CHAPTER 8
Vascular Medicine and Rehabilitation
Debra Kohlman-Trigoboff
First Edition Authors: Debra Kohlman-Trigoboff, Mitzi Ekers, and Cynthia Christensen
OBJECTIVES
1. Identify risk factors associated with development of atherosclerosis.
2. Describe elements of a comprehensive treatment plan for management of hypertension, dyslipidemia, diabetes, and smoking cessation.
3. Explain the role of exercise in the management of peripheral artery disease (PAD) risk factors and claudication symptoms.
4. List the components of each phase of a structured PAD Rehabilitation Program (PRP), including strategies for in-patient education, as well as outpatient assessment, physical reconditioning, education, and evaluation methods.
Introduction
Atherosclerosis is a systemic disease affecting the peripheral, coronary, and cerebral circulation resulting in myocardial infarction (MI), stroke, amputation, renal failure, pain, and death. PAD coexisting with other cardiovascular diseases are seen as follows: patients with symptomatic PAD are more likely to have symptomatic coronary artery disease (CAD) in the form of angina, PAD may be a stronger predictor of concurrent cerebrovascular (CVD) risk than is CAD, 11% of patients with CAD have cerebrovascular disease (CVD), while 9% have abdominal aortic aneurysms. PAD affects over 8.3 million people in the United States affecting 5% to 10% of adults over age 40, with over 4 million people being symptomatic with intermittent claudication (Go, Mozaffarian et al., 2013; Smith et al., 2006). Even though PAD is a marker for systemic atherosclerosis, it often goes unrecognized or is ineffectively treated (Hirsch et al., 2006; Rosamond et al., 2008). Many clinicians fail to understand the serious impact of PAD on the functional capacity, quality of life, and overall mortality of those it affects. The presence of PAD has been identified as an independent risk factor of mortality, posing a 3 to 10 times greater risk of cardiac events or death (Hirsch et al., 2006). Major risk factors for vascular disease are smoking, diabetes, dyslipidemia, and hypertension.
• Smoking appears to be the single most important modifiable risk factor for PAD and its progression toward ischemic events (Hennrikus et al., 2010). The diagnosis of PAD is made a decade earlier in smokers, and intermittent claudication is three times more common in smokers than in nonsmokers (Fiore et al., 2008).
• Diabetes accelerates atherosclerosis 200% to 400%. Diabetics have a fourfold increased risk of developing PAD or intermittent claudication. Diabetic patients have a sevenfold higher rate of amputations than those without the disease (American Diabetes Association [ADA], 2013). Elevated hemoglobin A1C is an established predictor for developing atherosclerosis (ADA, 2013; Gerstein et al., 2003).
• Dyslipidemia indicates elevated levels of total cholesterol, low-density lipoprotein (LDL), and triglycerides or lower levels of high-density lipoprotein (HDL), all of which increase the likelihood of cardiovascular disease (Grundy et al., 2002; Hirsh et al., 2006). However, new ACC/AHA guidelines focus on statin therapy to lower LDL according to patients’ atherosclerotic cardiovascular risk (Stone et al., 2013).
• Hypertension is a constant and independent risk factor for vascular disease (Chobanian et al., 2003). Individuals with hypertension have about a twofold increased risk of developing claudication, and the risk of stroke, heart attack, abdominal aortic aneurysm expansion, kidney disease, and death from uncontrolled hypertension is magnified in individuals with PAD (Chobanian et al., 2003). Antihypertensive therapy must be directed at both the reduction of blood pressure as well as improved endothelial function and arterial compliance (Chobanian et al., 2003). In the Heart Outcomes Prevention Evaluation (HOPE) study, patients with symptomatic PAD who received ramipril had a 25% risk reduction of MI, stroke, or vascular death (Yusuf et al., 2000).
The successful long-term management of PAD is enhanced by a medical management program that may delay disease progression, decrease morbidity and mortality, and improve quality of life. The vascular healthcare team, the patients, and the patients’ families should develop a partnership to help negotiate the steps involved in these major lifestyle changes. An ideal resource for this partnership is a well-designed PRP which includes exercise and cardiovascular risk factor management.
 I. Comprehensive Vascular Medical Therapeutic Approach
I. Comprehensive Vascular Medical Therapeutic Approach
A. Purpose: To recognize and treat preventable vascular disease risk factors to reduce disease incidence and complications
B. Smoking and Peripheral Artery Disease
1. Incidence
a. In 2011, an estimated 19.0% (43.8 million) US adults were current cigarette smokers (Agaku, King, & Dube, 2012)
b. Deaths resulting from cigarette smoking: over 400,000 accounting for about 20% of cardiovascular deaths (Benowitz, 2003)
c. Approximately 443,000 US adults die from smoking-related illnesses each year (Agaku et al., 2012; U.S. Department of Health and Human Services, 2010)
d. The Framingham study: 78% of cases of intermittent claudication could be contributed to smoking (Tendera et al., 2011)
2. Cigarette smoking accelerates atherosclerosis; increases risk of ischemic and thrombotic events (US Dept HHS, 2010)
a. Endothelial dysfunction occurs from a reduction in nitric oxide resulting in impaired vasodilatation
b. Carbon monoxide
1) Reduces the capacity of hemoglobin to carry oxygen
2) Impairs release of oxygen to body tissues
3) Results in a state of relative hypoxemia
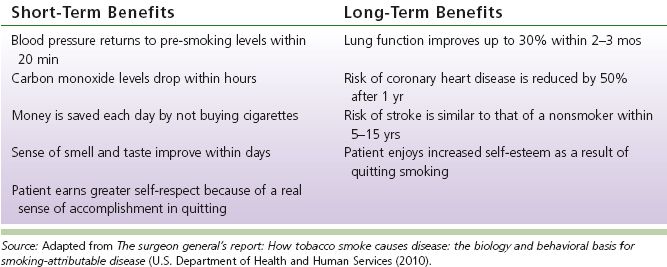
TABLE 8-1 Benefits of Smoking Cessation
c. Increased blood viscosity: increases risk of thrombosis
1) Polycythemia develops as a reaction to induced hypoxemia
2) Fibrinogen levels also increase, contributing to increased viscosity
d. Smoking induces a chronic inflammatory state
1) Evidenced by increased blood levels of C-reactive protein
2) Chronic inflammation is one theory of athrogenesis
e. Nicotine
1) Increases the heart rate and transiently increases blood pressure
2) Adds to the injury theory of athrogenesis
f. The gas and tar phases of tobacco smoke
1) Contain many reactive oxidizing species which enhance the free radical load of smokers
2) Results in increased oxidation of fatty acids and athrogenicity of LDL cholesterol (Benowitz, 2003)
3. Benefits of smoking cessation (see Table 8-1)
4. Smoking cessation: four crucial factors
a. Make the decision to quit; patient more likely to quit if
1) Believes he could get a tobacco-related disease
2) Knows someone who has health problems related to smoking
3) Believes the benefits of quitting outweigh the benefits of smoking
b. Setting a quit date
1) Pick a date and mark it on the calendar
2) Tell friends and family about quit day
3) Stock up on oral substitutes such as sugar-free gum and candy, carrot sticks
c. Decide on a plan
1) Obtain nicotine replacement if desired
2) Sign up for a class, set up a support system
d. Develop a maintenance plan
1) Review reasons for quitting
2) Develop alternatives to smoking: increase activity, find alternate stress relievers
3) Don’t worry about weight gain. Even without special attempts to diet, weight gain usually less than 10 pounds; more dangerous to continue smoking (AHA, 2010)
5. Symptoms of withdrawal
a. Depression
b. Feelings of frustration and anger
c. Irritability
d. Insomnia
e. Trouble concentrating
f. Restlessness
g. Headache
h. Tiredness
i. Increased appetite (AHA, 2010)
6. Techniques available to assist with smoking cessation
a. According to the 2005 PAD guidelines and Zwar et al. (2011), individuals with lower extremity PAD who smoke cigarettes or use tobacco should be advised by their clinicians to stop smoking at each clinic visit. These patients should be offered comprehensive smoking cessation interventions (behavioral modifications, nicotine replacement therapy, or bupropion)
b. Five components of effective smoking cessation (Five A’s) (Fiore et al., 2008)
1) Ask
2) Assess
3) Advise
4) Assist
5) Arrange follow-up
c. Nicotine replacement products and medications
1) Nicotine: drug found naturally in tobacco; as addictive as heroin or cocaine (National Institute on Drug Abuse, 2012)
2) When smokers try to quit or cut back, the absence of nicotine leads to withdrawal symptoms
3) Pharmacological interventions such as nicotine replacement therapy and bupropion achieve 1-year smoking cessation rates of approximately 16% and 30%, respectively (Hirsch et al., 2006)
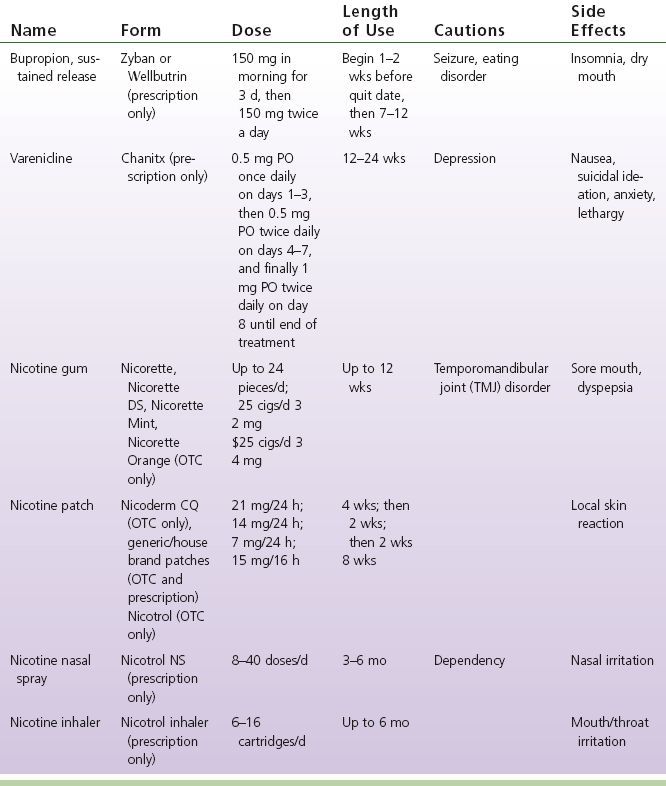
4) Medications helpful for successful smoking cessation and control of withdrawal (see Table 8-2)
d. Telephone “Quit lines”
1) Quit lines link smokers to counselors; help develop a plan for each person and help avoid common mistakes that may hurt a quit attempt
2) Convenient and accessible
3) People who use telephone counseling quit at twice the rate of those who do not get this type of help (AHA, 2010)
e. Self-help materials are the most common type of counseling but have not been found to be effective (Benowitz, 2003)
f. Both individual and group counseling have been found to increase rates of cessation (Benowitz, 2003)
g. Other tools for smoking cessation include
1) Hypnosis (useful for some people)
2) Acupuncture (no strong evidence to support effectiveness)
3) Filter to reduce tar and nicotine (not effective, as studies show smokers tend to smoke more with filters)
4) Over-the-counter products that change the taste of tobacco, combinations of vitamins (have little scientific evidence to support their claims) (AHA, 2010)
TABLE 8-2 Medications Used for Smoking Cessation (Rennard, Rigotti, & Doughton, 2013)
7. Regardless of technique used, certain information increases the chances of success
a. Thinking about the times of the day one is likely to smoke and plan distraction for those times; helps to start these distractions before one actually quits smoking
b. Social support in the form of caring, concern, and encouragement; this can come from healthcare providers, family, friends, and other community members (Rennard, Rigotti, & Doughton, 2013)
C. Antiplatelet and Antithrombotic Strategies (see Chapter 6, Medications Used in Patients with Peripheral Vascular Disease for full discussion)
1. Platelet aggregation starts with vascular trauma such as atherectomy, angioplasty, stent, surgery, hyperhomocysteinemia, smoking, external trauma, and burns
2. Platelet activation involves
a. Adhesion to the site of injury to the exposed collagen in the subendothelial layer
b. Release of intracellular granules containing chemical mediators
c. Aggregation of the other platelets caused by the chemical mediators, which forms a plug (Rooke et al., 2011)
3. Types of antiplatelet medications (Alberts et al., 2009)
a. Acetylsalicylic acid (aspirin)
b. Thienopyridines
1) Clopidogrel
2) Ticlopidine
3) Prasugrel
c. Ticagrelor
d. Dipyridamole
4. Goal of antiplatelet therapy: to reduce the risk of MI, stroke, or vascular death in individuals with atherosclerotic lower extremity PAD (Rooke et al., 2011)
a. Individuals with symptomatic lower extremity PAD
1) Intermittent claudication
2) Critical limb ischemia
3) Prior lower extremity revascularization (endovascular or surgical)
4) Prior amputation for lower extremity ischemia
b. In asymptomatic individuals with an ABI <0.90
1) Use in asymptomatic individuals with borderline abnormal ABI, defined as 0.91 to 0.99, not well established
5. Dual antiplatelet therapy (DAT): ASA + plavix may be considered to reduce the risk of cardiovascular (CV) events in patients with symptomatic atherosclerotic lower extremity PAD who are at high perceived CV risk (Rooke et al., 2011)
6. Oral anticoagulation therapy with warfarin is not indicated to reduce the risk of adverse CV ischemic events in individuals with atherosclerotic lower extremity PAD (Rooke et al., 2011)
D. Dyslipidemia Strategies
1. Body lipids: building blocks of fats (Galton, 2003; Jellinger et al., 2012).
a. In animals, lipids are spheres of oil stored for energy
1) Surface: phospholipid, free cholesterol, protein
2) Core: triglycerides and cholesterol
3) Triglycerides: fatty acid molecules contained in fats of both animals and plants
4) Difficult to transport in blood because of insolubility
b. Bound to proteins (albumin) and transported as lipoproteins
1) Complex aggregates of protein and lipid molecules transporting triglycerides and cholesterol from the intestines for use in peripheral tissues
2) Categorized into five types according to size and density, and whether they carry cholesterol or triglycerides
3) Cholesterol-carrying lipoproteins
a) Low density (LDL): “bad,” mostly cholesterol; lesions arise from transport and retention of plasma LDL through endothelium layer into subendothelial space, where LDL is then oxidized and initiates an inflammatory response; arterial wall becomes a scavenger for more LDL
• Apolipoprotein is a protein that binds to fats (lipids) forming lipoproteins. The lipoproteins then transport dietary fats through the bloodstream
• Apolipoprotein measurements (especially apolipoprotein B (apoB)) in several studies significantly predicted coronary heart disease (CHD) death, independently of conventional lipids and other cardiovascular risk factors (smoking, hypertension dyslipidemia, diabetes, and C-reactive protein). Sierra-Johnson et al. (2009) also found that the predictive ability of apoB alone to detect CHD death is better than any of the routine clinical lipid measurements
b) High density (HDL): “good,” mostly protein, large molecules that remove cholesterol from walls of arteries and transport to liver; prevent oxidation of LDL (antioxidant). High levels (over 60 mg/dL) are as important as low levels of LDL in prevention of disease
4) Triglyceride-carrying lipoproteins
a) Intermediate (IDL) and very low density (VLDL)
b) Chylomicrons: largest in size, lowest in density
c) Interact with HDL to cause fall in HDL as triglycerides rise
d) Also associated with arterial wall inflammation and thrombosis
5) Lipoprotein (a)
a) Size and density between LDL and HDL
b) Carry a protein that may deter the body’s ability to dissolve blood clots
c) Under investigation as either a marker or cause for arterial disease
d) Usually inherited; does not respond to diet/exercise
e) Older women and African Americans more at risk
6) Remnant lipoproteins: byproducts of chylomicrons and/or VLDLs; may be marker of arterial disease when cholesterol is normal
c. Two forms of metabolism of cholesterol and lipoproteins: endogenous and exogenous
1) Endogenous pathway: handles cholesterol produced by liver and chylomicron remnants that result from digestion of fat; chylomicron remnants converted in liver to VLDL, IDL, and LDL
2) Exogenous pathway: fats from diet absorbed in gut, incorporated into chylomicron particles made from triglycerides and apolipoproteins that are soluble in blood; chylomicrons are broken down by enzyme, lipoprotein lipase in capillaries, taken to liver where they enter endogenous pathway of lipid metabolism or are excreted through bile acids
d. Cholesterol sources
1) Dietary fats
2) Two thirds manufactured by liver
3) Production stimulated by saturated fat—animal products, meat, dairy
e. Cholesterol, an essential nutrient for
1) Repair of cell membrane (biogenesis)
2) Manufacturing vitamin D on skin’s surface
3) Production of hormones (estrogen, testosterone)
4) Steroid synthesis
5) Formation of bile acids (Jellinger et al., 2012)
f. Normal values for healthy people
1) Total cholesterol: 180 to 200 mg/dL
2) LDL, 130 mg/dL (70 mg/dL for individuals with atherosclerosis or diabetes mellitus)
3) HDL, 40 mg/dL (the higher, the better); for each 4-mg/dL decline, there is a 10% increase in cardiovascular disease
4) Triglycerides, 150 mg/dL or less
g. LDL particle size (LDL-P) (Jellinger et al., 2012)
1) The small and dense LDL particle is thought to be atherogenic. However, researchers have found that compared to LDL or non-HDL assessments, the number of LDL particles is a more sensitive indicator of CAD risk
2) Patients who have therapeutic LDL concentration on the standard lipid panel may still have a large number of circulating atherogenic LDL particles
3) LDL-P values: low = <1,000 nmol/L; borderline = 1,300 to –1,599 nmol/L; high = >2,000 nmol/L
4) Testing for particle size can be expensive and may not be needed since triglyceride levels >120 mg/dL and low HDL levels (<40 mg/dL in men, <50 mg/dL in women) are usually associated with small, dense LDL particles
2. Dyslipidemia
a. Defined as either elevated levels of total cholesterol, LDL, and triglycerides or lower levels of HDL
b. Fat transport: complicated system of proteins, enzymes, and receptors evolved to optimize transport and delivery of fat to peripheral tissue; when system breaks down, common disorders of lipid transport (dyslipidemia) and lipid storage (atherosclerosis) occur (Galton, 2003)
1) All abnormalities increase likelihood of cardiovascular disease (Grundy et al., 2002)
2) 50% of atherosclerosis deaths related to lipid disorders
c. Independent risk factor for vascular disease; when controlled, can lead to decreased development and progression of atherosclerotic plaque (Jellinger et al., 2012)
3. Role of dyslipidemia in atherosclerosis (Jellinger et al., 2012)
a. Evolution of atherosclerotic plaque is lifelong; a fatty streak in aorta evident by autopsy as early as age 10
b. Leading theory: injury to arterial endothelium followed by infiltration of lipids
c. Inflammation occurs after endothelial injury, making endothelium prothrombotic and susceptible to monocyte migration to the subendothelial or intimal space
d. Monocytes differentiate to macrophages that promote lipid oxidation and uptake; eventually become lipid-laden foam cells that characterize fatty streak
e. Smooth muscle cells may proliferate over the early atherosclerotic lesion or fatty streak and create a fibrous cap
f. Bang, Saver, Liebeskind, Pineda, and Ovbiagele (2008) found that patients with high cholesterol had an increased thrombotic potential compared with patients who had normal cholesterol
1) When aggressive cholesterol management achieved, evidence of decreased platelet thrombus formation seen
2) Increased thrombotic potential can accelerate the atherosclerotic process
3) Management of dyslipidemia should include some form of antiplatelet therapy
4. Dyslipidemia categories: Classification of dyslipidemias according to etiology can help clarify origin and management of lipid disorders (Galton, 2003)
a. Inherited: genetic predisposition to have an elevation in either triglycerides or cholesterol by defect of LDL receptor, lipase, or apolipoprotein
b. Acquired: elevation of cholesterol, triglycerides, or both
1) Elevated cholesterol caused by hypothyroidism, chronic renal failure, cholestatis, diabetes mellitus, anorexia nervosa, biliary cirrhosis
2) Elevated triglycerides caused by diabetes mellitus, alcoholism, obesity, and diet
3) Elevated cholesterol and triglycerides cause by obesity, insulin resistance, nephritic syndrome, alcoholism, hypothyroidism, oral estrogen treatment, pregnancy, Cushing’s syndrome, or drugs such as steroids, thiazides, or beta-blockers (BBs)
5. Screening for dyslipidemia risk assessment (Galton, 2003; Jellinger et al., 2012)
a. Modifiable risk factors—personal habits: smoking, exercise tolerance, alcohol, diet and occupation, obesity (BMI >30)
b. Current medications
c. Family history of dyslipidemia and evidence of atherosclerosis (e.g., MI, claudication, or stroke)
d. Other medical comorbidities, especially PAD and cerebral or cardiac vascular disease, hypertension, diabetes mellitus
e. Nonmodifiable risk factors: age (men <55; women <65), sex (higher risk in men than women until menopause), early menopause, positive family history
6. Diseases associated with dyslipidemia (Galton, 2003; Jellinger et al., 2012)
a. See above for acquired dyslipidemias
b. Multiple myeloma: abnormal gamma globulins secreted interfere with clearance of lipids from bloodstream
c. Metabolic syndrome (syndrome X): collection of health risks that increase likelihood of CAD, stroke, DM; characterized by central obesity, atherogenic dyslipidemia (high triglycerides plus low HDL), hypertension >130/85, insulin resistance, prothrombotic state (high fibrinogen), and proinflammatory state (elevated high-sensitivity C-reactive protein). Caused by obesity, inactivity, genetic factors predisposing to insulin resistance; 70 to 80 million Americans
7. Medications that interfere with lipids (Galton, 2003)
a. Diuretics: thiazides and spironolactone (increase both cholesterol and triglycerides)
b. BBs (increase triglycerides and decrease HDL)
c. Sympatholytics: prazosin or clonidine (decrease cholesterol)
8. ACC/AHA Cardiovascular Risk (Goff et al., 2013)
a. Calculation of risk of atherosclerotic cardiovascular disease (ASCVD) (10 years and lifetime risk). Those with 10-year risk of ASCVD ≥7.5%—need aggressive treatment
b. Assess risk factors every 4 to 6 years in patients 20 to 79 years of age
c. Variables
1) Sex
2) Age
3) Race (African American or Caucasian and all others)
4) TC
5) HDL
6) Systolic blood pressure (SBP)
7) Treatment for HTN
8) DM
9) Smoking
9. ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults (Stone et al., 2013)
a. Evidence no longer supports specific LDL or HDL treatment targets
b. Focuses on statin therapy to provide acceptable reduction in cardiovascular risk
c. Devised four statin benefit groups treated by either high-intensity or moderate-intensity statin therapy
d. Atherosclerotic cardiovascular disease (ASCVD): Defined as acute coronary syndrome (ACS), history of MI, stable or unstable angina (USA), CAD, vascular disease, history of transient ischemic attack (TIA) or stroke (CVA) thought vascular in origin
e. Statin therapy goals
1) High-intensity-lowering LCL-C by ≥50%
2) Moderate-intensity-lowering LDL-C by 30% to <50%
f. Four statin benefit groups
1) Patients with ASCVD
2) LDL ≥190 mg/dL
3) DM ages 40 to 79 with LDL 70 to 189 mg/dL without ASCVD
4) No ASCVD or DM and LDL 70 to 189 mg/dL with 10-year ASCVD of ≥7.5%
g. Types of statin therapy
1) High-intensity statin therapy
a) Population
• > 21 and ≤75 years with ASCVD
• LDL-C ≥190 mg/dL
• DM (I or II) ages 40 to 75 years with and estimated cardiovascular risk score ≥7.5%
b) Medications: goal: lower LDL-C >50%
• Atorvastatin 40 to 80 mg
• Rosuvastatin 20 to 40 mg
2) Moderate-intensity statin therapy
a) Population
• >75 years with ASCVD or not a candidate for high-intensity statin
• LDL-C ≥190 mg/dL and not a candidate for high-intensity statin
• DM (I or II) ages 40 to 75 years
b) Medications: goal: lower LDL-C 30% to <50%
• Atorvastatin 10 to 20 mg
• Rosuvastatin 5 to 10 mg
• Simvastatin 20 to 40 mg
• Pravastatin 40 to 80 mg
• Lovastatin 40 mg
• Fluvastatin XL 80 mg
• Fluvastatin 40 mg BID
• Pitavastatin 2 to 4 mg
3) Low-intensity statin therapy
a) Medications: goal: lower LDL-C <30%
• Simvastatin 10 mg
• Pravastatin 10 to 20 mg
• Lovastatin 20 mg
• Fluvastatin 20 to 40 mg
• Pitavastatin 1 mg
10. Lifestyle modification, the foundation for ASCVD risk factor reduction (Stone et al., 2013), includes
a. Heart healthy diet
1) Diet often neglected in advent of drug therapy despite recommendations (Jellinger et al., 2012; Kreisberg & Oberman, 2003)
a) Patients would rather take pill; providers see diet as ineffective/unimportant
b) Diet control allows lower drug dosage; minimizes adverse side effect potential
c) Lipid-lowering diet also aids control of other risk factors: hypertension, obesity, diabetes
2) General principles of lipid-lowering diet (Galton, 2003; Jellinger et al., 2012)
a) Decrease total and saturated fat intake
b) Increase high protein
c) Increase complex carbohydrates
d) Increase fruit and vegetables
e) Decrease dietary cholesterol
f) Moderately decrease sodium intake
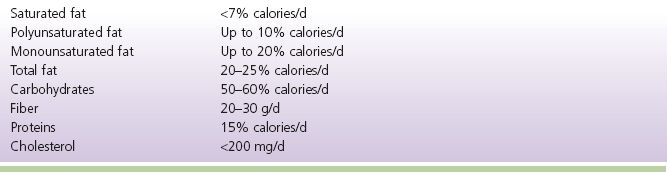
3) Goals of dietary treatment of dyslipidemia: lower ingested fat content and reduce operation of metabolic pathways that increase circulating LDL and triglycerides (Grundy et al., 2002; Jellinger et al., 2012). See Table 8-3 for recommended dietary goals
a) Saturated fatty acids found in high-fat dairy products, meats, tropical oils, and hard shortening increase LDL levels
b) Trans-fatty acids found in hydrogenated vegetable oils increase LDL and triglycerides and lower HDL
c) Substituting unsaturated fat for saturated fat improves cardiovascular risk and may improve insulin sensitivity
d) Exercise combined with diet can decrease triglycerides and increase HDL levels and will increase HDL levels with incremental advances in energy expenditure
e) Disadvantage: high carbohydrate intake may raise triglycerides and lower HDL (Jellinger et al., 2012)
TABLE 8-3 ATP III Diet Guidelines
4) Regular exercise: 30 to 45 minutes, in sessions performed at least three to five times per week (raises HDL, lowers triglycerides; inactivity is independent risk factor)
5) Maintain healthy weight (weight loss: improves insulin sensitivity and serum glucose uptake, lessens DM risk; lowers LDL)
6) Avoid tobacco products (smoking cessation: 30% increase in HDL)
11. Medication therapy (see also Chapter 6, Medications Used in Patients with Peripheral Vascular Disease)
a. Statins (Galton, 2003; Jellinger et al., 2012; Mihaylova et al., 2012; Stone et al., 2013)
1) Also known as HMGCoA (hydroxymethylglutaryl coenzyme A) reductase inhibitors
2) Directly inhibit cholesterol synthesis in the liver and peripheral tissues, as well as induces the synthesis of LDL receptors on cell membranes of the liver to remove LDL cholesterol
3) Most powerful agent for LDL lowering: 20% to 60% depending on medication used
4) Other benefits
a) Anti-inflammatory effects (pravastatin)
b) Stabilize the atherosclerotic plaque (by decreasing smooth muscle cell proliferation)
c) Alter vasomotor responsiveness and their antithrombotic effects (influencing platelet aggregation)
d) Recommended to be the first line of treatment in diabetic patients and those with increased triglycerides
5) Optimal dosing schedule: after the evening meal so that the synthesis of the liver is inhibited overnight
6) Examples of statins: atorvastatin (Lipitor), fluvastatin (Lescol), lovastatin (Mevacor), pravastatin (Lipostat), rosuvastatin (Crestor), simvastatin (Zocor)
7) Caution in patients with liver and renal dysfunction, pregnancy, or a high alcohol intake
8) Side effects of statins include reversible myositis; can ultimately lead to severe myopathy with rhabdomyolysis if drug is not discontinued
a) Individuals at higher risk for myopathy/rhabdomyolysis have
• Advanced age (>65 years)
• Hypothyroidism
• Renal insufficiency
b) CPK will become elevated
c) Crestor revised their labeling following an investigation by the FDA for myopathy and renal failure. Recommend starting patient on lowest dose initially
d) Dietary caution: grapefruit juice interferes with activity; 4 oz. of juice or 1/2 grapefruit is acceptable
e) Drug–drug interaction: atorvastatin may impair antiplatelet activity of clopidogrel
• The proposed mechanism is competitive inhibition of CYP450 3A4 enzymatic activity, which is responsible for the conversion of clopidogrel to its active metabolite
• Pravastatin (Pravachol), fluvastatin (Lescol), and rosuvastatin (Crestor) are not metabolized by CYP450 3A4 and are theoretically not expected to interact with clopidogrel
b. Nonstatin dyslipidemic medications: Research controlled trials show that nonstatin therapies do not provide acceptable ASCVD risk reduction benefits compared to their potential for adverse effects in the routine prevention of ASCVD (Stone et al., 2013)
1) Nicotinates (Galton, 2003; Jellinger et al., 2012)
a) Potent antilipolytic agents that inhibit the release of free fatty acids from adipose tissue, thus reducing the flux of free fatty acids to the liver
b) Niacin has been shown to reduce LDL and triglycerides and to increase HDL
c) Contraindications: chronic liver disease, arterial bleeding, and peptic ulcer disease
d) Side effects
• Skin flushing, dizziness, and palpitations; aspirin 30 minutes prior to niacin may decrease flushing
• Increasing insulin resistance in diabetics
• Increasing serum homocysteine
• Increasing uric acid levels
e) Examples of nicotinates
• Nicotinic acid (niacin)
• Advicor (lovastatin/niacin)
• Niaspan, Slo-Niacin, Niacin SR, Niacor
2) Fibrates (Galton, 2003; Jellinger et al., 2012)
a) Aid in lowering triglycerides and increasing HDL
b) Bind to peroxisomal proliferator–activated nuclear receptors to form transcription factors that regulate genes to drive fat utilization
c) Contraindications
• Hepatic or renal dysfunction
• Primary biliary cirrhosis
• Pre-existing gallbladder disease
d) Side effects (rare)
• Skin rashes
• Bone marrow dysplasias
• Gallstones
• Myositis
• Use with caution when combined with statins (statin doses should be reduced 25% to 50%) because of the increased risk of rhabdomyolysis
e) Examples of fibrates
• Clofibrate (Atromid)
• Bezafibrate (Bezalip)
• Fenofibrate (Tricor, procetophen)
• Gemfibrozil (Lopid)
• Ciprofibrate (Modalim)
3) Bile acid resins (Galton, 2003; Jellinger et al., 2012)
a) Bind bile acids in the small intestines and interrupt the circulation of bile in the liver, promoting its excretion in the feces
b) Without reabsorption of bile, there is an increased intracellular conversion of cholesterol to bile acids, ultimately leading to an increased catabolism of LDL particles
c) Contraindications
• Biliary obstruction
• Familial dysbetalipoproteinemia (triglycerides >500 mg/dL)
d) Side effects
• Increase in triglycerides
• Constipation, back pain, fatigue, cough
• Interference with the absorption of fat-soluble vitamins and some medications
• Bile acid resins are not directly absorbed into the body; systemic side effects do not occur
e) Examples
• Colestyramine (Questran)
• Colestipol (Colestid)
• May use in combination with statins or nicotinic acid but not fibrates (drug interaction)
4) Cholesterol absorption inhibitors (Jellinger et al., 2012; Kreisberg & Oberman, 2003)
a) A new class of dyslipidemic medication
b) Interferes with the absorption of cholesterol secreted in the bile and enterohepatic circulation
c) Zetia (Ezetimibe) is the only drug in this class available
d) Should be used adjunctively in patients whose LDL reduction is suboptimal
e) Cardiovascular end points of the effects of Zetia are yet unknown
f) A recent study Vytorin (Zetia/Simvastatin) vs. Simvastatin did not reduce carotid intima-media thickness. More research needs to be done (Kastelein et al., 2008)
12. Special considerations
a. Diabetics (ADA, 2013; Faxon et al., 2004)
1) Dyslipidemia commonly associated with type II diabetes; believed to be a major source of increased risk of vascular events
2) Diabetic patients tend to produce small, dense LDL that is more vulnerable to oxidation; LDL particles of diabetics contain more triglycerides than those of nondiabetics
3) According to the ATP, other mechanisms in diabetics that promote vascular disease are glycation of arterial wall proteins, change in arterial cellular function, promotion of thrombogenesis, and enhancement of LDL oxidation
4) 19% of poorly controlled diabetics have hypertriglyceridemia and low HDL levels; insulin deficiency leads to the impairment in clearance of plasma triglycerides
5) Because of the magnitude of the problem, dyslipidemia must be managed aggressively in diabetics; most recent American Diabetes Association guidelines recommend lipid-lowering therapy initiated in patients with type II diabetes and LDL levels ≥100 mg/dL
b. Women
1) Elevated triglyceride levels recognized as an independent predictor of CHD risk, especially in women and in patients with impaired glucose metabolism (Faxon et al., 2004)
2) Triglyceride levels are a more sensitive predictor of CHD risk in women than in men, and high triglyceride levels lead to greater atherogenic LDL particles and lower HDL levels; however, clinical trials on the vascular outcome of lowering triglycerides in women are lacking (Jellinger et al., 2012)
3) Postmenopausal women (Faxon et al., 2004)
a) Increased risk of ASCVD thought attributable to the loss of the “protective” effect of estrogen
b) Often gain weight and decrease their physical activity; when combined with the lower estrogen levels, can lead to increased lipids levels (Hsia et al., 2004)
c) Should have aggressive lipid-lowering therapy to include diet, exercise, and if necessary medication to lower their cholesterol levels
4) Women have increased regression of vascular plaques/lesions and similar improvement in survival as men on statin therapy
c. Elderly (Jellinger et al., 2012)
1) Providers may be reluctant to prescribe drug therapy for elderly patients; may feel patients have a short life span ahead or that their atherosclerosis is irreversible
2) Research controlled trials support continuing statins beyond 75 years of age in persons who are already taking and tolerating these drugs. More research data support the use of moderate-intensity statin therapy for secondary prevention in individuals with clinical ASCVD >75 years of age. However, there is less to support starting high-intensity statin therapy for secondary prevention in individuals >75 years (Stone et al., 2013)
3) Considerations in using lipid-lowering drugs in the elderly
a) Resins usually cause constipation, already an issue with most elderly
b) Renal function: most cases of myopathy with the use of statins associated with renal insufficiency
c) Fibrates can increase the risk of gall stones
d) Nicotinic acid: dry mouth and dry skin, along with flushing
d. Renal insufficiency (Jellinger et al., 2012)
1) Increased risk of cardiovascular events in patients with renal insufficiency and end-stage renal disease
2) Despite this increased risk, drug therapy for dyslipidemia in renal-diseased patients revealed a 28% reduction in major cardiovascular events
13. Summary
a. Dyslipidemia indicates elevated levels of total cholesterol, LDL, and triglycerides or lower levels of HDL, all of which increase the likelihood of cardiovascular disease
b. When associated with other vascular risk factors such as hypertension, smoking, diabetes, and a family history of vascular problems, dyslipidemia is a substantial risk factor for the progression of vascular disease
c. Evidence no longer supports specific LDL or HDL treatment targets. Guidelines now focus on statin therapy to provide acceptable reduction in cardiovascular risk
d. Risk factors for atherosclerosis are the target for therapeutic intervention and the method of risk assessment in the prevention of atherosclerotic vascular disease
e. Modification of dyslipidemias through diet, exercise, and/or drug therapy can decrease the progression of atherosclerosis, decrease cardiovascular events, increase atherosclerotic plaque stability, and decrease hyperthrombotic potentials in vascular patients
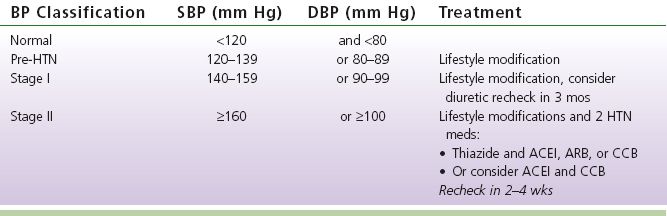
E. Hypertension Management (see Table 8-4)
1. Effects of elevated blood pressure, or Hypertension (HTN) (Chobanian et al., 2003)
a. One of the four major factors that contribute to the development of atherosclerosis and is an independent risk factor for cardiovascular disease.
Hypertension is a risk factor for
1) CAD
2) Heart failure (HF)
3) Stroke (CVA) or TIA
4) PAD
5) Chronic kidney disease (CKD)
6) Aortic regurgitation (AR/AI)
7) Atrial fibrillation/flutter
b. Incidence
1) Statistics: 81.5% of those with hypertension are aware they have it; 74.9% are being treated for HTN; 52.5% are under control (Go, Bauman et al., 2013)
2) Hypertension affects approximately 26.4% of the worldwide population and is the leading chronic risk factor for mortality, causing 13.5% of all deaths (Brook et al., 2013)
TABLE 8-4 Stages of Hypertension (Chobanian et al., 2003; Go, Bauman et al., 2013)