CHAPTER 5
Vascular Diagnostic Studies
Patricia A. Lewis
Marie Rossi
First Edition Author: Patricia A. Lewis
OBJECTIVES
1. Identify the purpose and clinical indications for invasive and noninvasive vascular diagnostic examinations.
2. List the elements of normal and abnormal results for vascular diagnostic examinations.
3. Correlate the findings from vascular examinations with appropriate nursing interventions.
Introduction
Vascular diagnostic studies are used as an adjunct to a thorough history and physical examination to detect disease in the arterial and venous systems, plan appropriate management or intervention, and follow disease progression. Invasive studies use various radiologic techniques combined with arterial or venous cannulation for instillation of radio-opaque contrast medium. Noninvasive testing uses ultrasound technology and Doppler principles to visualize vessels and analyze flow within the vascular bed under investigation. In addition to a discussion of the advantages and disadvantages of these general techniques, each test description provides information regarding test purpose, clinical indication, patient preparation, instrumentation, procedure, interpretation, limitations, complications and precautions, and nursing implications.
 I. Overview
I. Overview
A. Objective Evaluation of the Circulatory System
1. History and physical examination
2. Methods of documenting site(s) of involvement
3. Functional impact of disease on patient’s life
B. Invasive Vascular Examinations
1. Outline site(s) of disease
a. Catheter cannulation of vessel with instillation of radio-opaque contrast medium provides outline of arterial or venous vascular system
b. Highly accurate; considered “gold standard” for diagnosis of many disease conditions
2. Clinical benefits need to be weighed against risks and cost-effectiveness of procedure.
a. Potential iatrogenic injury to vascular system and organ beds; exposure to x-rays, and complications of contrast
b. Time consuming and expensive
3. Reserved for use when intervention is being considered
a. Not useful for screening
b. Limited use for following progression of disease or outcome of intervention because of cost and risks of procedure
C. Noninvasive Vascular Examinations
1. Based on Doppler ultrasound principles (Christian Doppler, circa 1840).
a. Audible sound waves: 20 to 20,000 Hz. 1 Hz = 1 sound wave cycle/second
b. Ultrasound: 1 to 30 MHz. Vascular ultrasound equipment uses 2 to 10 MHz
c. Doppler effect: high-frequency sound beam generated from vibrating piezoelectric crystal in transducer meets moving structure (blood cell), reflected sound returns at different frequency (frequency shift) (see Fig. 5-1)
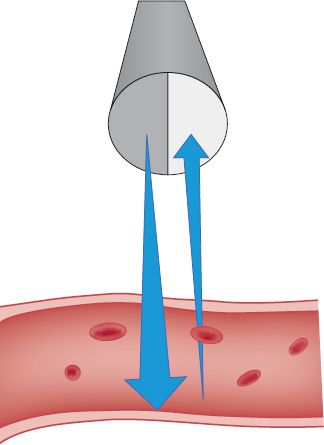
FIGURE 5.1 Continuous wave Doppler.
1) Speed of moving structure can be calculated from this frequency shift
2) Returning frequency increases if flow is toward the sound source (transducer); decreases if flow is away from sound source
3) Doppler equation: flow = returning frequency – sending frequency; dependent on angle of ultrasound beam directed at vessel (ideally, 60 degrees for most accurate signal processing) (Sanders, 1998)
4) Frequency of returning wave is converted into audible signal and visible waveform or sound spectrum on a screen that can be recorded as analog waveforms and analyzed by computer (spectral analysis)
5) Poiseuille law: speed of blood flow increases across an area of narrowing in a blood vessel, which results in higher frequency shifts on Doppler evaluation. At the same time, blood pressure in the vessel decreases beyond the area of narrowing because of loss of energy across the stenotic lesion. This is the basis for categorization of vascular stenosis
d. B-mode (brightness mode) ultrasound: reflected ultrasound produces two-dimensional (2D) image of anatomic structures (Ridgway, 1992; Rumwell & McPharlin, 1998)
1) Reflected ultrasound displayed in shades of gray; the denser the tissue, the brighter the image. Example: calcified plaque is bright white; blood or fluid-filled lumen is black
2) Real time: constantly rotating transducer produces moving images (pulsating vessel; moving organs)
e. Duplex ultrasound: combination of B-mode imaging with Doppler spectral analysis
1) Anatomic and hemodynamic/physiologic information
2) Color duplex imaging: frequency shift from moving red cells displayed with colors corresponding to direction of flow; blue, away from transducer (venous); red, toward transducer (arterial); mixed color, turbulent flow from mixed velocities across stenotic lesion (Wolf & Fobbe, 1995)
2. Advantages
a. Provide noninvasive quantitative determination of severity of disease
b. Suitable for screening, diagnosis, and follow-up evaluation
c. Highly accurate with experienced technologist
d. Relatively inexpensive compared with invasive testing without risks or complications
3. Disadvantages
a. Results are operator dependent
b. May not be adequate for operative intervention, although some surgeons rely solely on carotid duplex scanning results before endarterectomy procedures
 II. Noninvasive Diagnostic Studies
II. Noninvasive Diagnostic Studies
A. Arterial Examinations
1. Ankle–brachial index (ABI)
a. Purpose is to assess the presence of peripheral artery occlusive disease (PAD) in the lower extremities by using Doppler techniques combined with blood pressure evaluation of flow
b. Clinical indications
1) Assess the patient who has complaints of claudication
2) Evaluate patients who have suspected PAD by grading the severity of blood flow reduction
3) Aid in determination of intervention methods for treating PAD
4) Surveillance of disease progression before and after intervention
5) Screening general population for presence of PAD
c. Patient preparation
1) Explain procedure and complete history
2) Remove appropriate clothing: shoes, socks, and long-sleeved shirts to expose feet, ankles, and arms
3) Have patient lie supine with head slightly elevated in warm room for ≥10 minutes. Position for comfort and relaxation to minimize tremor, restlessness. Must be able to access all extremities easily
d. Instrumentation
1) 5- to 10-MHz continuous wave Doppler and coupling gel
2) Analog waveform equipment (desired but not absolute)
3) Appropriate blood pressure cuffs for arms and ankles and sphygmomanometer
e. Procedure (see Figs. 5-2 and 5-3)
1) Inflate blood pressure cuff to 20 mm Hg above systolic pressure, record systolic pressure as cuff is deflated using Doppler in right and left brachial, dorsalis pedis, and posterior tibial arteries
2) If analog waveform is to be recorded, obtain tracings in all vessels before blood pressure recordings
3) Calculate ratio of distal pressure/proximal pressure by dividing highest distal pressures on each limb by the highest brachial pressure. Example: ankle pressure of 60 mm Hg divided by brachial pressure of 120 mm Hg = 0.50
f. Findings/interpretation
1) In absence of disease, no difference between proximal (brachial) and distal (ankle) pressures. Distal pressures may be slightly higher because of normal vascular resistance. Therefore, normal index = 1.00 to 1.20.
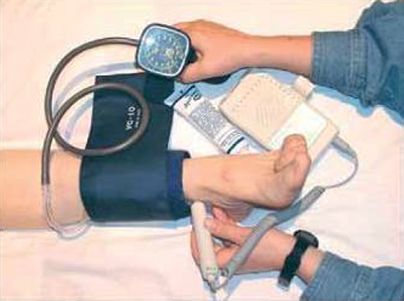
FIGURE 5.2 Performing an ABI.
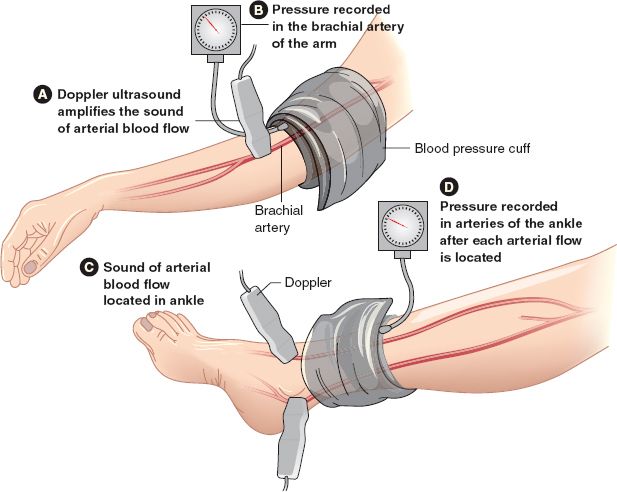
FIGURE 5.3 Cuff and Doppler placement for ABI.
2) As disease progresses, index drops, indicating reduced flow across stenotic lesion in arterial system; below diaphragm
3) Ratio is proportionate to degree of ischemia and is classified as follows
| Normal | 1.0 to 0.95 |
| Mild | 0.94 to 0.80 |
| Moderate | 0.75 to 0.40 (claudication level) |
| Ischemic | < 0.40 (threatened tissue/limb loss) |
Blackburn, D. R., & Peterson-Kennedy, L. (2004). Noninvasive vascular testing. In V. Fahey (Ed.), Vascular nursing (4th ed., pp. 73–96). St. Louis, MO: Saunders.
4) Ratio is useful for comparison studies/serial follow-up. Change of >0.15 (±) is indicative of deterioration or improvement
5) Analog waveform analysis (see Fig. 5-4)
a) Normal: sharp systolic upstroke, narrow waveform, one or more diastolic (reversed) wave components
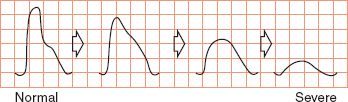
FIGURE 5.4 Analog readings.
b) Stenotic: absent diastolic component, widened systolic wave with blunting
c) Occluded or near occluded: marked flattening or widening; no diastolic component
d) Abnormal signal indicates stenosis proximal to or above site of recording
g. Limitations
1) Noncompressible vessels secondary to calcification of diabetes (Salles-Cunha et al., 2012), endstage renal disease—cause artificial elevation of pressures with index of ≥1.30. Must base examination on waveform analysis only.
2) Obesity: too large for appropriate cuff sizing
3) Acute deep vein thrombosis
4) Acute trauma, ulcerations, or indwelling intravenous (IV) line placement in areas needed for testing
5) Extensive dressings or casts at sites to be tested
6) Restlessness, tremor, inability to cooperate
h. Complications/precautions
1) Obtaining pressure readings immediately postoperatively following distal bypass revascularization may be contraindicated—check with surgeon. Waveform analysis acceptable
2) Appropriate precautions for wound infections. Avoid coupling gel in open wound
3) Avoid prolonged cuff inflation on any extremity
i. Nursing implications
1) Safe, accurate, reproducible, portable bedside test to assess vascular status on most patients, regardless of comorbidities
2) Implement appropriate vascular precautions and wound care as indicated
2. Segmental pressures and analog waveforms (see Fig. 5-5)
a. Purpose: to evaluate four segments of the arterial system in the lower extremity to identify the level of stenosis in the patient who is suspected of having PAD—aortoiliac, ileofemoral, femoral-popliteal, and distal circulation
b. Clinical indication: as above for ABI except not used for routine screening of general population
c. Patient preparation: as above for ABI
d. Instrumentation: as above for ABI with addition of blood pressure cuffs for upper thigh, lower thigh, below knee, and ankle
e. Procedure: After obtaining both brachial blood pressures as above, sequentially obtain analog waveforms and blood pressure on each segment of the lower extremities
1) All distal pressures are heard with the Doppler placed over the posterior tibial or dorsalis pedis artery, whichever is most audible
2) Calculate ABI as above for each extremity
f. Findings/interpretation: systolic pressure decreases and waveform changes below level of stenosis. Example: If pressures are diminished at high thigh, suspect aortoiliac disease. If not diminished until calf, suspect popliteal disease
1) If multiple levels of stenosis, systolic pressure measured distal to last area reflects additive changes, likely with ABI <0.5
2) Difference of >30 mm Hg between segments indicates disease
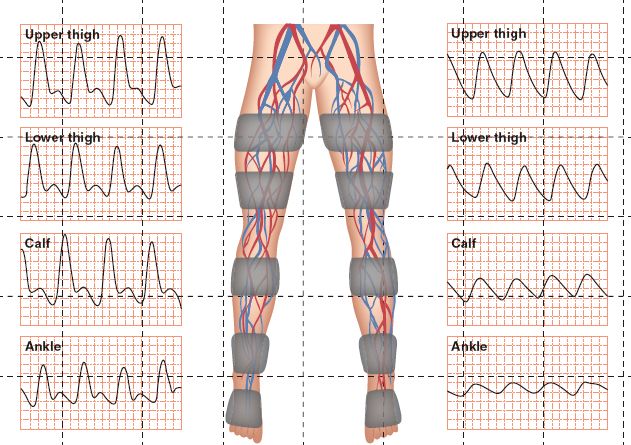
FIGURE 5.5 Segmental pressures and analog waveforms, Right leg = normal; Left leg = abnormal.
g. Limitations: as above for ABI
h. Complications/precautions: as above for ABI. Thigh cuff inflation may be painful; deflate as rapidly as possible while maintaining test accuracy
1) Nursing implications: as above for ABI. Useful test to predict healing potential for amputation level. If thigh pressure exceeds 60 mm Hg, below knee amputation should heal
3. Toe pressures (Strandness, 1996)
a. Purpose: assess distal arterial flow
b. Clinical indications: patients who have diabetes or calcification of larger vessels of lower extremity, which make ABI calculation falsely elevated. Also useful for prediction of healing toe, forefoot, or digit amputation wounds
c. Patient preparation: as for ABI
d. Instrumentation: as for ABI with addition of 2.5 × 9 cm toe cuff
e. Procedure: Obtain brachial systolic Doppler pressures as above. Place toe cuff around great toe of each foot, auscultate for arterial signal, obtain waveform tracing and pressure. Calculate toe/brachial index
f. Findings/interpretation: normal toe pressure = 60 mm Hg
1) Normal toe/brachial index (toe systolic pressure index) ≥0.60
2) Variability of measurement is ± 0.17
3) Hemodynamically significant lesion ≥0.60 or toe pressure between 30 and 60 mm Hg
4) Rest pain/ischemia ≥0.15 or toe pressure less than 30 mm Hg
g. Limitations: often difficult to auscultate toe arterial signal, especially when abnormal or if patient cannot maintain immobility during test. Pencil probe–style of Doppler is helpful
h. Complications/precautions: as above for ABI
i. Nursing implications: helpful in predicting healing of open lesions. If toe systolic pressure is less than 30 mm Hg, healing is unlikely without revascularization
4. Exercise testing
a. Purpose: to differentiate between circulatory and neurologic source (e.g., spinal stenosis) of leg pain
b. Indication: subclinical stenosis. Resting ABI is normal or near normal, but the patient complains of claudication symptoms
c. Patient preparation: as for ABI, leaving cuffs in place after baseline (resting) supine ABI is calculated
d. Instrumentation: as for ABI, plus watch or timing device and treadmill capable of adjusted speed and grade
e. Procedure
1) Obtain careful history: Neurospinal problems are not usually as reproducible as true claudication (good days, bad days; pain brought on with sitting or standing as well as walking; have to lie down for relief)
2) Obtain resting supine ABI and waveforms as above
3) Patient walks for 5 minutes or until disabled by leg pain at standardized speed and grade on treadmill: 1.5 to 2.0 mph with grade of 10% to 12%. Note time, symptoms, and walking distance/time at end of test
4) Record ABI on each limb every minute until pre-exercise level is obtained. Terminate test at 10 minutes after exercise
f. Findings/interpretation
1) Exercise normally increases flow; occlusive disease does not allow for this increase and pressures decrease with exercise
2) Pressure reduction proportionate to degree of stenosis and recovering time
3) Multilevel disease is additive, as in segmental examination
4) If pressures do not decrease with exercise, suspect noncirculatory origin of leg pain
g. Limitations: reserve test for patients who are physically able to perform exercise safely. Contraindicated for patients who have angina, known abnormal cardiac stress test, recent coronary artery bypass graft without normal postoperative stress test, significant dyspnea, uncontrolled hypertension, or unable to ambulate unassisted
h. Complications/precautions: Patients who have unknown cardiac or pulmonary disease may experience cardiac or respiratory symptoms during testing. Be prepared to abort testing; have rescue equipment/system available. Because of the high percentage of cardiac disease, cardiac monitoring during testing is recommended
i. Nursing implications
1) Constant observation, monitoring recommended
2) Rapid pressure recordings required for accuracy of results
3) Contact referring provider if patient is not appropriate candidate for exercise
5. Pulse volume recording (PVR) (see Fig. 5-6)
a. Purpose: Record volume changes in limb with each arterial pulsation. May be used as alternative waveform to analog waveforms or used to supplement arterial analysis
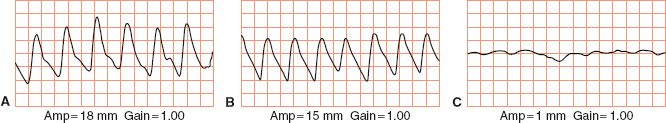
FIGURE 5.6 Pulse volume recordings. A. Normal. B. Mild arterial disease. C. Severe arterial disease.
b. Clinical indication: used with ABI, segmental, or toe pressure studies
c. Patient preparation: as for ABI, segmental, or toe pressure study
d. Instrumentation: air plethysmography. Pneumatic (air-filled) cuff applied to extremity, inflated to low pressure (∼60 mm Hg) to ensure contact with skin and compression of venous component of flow. Volume changes in limb with each pulsation result in pressure changes within air-filled cuff. These are recorded as waveforms and analyzed for normal configuration
e. Procedure: as for ABI or segmental pressure examination. A small cushion is placed beneath heels. Large thigh cuff is placed as proximally as possible on thigh. Cuffs are inflated one at a time, to a pressure of 60 to 65 mm Hg. Volume recordings are obtained at each level for five or six cardiac cycles
f. Findings/interpretation
1) Normal: sharp systolic peak; prominent dicrotic wave
2) Mildly diminished: sharp systolic peak; absent dicrotic notch; downslope bowed away from baseline
3) Moderately diminished: flattened systolic peak; upslope and downslope nearly equal; dicrotic wave absent
4) Severely abnormal: pulse wave of very low amplitude or entirely absent; if present, equal upslope and downslope time
g. Limitations: patient must be able to lie still; tremor and motion interfere with accuracy of recording. Obesity may limit cuff placement as with segmental examination. Pain is not usually a limitation because the cuffs are not inflated above arterial systolic pressure
h. Complications/precautions: usually acceptable to perform following distal bypass graft procedure; check with surgeon as indicated
i. Nursing implications: analysis of PVR from different levels provides information regarding level or site of disease and estimate of severity of stenotic process
6. Arterial duplex scan with color Doppler of lower extremity (see Fig. 5-7)
a. Purpose: localizing and grading stenotic lesions in the arterial system or bypass (Hodgkiss-Harlow & Bandyk, 2012) grafts in the lower extremities. Used in conjunction with ABIs, segmental pressures, or PVRs
b. Indications
1) Native vessels: claudication, rest pain, absent/diminished pulses, edema limiting arterial examination, cold sensitivity, identification of isolated stenotic lesions suitable for angioplasty/stent placement, suspected peripheral aneurysm with or without microemboli to foot, and documentation of peripheral flow in critically ill patient with balloon pump in place
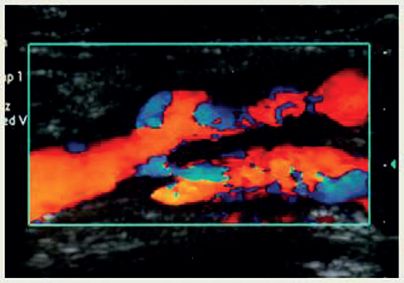
FIGURE 5.7 Arterial duplex.
2) Evaluation of bypass graft flow: Immediately postoperatively and annually for baseline evaluation; decrease in ABI value, new claudication or pain, bruit/thrill over graft
3) Evaluation of pulsatile mass—aneurysm, pseudoaneurysm, or hematoma—especially following groin cannulation for invasive procedure
c. Patient preparation
1) Explain procedure to patient
2) Remove all unnecessary clothing below waist; drape patient and keep warm
3) Position patient supine with head resting on pillow
d. Instrumentation: high-resolution duplex scanner with color flow capabilities
1) 5- to 7.5-MHz liner array probe. Low-frequency (2 to 3 MHz) probe if the aortoiliac segment is to be examined
2) Acoustic gel
3) 10-cm cuffs for ABI/brachial pressures; hand-held Doppler
e. Procedure
1) Record ABI or segmental pressures as above
2) Each vessel, common femoral artery (CFA), proximal, mid, and distal superficial femoral artery (SFA), popliteal, posterior tibial artery (PT), and dorsalis pedis artery (DP) is imaged along entire length using 2D color duplex and duplex Doppler in sagittal views. Bypass grafts are imaged in longitudinal view, including proximal and distal anastomosis and flow in native vessels
3) Color imaging is used to visualize any velocity increases that would suggest narrowing. Significant stenosis (usually ≥75%) will produce color aliasing—multiple colors mixed together combined with high-frequency signal >200 cm/sec
4) Doppler velocity samples are obtained proximal to, at, and distal to, any areas of stenosis. Doppler angle is set at 45 to 60 degrees. Smallest sample volume should be used with sample volume placed in center of vessel. Maintain Doppler angle parallel to blood flow. Obtain vessel diameter measurements
5) If no stenotic lesions are identified, velocities are recorded in the midportion of each segment
f. Findings/interpretation
1) Normal flow: laminar pattern with highest frequency/velocity in center of lumen, lower frequencies/velocities along wall. Similar frequencies/velocities throughout vessel if unobstructed. Spectrum displayed on screen has sharp systolic peak, black “acoustic window” under peak—looks similar to analog waveform
2) Stenotic flow: higher frequencies/velocities with more variable frequencies because of turbulence. The more stenosis, the higher the systolic frequencies and the more turbulence that is detected, especially in the segment just distal to the area of stenosis. Acoustic window becomes filled in with mixed frequency shifts (“spectral broadening”)
3) Occluded flow: dull “thump” with markedly reduced systolic flow, no diastolic flow proximal to the occlusion. At and distal to occlusion, no signal is obtained
| Diameter reduction | Peak systolic velocity (PSV) |
| 0% to 19% | Normal velocity pattern without increase in flow |
| 20% to 49% | PSV increased > 30% but < 100% from normal proximal arterial segment |
| 50% to 99% | PSV increased > 100% or doubled baseline velocity (hemodynamically significant stenosis) |
| Occluded | No flow in imaged vessel |
| Waveform analysis | |
| 0% to 19% | Triphasic; no spectral broadening |
| 20% to 49% | Reverse component of waveform present; spectral broadening present but mild |
| 50% to 99% | Loss of reverse component, spectral broadening, and velocity increase at stenosis; monophasic waveform and decreased systolic velocity distally |
| Occlusion | Monophasic, preocclusive “thump” heard proximally to stenosis; diminished velocity and monophasic waveform distally |
| Diameter enlargement Twice expected size | Suspect aneurysm; flow: often diminished velocity with midstream turbulence; thrombus seen along vessel wall |
| Pulsatile mass adjacent to vessel | Suspect pseudoaneurysm, may be able to identify attachment or neck, varying color flow intraluminal velocities with turbulence |
g. Limitations
1) Staples on skin may obscure imaging
2) Time consuming
h. Complications/precautions: no adverse effects of diagnostic ultrasound have been identified
1) Pain control measures may be needed for patients who have rest pain or postoperative pain
2) Avoid acoustic gel in open wounds or incision line
i. Nursing implications
1) Accurate identification and location of stenotic segments in native and conduit vessels, as well as postoperative fistula
2) Because of time required, patients who cannot lie still may not be candidates for examination
3) Ultrasound-guided compression of catheter-related femoral pseudoaneurysms possible with same scanning probe by manually compressing aneurysm at the point of communication with native artery. Several cycles of 10 minutes each may be needed to induce thrombosis unless pseudoaneurysm is chronic. Careful monitoring of site needed with repeat study 24 hours after compression. Monitor limb and distal pulses for evidence of change in perfusion

FIGURE 5.8 Carotid duplex with fibromuscular dysplasia.
7. Arterial duplex scan of extracranial arterial system (carotid, vertebrobasilar) (see Fig. 5-8)
a. Purpose: examination of the extracranial arterial system for anatomic and physiologic evidence of arterial disease; helpful in detecting lesions amenable to operative intervention and following disease progression (Porreca, Rodriguez-Wong, & Hughes, 2012)
b. Clinical indications
1) Hemispheric symptoms of stroke
2) Transient ischemic attacks; resolving ischemic neurologic deficit
3) Amaurosis fugax or transient monocular blindness
4) Nonhemispheric symptoms: dizziness, loss of memory, drop attacks, blurred or double vision, sudden nausea/vomiting, ataxia, or other symptoms suggestive of vertebrobasilar disease
5) Discrepancy of brachial blood pressures of >20 mm Hg or symptoms of unilateral arm fatigue, diminished pulse, pallor, coolness, or other symptoms of subclavian steel syndrome
6) Asymptomatic carotid bruit
7) Intra- and postoperative surveillance of carotid endarterectomy and stent placement
8) Evaluation for follow-up of known disease for progression
9) Palpable pulsatile neck mass
10) Neck trauma to assess for intimal tears (e.g., motor vehicle accident seat belt injury)
11) Asymptomatic patient with planned cardiac bypass surgery to evaluate for comorbid carotid disease. Often surgically corrected simultaneously
c. Patient preparation
1) Explain procedure to patient
2) Place patient supine with neck hyperextended (small pillow or towel roll may be used), head turned away from side to be examined. If not able to cooperate with this position, scanning may be done with patient sitting
3) Obtain bilateral brachial blood pressures; assess for presence of carotid bruit
d. Instrumentation: high-resolution duplex scanner with color flow capabilities, 5- to 10-MHz linear array transducer, acoustic gel
e. Procedure
1) Examiner is positioned at head of bed or at level of patient’s shoulder
2) Transducer is placed in transverse plane at base of neck, vessels identified to level of bifurcation. Color may be turned off to better image atherosclerotic plaque. Diameter reduction measurements are obtained
3) In sagittal plane, images and duplex Doppler velocity measurements are obtained in the common, internal, and external carotid arteries
4) If plaque is identified, velocity measurements are obtained proximal, mid, and distal to plaque formation. Highest velocity measurement used for calculation of internal carotid to common carotid ratio. Angle of insonation should be maintained as close to 60 degrees as possible
5) Vertebral arteries are imaged in transverse and sagittal planes, imaging from the upper neck to the subclavian. Subclavian Doppler signals are also recorded
f. Findings/interpretation
1) Images: intact intima visualized throughout common, internal, and external carotid vessels (Kingstone, Torres, & Currie, 2012)
2) If plaque is identified, diameter reduction is measured in transverse plane by measuring with electronic calipers. Plaque is classified as homogenous (uniform composition), heterogenous (mixed composition), smooth, irregular, calcified, and ulcerated with or without thrombus.
3) Doppler analysis
4) Intraoperative carotid endarterectomy examination: moderate flow disturbance (peak systolic flow 125 to 140 cm/sec) with abnormal image warrants assessment with intraoperative arteriogram. Severe flow disturbance with marked spectral broadening and abnormal image warrants immediate revision
g. Limitations
1) Requires experienced ultrasonographer and expensive scanning equipment
2) Calcifications may obscure accuracy of diameter reduction measurements
3) Vessel tortuosity and aberrant anatomy may limit accuracy; may confuse internal carotid artery (ICA) and external carotid artery (ECA) on imaging; need to rely on signal processing. ICA flow is low resistance (windy sound) with elevation off baseline; ECA flow is high resistance, similar to peripheral arterial signals
4) High bifurcation under jaw limits complete evaluation
5) Unable to examine above jaw into carotid siphon level
6) May be difficult to identify vertebral system, especially in patient who has a thick neck or significant arthritic changes of spine
7) Calculation of ICA/CCA ratio may be difficult in a patient who has arrhythmia, especially atrial fibrillation, because peak systolic velocities vary
8) Occluded ICA may lead to “internalization” of ECA signal and mask diagnosis
h. Complications/precautions
1) No adverse effects of diagnostic ultrasound
2) Avoid excess pressure on carotid sinus during examination, which may provoke syncopal episode
3) Patients who have sustained trauma should not undergo neck manipulation without physician clearance
i. Nursing implications
1) Careful pretesting assessment, noting uniformity of blood pressures, neurologic status, presence of carotid bruit, and thorough history for risk factors and symptoms
2) Comfort measures as indicated, especially in agitated patient, to ensure accuracy of results and avoid unnecessarily long examination time
3) Correlation of test results with clinical indications, history, and physical examination; asymptomatic total occlusion is rare, but possible
8. Arterial duplex scan of renal and mesenteric arterial system and aorta (see Fig. 5-9)
a. Purpose: anatomic and physiologic assessment of the mesenteric (splanchnic) and renal arterial systems (Neumyer, 2012)
b. Clinical indications
1) Abdominal bruit
2) Unexplained weight loss
3) Persistent postprandial nausea, vomiting, and abdominal pain
4) Uncontrolled hypertension on antihypertensive medication therapy (suspected renovascular hypertension)
5) Renal allograft rejection suspected
6) Abdominal aortic aneurysm detection; identification of size and location
7) Visualization of aortic endograft; documentation of position, patency, presence of endograft leak (Blackburn & Peterson-Kennedy, 2004; Iezzi et al., 2009)
c. Patient preparation
1) Fast for 6 to 8 hours before examination; preferable to perform study early in morning after overnight fast when bowel gas is at minimum
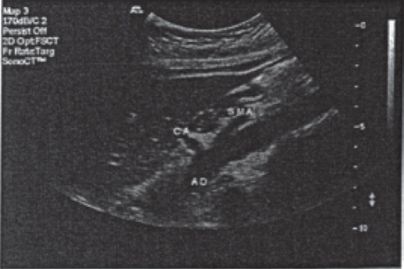
FIGURE 5.9 Normal duplex exam.
2) Supine position with head resting on pillow, slightly elevated. Will need to turn to right and left lateral decubitus position during renal examination
d. Instrumentation: high-resolution duplex scanner with color Doppler capabilities, low-frequency pulsed-Doppler probe (2 to 4 MHz), acoustic gel
e. Procedure
1) Beginning at xyphoid process, using transverse view, identify proximal abdominal aorta; record Doppler signal in sagittal view; note any plaque or aneurysmal changes
2) Image celiac artery and obtain Doppler signals—first branch of abdominal aorta (Roberts, Alonso, & Khatib, 2012)
3) Image superior mesenteric artery and obtain Doppler signals—second branch of abdominal aorta just distal to celiac trunk
4) Image and obtain signals in hepatic and splenic arteries
5) If an enlarged inferior mesenteric artery is noted, this may suggest superior mesenteric artery (SMA) occlusive disease
6) If scanning for suspicion of mesenteric ischemia, have patient ingest high-calorie liquid (protein shake). Repeat celiac and SMA measurements in 30 to 40 minutes
7) If scanning for suspicion of renal artery stenosis, obtain vessel images and signals at origin (ostium), mid, and distal portions of both renal arteries, noting any plaque formations. Patient may be turned to right and left lateral decubitus positions to maximize imaging. Obtain images of each kidney, measuring pole to pole, and velocities from medulla, cortex, and renal hilum
f. Findings/interpretation
1) Celiac signals: normally low resistance with open acoustic window in spectral wave, continuous forward flow throughout cardiac cycle. Systolic and diastolic velocities increase with protein supplement meal. Celiac systolic velocity >200 cm/sec is abnormal and indicates stenosis
2) SMA signals: in fasting state, normally high resistance (triphasic) with early diastolic flow reversal and late diastolic forward flow. Postprandially, flow becomes low resistance because of vasodilatation of mesenteric vessels. Systolic velocity >275 to 300 cm/sec suggests stenosis of >70%. Postprandial PSV nearly doubles the fasting velocity but should be <160 cm/se. Velocities should return to baseline in 90 minutes
3) Renal size: fairly uniform measurements of 10 cm in length. A pole-to-pole length of <9 cm or difference of 3 cm suggests stenosis or occlusion. Very small kidney length (5 to 6 cm) suggests chronic occlusion
4) Renal signals: low-resistance pattern with continuous forward flow throughout cardiac cycle, similar to ICA or celiac. Normal PSV is <180 cm/se. Normal aorta systolic velocity is ∼60 ± 15 cm/sec. Calculate peak systolic renal artery velocity/peak systolic aorta velocity ratio to obtain renal/aorta ratio. Normally, ratio is <3.5; if >3.5, stenosis is suspected
5) Renal parenchymal signals (medulla and cortex): use highest amplitude and velocity to calculate end-diastolic/peak systolic ratio to determine parenchymal resistance. A ratio of <0.2 indicates intrinsic renal parenchymal disease (e.g., diabetic nephropathy)
6) Aorta: dilatation over twice normal size of 2 cm is indicative of aneurysmal changes; thrombus lining wall of aneurysm may be identified, as well as location and flow. Endograft surveillance should demonstrate reduction of aneurysmal sac over time; enlargement indicates endograft leak (Blackburn & Peterson-Kennedy, 2004)
g. Limitations
1) Large body habitus
2) Bowel gas artifact
3) Adipose distribution
4) Respiratory movements
5) Unpredictable vessel course
6) Recent abdominal surgery
7) Requires very experienced ultrasonographer and expensive equipment
8) Time consuming; adequate patient preparation is required
9) Coexistent abdominal aorta may limit visualization of splanchnic and renal branches
h. Complications/precautions
1) Patients who have true mesenteric ischemia may not tolerate high-calorie meal challenge; will cause significant pain or vomiting. Use clinical judgment
2) Patients who have chronic obstructive pulmonary disease combined with obesity will not tolerate supine position for lengthy renal scan procedure—often 1 hour or more
i. Nursing implication
1) Careful explanation of examination is required to ensure patient cooperation
2) Comfort measures during lengthy examination, especially monitoring for respiratory distress as indicated by patient condition
B. Venous Examinations (see Fig. 5-10)
1. Venous duplex scan (Pellerito & Polak, 2012; Salles-Cunha & Beebe, 2001)
a. Purpose: determine the presence and assess the location and extent of acute or chronic venous disease in the deep and superficial venous system by using ultrasound techniques
1) Examination of choice for detection of deep vein thrombosis
2) Visualization and localization of acute and chronic thrombosis
3) Hemodynamic information of flow patterns in venous system
b. Indication: evaluation of patients who have suspicion or clinical evidence of
1) Deep venous thrombosis
2) Postphlebitic syndrome
3) Venous insufficiency
4) Varicose veins
5) Edema of the extremities of unknown etiology
6) Mapping of greater and lesser saphenous, cephalic, and basilic veins before harvest for bypass surgery
c. Patient preparation
1) Explain procedure
2) Remove all unnecessary clothing to expose extremity(ies) or area to be examined (abdomen, thorax) adequately
3) Supine position
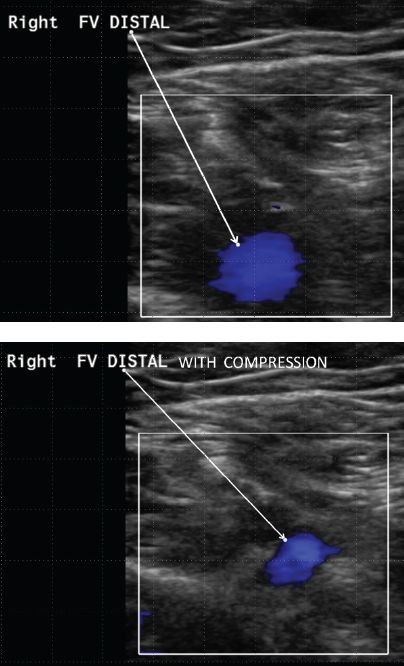
FIGURE 5.10 Normal duplex examination at the common femoral level.



