasive ventilation104
| Priority | Assessment |
|---|---|
| Patient comfort | |
| Conscious level | Glasgow coma score |
| Work of breathing | |
| Gas exchange parameters | |
| Haemodynamic status | |
| Ventilator parameters |
SpO2: saturation of peripheral oxygen; VT: tidal volume; PaCO2: partial pressure of carbon dioxide in arterial blood; PaO2: partial pressure of oxygen in arterial blood.
Potential Complications
Masks need to be tight-fitting to reduce air leaks; however, this contributes to pressure ulceration on the bridge of the nose or above the ears (due to mask straps/headgear). Air leaks may cause conjunctival irritation and the high flow of dry medical gas results in nasal congestion, oral or nasal dryness and insufflation of air into the stomach. Claustrophobia associated with the NIV interface may also lead to agitation reducing the efficacy of NIV treatment due to poor coordination of respiratory cycling between the patient and NIV unit.79 More serious, yet infrequent, complications include aspiration pneumonia, haemodynamic compromise associated with increased intrathoracic pressures and pneumothorax.80
Detecting NIV Failure
Failure to respond to NIV within 1–2 hours of commencement is demonstrated by unchanged or worsening gas exchange, as well as ongoing or new onset of rapid shallow breathing and increased haemodynamic instability.111 A decreased level of consciousness may be indicative of imminent respiratory arrest.
Invasive Mechanical Ventilation
Critically ill patients with persistent respiratory insufficiency (hypoxaemia and/or hypercapnia), due to drugs, disease or other conditions, may require intubation and mechanical ventilation to support oxygenation and ventilatory demands.115,116 Clinical criteria for intubation and ventilation should be based on individual patient assessment and patient response to measures aimed at reversing hypoxaemia.
Indications
Indications for intubation and mechanical ventilation include:
• inability to protect airway; e.g. loss of gag/cough reflex; decreased Glasgow Coma Scale (GCS) score
• clinical signs indicating respiratory distress; e.g. tachypnoea,117 activation of accessory and expiratory muscles, abnormal chest wall movements,118 tachycardia and hypertension
• inability to sustain adequate oxygenation for metabolic demands; e.g. cyanosis, SpO2 <88%, with supplemental FiO2 ≥0.5
• respiratory acidosis (e.g. acute decrease in pH <7.25)
• postoperative respiratory failure
Mechanical Ventilators
Contemporary ventilators use sophisticated microprocessor controls with sensitive detection, response and control of pressure and gas flow characteristics. These mechanical ventilators are more sensitive to patient ventilatory demands, enabling improved patient–ventilator synchrony during both inspiratory and expiratory breath phases. Parameters commonly manipulated during mechanical ventilation are detailed in Table 15.5. Parameters often observed and documented are discussed below.
TABLE 15.5 Set ventilator parameters
| Parameter | Description |
|---|---|
| Fraction of inspired oxygen (FiO2) | The fraction of inspired oxygen delivered on inspiration to the patient. |
| Tidal volume (VT) | Volume (mL) of each breath. |
| Set breath rate (f) | The clinician determined set rate of breaths delivered by the ventilator (bpm). |
| Inspiratory trigger or sensitivity | Mechanism by which the ventilator senses the patient’s inspiratory effort. May be measured in terms of a change in pressure or flow. |
| Inspiratory pressure (Pinsp, Phigh) | Clinician determined pressure that is targeted during inspiration. |
| Inspiratory time (Tinsp) | The duration of inspiration (sec). |
| Inspiratory : expiratory ratio (I : E) | The ratio of the inspiratory time to expiratory time. |
| Flow (V) | The speed gas travels during inspiration. (L/min). |
| Pressure support (PS) | The flow of gas that augments a patient’s spontaneously initiated breath to a clinician-determined pressure (cmH2O). |
| Positive end-expiratory pressure (PEEP) | Application of airway pressure above atmospheric pressure at the end of expiration (cmH2O). |
| Rise time | Time to achieve maximal flow at the onset of inspiration for pressure-targeted breaths. |
| Expiratory sensitivity | During a spontaneous breath, the ventilator cycles from inspiration to expiration once flow has decelerated to percentage of initial peak flow. |
| Minute volume (VE) | Generally not set directly but is determined by VT and f settings. Tidal volume multiplied by the respiratory rate over one minute (L/min). |
| Airway pressure (Paw) | The pressure measured in cmH2O by the ventilator in the proximal airway. |
| Plateau pressure (Pplat) | The pressure, measured in cmH2O, applied to the small airways and alveoli. Pplat is not set but can be measured by performing an inspiratory hold manoevre. |
Fraction of Inspired Oxygen
The fraction of inspired oxygen (FiO2) is expressed as a decimal, between 0.21 and 1, when supplemental oxygen is applied. Room air has an oxygen content of 0.21 (21%). Ventilation is commonly commenced on a high FiO2 setting, but as noted earlier, consideration is given to the risks of oxygen toxicity which include disruption to the alveolar-capillary membrane and fibrosis of the alveolar wall.119
Tidal Volume
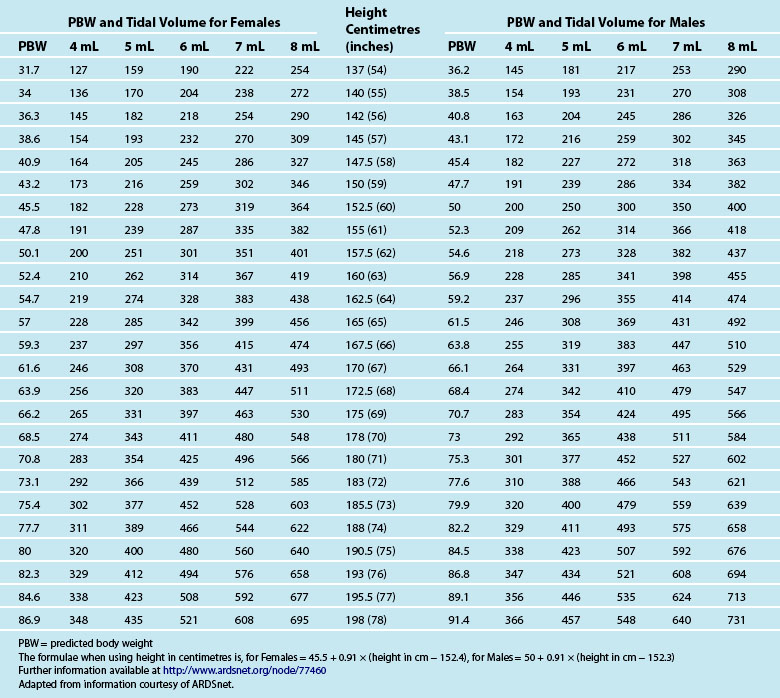
Tidal volume (VT) is the volume, measured in mL, of each breath. The VT is calculated using the patient’s ideal body weight using height and gender-specific tables120 to achieve 6–8 mL/kg (see Table 15.6). Strong evidence indicates a mortality benefit for using 6 mL/kg in patients with acute respiratory distress syndrome (ARDS).121 Some evidence also indicates 6 mL/kg as a target for patients without ARDS or acute lung injury (ALI).122,123 While further studies are required, clinicians should consider aiming for 6–8 mL/kg in all ventilated patients.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree