23 Trauma Management
After reading this chapter, you should be able to:
• identify the benefits and limitations of an organised trauma system
• describe the rationale for a systematic approach to the patient who has sustained injuries
• discuss the benefits of appropriate nursing care of the patient with serious injury and/or multitrauma
• describe the acute nursing management of the patient with multiple serious fractures
• describe the acute nursing management of patients with burn injuries, abdominal injuries and chest trauma
• describe the nurse’s role in managing the trauma patient undergoing interim damage-control surgery.
Introduction
Trauma refers to physical injury that is caused by mechanical injury, also known as kinetic injury. Injury remains the leading cause of death in adults under 45 years of age, and is a leading cause of preventable mortality and morbidity in Australia and New Zealand, as well as the rest of the world.1–4 Furthermore, injury represents a major cost to injured individuals, the healthcare system and society.5,6 More than 5.2 million people throughout the world die due to injury, with 90% occurring in low- to middle-income countries. According to the World Health Organization, injury accounts for 16% of the world’s disease burden.7
Trauma Systems and Processes
A trauma system can be defined as:
an assembly of health care processes intended to improve survival among injured patients by reducing the time interval between injury and definitive treatment, and by assuring that appropriate resources and personnel are immediately available when a patient presents to a hospital’.8, p. 643
Without trauma systems in place, a range of organisational and clinical errors in the management of trauma patients have been identified. These errors occur at all stages of care, including prehospital, emergency, operating theatre, intensive care unit, wards, and during transfers between hospitals.9 The majority of errors identified were errors in management of patients, although approximately 20% of errors occurred as a result of system inadequacies. A smaller number of technique or diagnostic errors occurred.
Over the past 20 years there has been increasing emphasis on the development of trauma systems that cover geographical areas, such as a nominated state or region. The introduction of trauma systems has resulted in a 15–30% reduction in the risk of death, primarily in the area of preventable deaths.10 Although this reduction appears widespread, it has not been replicated in remote areas,11 and is limited by the lack of examination of deaths that occur before a patient reaches hospital or after discharge. Additionally, the lack of examination of functional outcomes limits interpretation of the trauma system, as it is not clear whether the patients who survive have altered functional capacity. Despite these limitations, there is widespread agreement on the benefits of trauma system implementation, although the contribution of nursing care in such trauma systems is rarely considered or measured. Furthermore, the precise components of a trauma system that prove beneficial have not been identified.12
Prehospital Care
The debate regarding the relative benefits of stabilising a patient at the scene versus proceeding to the hospital as quickly as possible, is not new.13 Benefits are somewhat dependent on the proximity of effective trauma facilities, the level of knowledge and skills of the prehospital personnel available and the specific injuries and condition of the patient. The principle of the ‘golden hour’ remains in place today and suggests that, in order to improve outcomes, definitive care should be provided to patients as soon as possible, and preferably within 1 hour of the injury being sustained.13,14 In countries with large distances and sparse populations this aim presents particular challenges and cannot be met in many regions. Despite these distances and transport challenges, recognition of life-threatening conditions, application of appropriate emergency interventions and prompt transport to the nearest appropriate hospital remain the principles of prehospital care.13–15
In a number of regions, processes are in place to facilitate prehospital admission: personnel can notify the receiving hospital in advance, for those patients who meet predefined criteria. Identified patients generally have severe physiological compromise, or injuries from high-velocity causes that result in significant injury and associated poor outcomes. Early notification allows the assembly of a multidisciplinary group of health professionals who can provide immediate, expert assessment, resuscitation and treatment of critically injured patients.16,17 Such trauma teams have been shown to provide benefit in the early management of multiply-injured trauma patients, and are reviewed later in this chapter.10,16
Transport of the Critically Ill Trauma Patient
Transport of critically injured patients occurs at two stages in the patient’s care. Primary transport occurs from the place of injury to the first healthcare facility to provide care to the patient; this is sometimes referred to as prehospital transport. Secondary transport occurs between healthcare facilities; this is sometimes referred to as interhospital transport. This chapter concentrates on secondary transport, although many of the principles are similar for both stages of transport. Intrahospital transport principles are also relevant for critically injured patients being transferred within departments in a healthcare facility (see Chapter 6). Transport of a patient between healthcare facilities may occur for clinical reasons, such as specialist or higher levels of care being required, or for non-clinical reasons, such as bed availability. It is preferable for patient transfer to be for clinical reasons only; however non-clinical transfer is sometimes unavoidable.
• the condition of the patient
• the potential impact of the transport medium on the patient
• the urgency of the transport
• the environmental conditions
Amenities such as landing sites, particularly for helicopters, being in close proximity to healthcare facilities must also be considered. Different jurisdictions activate air retrieval using helicopters when the distance for the transport is beyond a certain point, with the minimum distance ranging from 16–80 km.14,15,19,20
It is essential that the standard of care is not compromised during transport of critically injured patients. Minimum standards exist that outline the requirements for transport of critically injured patients, and these should be referred to for full details.13,18,19 The following principles apply during such phases of care:
• There must be adequate preparation of the patient and equipment.
• Transport must occur by personnel with appropriate levels of expertise.
• Necessary equipment, including batteries and pumps, should be secured.
• Patients should be stabilised prior to transport (whilst balancing the need for timely transport).
• Monitoring of relevant aspects of the patient’s care is essential.
• Adequate vascular access and airway control must be secured prior to commencing transport.
• Effective communication is mandatory between referring, transporting and receiving personnel.
• Documentation, including X-rays and scans, should accompany the patient and should cover the patient’s status, assessment and treatment before, during, and on completion of, the transport.
• Relatives should be informed of the transfer, including destination, and provided with assistance for their own travel arrangements.18 Checklists itemising many of these principles, sometimes attached to an envelope containing all transfer documentation, are often used to ensure that all necessary actions are undertaken.20
Trauma Reception
The formal process of triage provides a means of categorising patients based on threat to life. Although there are many different triage systems in use, within Australia and New Zealand, the five-level triage categorisation of the Australasian Triage Scale (ATS) is widely used.23 See Chapter 22 for further description of the ATS.
Primary Survey
Priorities of care are similar to those in all health settings, with airway, breathing and circulation taking precedence, and disability and exposure/environment being part of the primary survey (see Chapter 22). These components of care will often occur simultaneously rather than sequentially. Compromise to airway and breathing may result from direct injury, for example to the trachea, or indirectly through decreased level of consciousness. Compromise to circulation is usually as a result of significant blood loss, although it may occur as a result of injuries, such as cardiac contusions in chest trauma, or the patient’s preexisting disease. The priorities of care during this time reflect the principles of care in any setting, and include:
Secondary Survey
Following stabilisation of the life-threatening problems identified during the primary survey, patients should undergo a secondary survey (see Chapter 22). This is a systematic examination of the body regions to identify injuries that have not yet been recognised. It is essential that both the front and the back of the patient, as well as areas covered by clothing, are examined during this process.
Radiological and Other Investigations
If the patient is sufficiently stable after the secondary survey, more extensive investigation in the radiology department should be undertaken. This will include CT scans. It is essential that clinicians consider investigations carefully, to ensure that all necessary imaging is undertaken; for example, where a CT scan of the brain is required it is often prudent to also undertake a CT scan of the cervical spine. However care should be taken to avoid investigations that will not change the planned treatment but may delay urgent interventions such as surgery. Current controversies in radiation exposure and lifetime-associated cancer risks need to be considered.21 Furthermore, the implications of moving the patient on and off imaging tables for repeated imaging is problematic. The patient should be accompanied and monitored by an appropriately competent nurse during all transfers for investigation. Where the patient is requiring ongoing advanced life support such as fluid resuscitation or airway monitoring, it may also be appropriate for a medical officer to accompany the patient.
Focused assessment with sonography for trauma
Where abdominal trauma is suspected, a focused assessment with sonography for trauma (FAST) examination22,23 is likely to be used as part of the secondary survey to determine whether free fluid is present in the abdominal cavity. The abdomen is scanned in four zones – pericardial, Morison’s pouch (right upper quandrant), splenorenal (left upper quadrant), and pelvis (Douglas’ pouch). This generally takes 1–2 minutes when performed by an experienced, credentialled clinician. Findings are regarded as positive (fluid [blood] observed), negative or equivocal. Technical difficulties can be experienced with obese patients. While a positive FAST is useful in identifying if a patient should receive urgent surgical intervention, a negative FAST does not rule out significant abdominal trauma, and the low sensitivity of FAST remains a concern for trauma clinicians.22 Where a patient is undergoing a prolonged trauma resuscitation phase, there may be an indication to repeat the FAST after 20 minutes. The use of FAST examination outside the trauma resuscitation and reception phase is occurring more often and can be undertaken in any clinician setting where there is a suspicion of internal haemorrhage or pneumothorax.24
Trauma Teams
There are a number of different ways to organise the early care of trauma patients. The most common method used is through the establishment of multidisciplinary trauma teams that can provide immediate, expert assessment, resuscitation and treatment of traumatised patients, especially those with multiple injuries. Many hospitals that receive trauma cases operate trauma teams that are either activated or placed on standby, via pagers or telephone, based on communications from paramedic personnel in the prehospital setting.25 This activation is based on a combination of physiological and injury criteria (see Table 23.1). Age is sometimes added to the patient criteria, with those under 5 years or over 65 years receiving particular attention. A number of hospitals have two levels of trauma team activation, with more severe injuries activating the full trauma team and less severe activating a partial team. The use of two-tiered trauma team activation has not been shown to affect patient outcomes.17
TABLE 23.1 Criteria for activation of trauma teams26,27
| Physiological criteria | Injury criteria |
|---|---|
| Heart rate <50 or >120 beats/min Respiratory rate <10 or >29 breaths/min Systolic blood pressure <90 mmHg Glasgow Coma Scale Score <10 Skin pale, cool or moist Paralysis Trauma arrest | Penetrating injury to head, neck or torso Burn to ≥20% body surface area Fall ≥5 metres Multiple trauma Crush or degloving injury to extremity Amputation proximal to the wrist or ankle Motor vehicle crash with ejection |
Common Clinical Presentations
Patients with multitrauma will also be cared for according to the principles of care for each specific injury, although consideration of priorities is essential. Care should follow the common principles of airway, breathing and circulation, therefore concentrating on respiratory and circulatory compromise first, before moving on to the treatment of other injuries. The relative importance of other injuries, for example neurological trauma or skeletal trauma, will vary for each individual patient and will be dependent on the physiological impact of the injuries. Neurological and spinal cord injury are reviewed in Chapter 17.
Mechanism of Injury
The most common causes of traumatic injury include road traffic crashes, falls and collisions. While falls account for the greatest number of injuries requiring hospitalisation,28 injuries sustained in road traffic crashes tend to be more severe given the high velocity of the trauma, and account for the greatest number of major injuries, including those injuries requiring a critical care admission.28–30
The mechanism of injury is recognised as affecting both survival and requirement for admission to the intensive care unit. Patients who are injured in a road traffic crash experience a similar mortality to those injured through falls (approx 3% in all patients and 10–17% in major injury patients), with both groups having a higher mortality than patients injured in assaults and collisions with objects (<1% in all patients and 12% in major injury patients).28,29 The older age group, with associated comorbidities, is likely to account for many of the deaths in the group injured through falls. In addition, patients injured in road traffic crashes tend to spend longer in the intensive care unit than patients injured through falls or assaults and collisions, and experience a greater number of injuries.28
Generic Nursing Practice
Positioning and Mobilisation of the Trauma Patient
Difficulty in positioning and mobilisation is often experienced when there is concern for the stability of the patient’s cervical spine, particularly in unconscious patients. Specific protocols for confirming the absence of injury to the cervical spine in unconscious patients, or those complaining of cervical soreness or abnormal neurology, vary between institutions and regions, but generally incorporate the following principles:31
• Obtain a detailed history of the injury wherever possible, including specific investigation of mechanisms of injury that might exert force on the cervical spine. A high index of suspicion should remain, particularly in the setting of injuries often associated with cervical spine injury, including craniofacial trauma rib fractures, pneumothoraces and damage to the great vessels and/or trachea.
• Undertake plain X-rays of the full length of the spine, interpreted by a radiologist.
• Where any abnormality exists in clinical or radiological assessment, or the patient remains unconscious, a CT or MRI may be undertaken, and this must be reported on by a radiologist.
• A correctly fitted hard collar should remain in place only until the patient is appropriately reviewed and the chance of a cervical spine injury is eliminated. If a collar is required for more than 4 hours, a long-term collar (e.g. Philadelphia, Aspen or Miami J) should be used.
• Maintain appropriate pressure area care to areas under the hard collar as well as usual pressure points until cervical clearance is gained.32
The two methods available for moving the trauma patient are staff manual handling and lifting hoists. Generally, trauma patients can be log-rolled (see Figure 23.1 for initial care and p. 635 for later care) as frequently as required for nursing care. Any restrictions to patient positioning and weight bearing due to injuries or physiological status must be considered through this process; it is essential that care be taken to prevent any worsening of injuries due to handling of the patient. Knowledge of the position restrictions for each limb, including all weight-bearing joints and the vertebrae, is imperative to avoid secondary iatrogenic injury. Certain injuries will impose position and mobility restrictions (see Table 23.2).
TABLE 23.2 Position and mobility restrictions in trauma patients
| Type of injury | Restrictions |
|---|---|
| Traumatic brain injury | |
| Facial trauma | |
| Chest trauma | |
| Abdominal trauma | |
| Pelvic trauma | • Position restrictions are dependent on severity of fracture(s), use of external fixateurs and degree of stabilisation. • Some patients may sit out of bed and ambulate with external pelvis fixateur in situ. • Position restrictions require regular review, as changed or loss of fixation may affect recovery. |
| Extremity trauma |
ICP = intracranial pressure.
Practice tip
The NEXUS low-risk criteria have been widely accepted as identifying patients in whom further examination is unnecessary and cervical spine injury can be excluded on the basis of clinical examination.82 These criteria include absence of midline cervical spine tenderness, no focal neurological deficit, no intoxication, no painful distracting injury and normal alertness.
The ‘Trauma Triad’
The critically injured patient can experience the ‘trauma triad’ of hypothermia, acidosis and coagulopathy. While it is possible to experience these pathophysiological conditions individually, they often occur simultaneously. Additionally, hypothermia is a common contributor to the exacerbation of both acidosis and coagulopathy.33–38 Acidosis has been discussed in earlier chapters so is reviewed here only as it interacts with hypothermia and coagulopathy in the trauma setting. Low cardiac output, hypotension, hypoxia, hypothermia and rhabdomyolysis are common causes of acidosis in the trauma setting. The increased recognition of the importance of this triad in the trauma setting has led to the development of damage control surgery. The principle of this surgery is reviewed below.
Hypothermia
Hypothermia is defined as a core temperature <35°C and is associated with high morbidity and mortality. Even in sub-tropical environments, hypothermia is identified in approximately 10% of major trauma cases during the prehospital or in-hospital phase of care.36,39
Uncontrolled causes of hypothermia can be endogenous or accidental.33,34,37,39 Endogenous causes include metabolic dysfunction with decreased heat production, or central nervous system dysfunction with insufficient thermoregulation such as in neurological trauma. Dermal dysfunction, such as a burn, is another endogenous cause of hypothermia.
Accidental hypothermia can occur without thermoregulatory dysfunction, and generally occurs in the trauma patient as a result of environmental exposure either at the injury site or during transport to, or between, healthcare facilities, as a result of large-volume fluid resuscitation or during prolonged surgical procedures. The pathophysiological changes associated with hypothermia vary depending on the severity, and are outlined in Chapter 22. Of particular relevance, shivering leads to increased oxygen consumption and acidosis, and platelet dysfunction leads to impaired clotting.33,36,39
• ensuring the patient is adequately covered during transport and hospital care
• using warm blankets or electrical warming blankets
• adjusting the temperature in the operating room where feasible.39
Coagulopathy
Coagulation is widespread in the trauma setting, and ranges from a mild defect in coagulation function to life-threatening coagulopathy. Defects in coagulation may be caused by dilution, hypothermia, acidosis, tissue damage or the effects of underlying disease.34,35
Dilution results from the transfusion of either crystalloid or colloid fluids, and occurs as the concentration of coagulation factors in the patient’s blood is diluted with the transfused fluid. It should be remembered that transfusion of red blood cells has the same effect, as whole blood or packed cells have undergone some dilution and have reduced viability of platelets.38 Hypothermia causes coagulopathy because many of the enzymatic reactions in coagulation are temperature-dependent. Platelet and thromboplastin function both decline with even moderate (34°C) hypothermia, while hypothermia stimulates fibrinolysis.34,40
Acidosis reduces the activity of both the extrinsic and the intrinsic coagulation pathways, as well as platelet function. This is particularly pronounced with a pH below 6.8.34 Tissue damage causes endothelial disruption and defibrination, which promote the systemic activation of coagulation; this is particularly profound in patients with brain injury due to the high level of thromboplastin in brain tissue.34,37,38 The final cause of coagulopathy in trauma is the underlying disease present in many patients. Patients may have a coagulation defect such as haemophilia or von Willebrand’s disease, or liver disease with resultant compromise to coagulation on an ongoing basis. Alternatively, patients may be taking anticoagulants, such as aspirin or warfarin, as treatment for other health conditions.37,41
Treatment of coagulopathy should focus first on prevention of coagulopathy and then on the treatment as required. Prevention strategies include:40
• maintaining normothermia in critically injured patients through the use of blankets, warming devices, and minimisation of exposure and theatre time
• administering as little resuscitation fluid as is necessary to maintain adequate circulation
• achieving control of haemorrhage as soon as possible, through techniques such as low-pressure resuscitation and damage-control surgery.
Treatment includes transfusion of platelets, fresh frozen plasma (FFP) and cryoprecipitate, as well as the plasma derivatives showing promise in this area of treatment.35 While transfusion of platelets is specifically directed towards increasing the circulating concentration of platelets, administration of FFP is directed at increasing the levels of fibrinogen and other coagulation factors. Cryoprecipitate is made by freezing and thawing individual units of FFP and collecting the precipitate, a process that concentrates fibrinogen, von Willebrand factor, factor VIII and factor XIII.
Damage-control Surgery
Damage-control surgery can be defined as a four-stage procedure, involving early recognition of relevant patients and ‘rapid termination of an operation after control of life-threatening bleeding and contamination followed by correction of physiological abnormalities and definitive management’.42,43 This approach to surgical correction of traumatic injuries gained favour through the latter part of the 1990s and is intended to reduce the development of the triad of complications of hypothermia, acidosis and coagulopathy. The intention is that surgery is initiated rapidly, only the most rapid and simplest interventions that are required to stop bleeding and contamination are undertaken, then surgery is completed and the patient moved to definitive care, usually in the ICU.42 Care can then be undertaken to ensure that hypothermia, acidosis and coagulopathy do not develop or, if present, are rapidly reversed, thereby ensuring correction of physiological abnormalities as quickly as possible. Definitive surgical correction of injuries is undertaken during the ensuing days when the patient is physiologically stable. Damage-control surgery can apply to a range of patients, including those with abdominal, skeletal and thoracic trauma.
Skeletal Trauma
Skeletal trauma involves injury to the bony structure of the body. While skeletal injuries alone rarely result in the patient being admitted to critical care, damage to surrounding blood vessels and nerves, as well as potential complications such as fat embolism syndrome (FES) and rhabdomyolysis, may cause the patient to become seriously ill. Patients with skeletal trauma who require admission to ICU include those with multiple injuries, severe pelvic fractures (often associated with significant blood loss), long bone fractures (often associated with FES) and thoracic injuries such as flail segment. A small number of people with crush injuries that cause significant damage to muscles, often resulting in rhabdomyolysis, also require admission to the ICU.44,45
Skeletal trauma is the form of trauma that causes the highest number of patients to be admitted to hospital for 24 hours or more, with approximately 50% of patients experiencing a fracture as their main injury.28 Of those patients admitted to an ICU, fractures are the second most common type of injury (after head injury), with approximately 20% of patients experiencing this type of injury.
Pathophysiology
Bone is composed of an organic matrix as well as bone salts. The majority of the organic matrix is collagen fibres and the remainder is ground substance, a homogeneous gelatinous medium composed of extracellular fluid plus proteoglycans.46 Calcium and phosphate are the primary bone salts, although there are smaller amounts of magnesium, sodium, potassium and carbonate ions. These ions combine to form a crystal known as hydroxyapatite.
A fracture is simply defined as a break in the continuity of a bone. Fractures generally occur when there is force applied that exceeds the tensile or compressive strength of the bone. In patients sustaining a major injury (injury severity score [ISS] ≥16) fractures are the primary injury in more than 15% of cases, although many patients experience a fracture in addition to other serious injury resulting in ICU admission.28
A fracture causes disruption to the periosteum, blood vessels, marrow and surrounding soft tissue, resulting in a loss of mechanical integrity of the bone. Bone is one of only two sites (the other being the liver) that will reform itself, not forming scar tissue when it heals.47 When a fracture occurs, there is initial bleeding and soft tissue damage around the site, with haematoma formation within the medullary canal. The healing sequence that follows a fracture depends on the type of fracture fixation that is used. When a fracture is fixed in a method that eliminates the interfragmentary gap and provides stability to the site, such as in screwing or wiring, primary healing takes place. When a fracture is fixed in a manner that reduces but does not eliminate movement around the fracture site, secondary healing takes place.48
In primary healing, also referred to as direct union, the haematoma that initially formed is eliminated by the apposition of fracture ends during reduction. Once the bone ends are intact, osteoclasts form cutting cones that in turn form new haversian canals across the fracture gap. These contain blood vessels that are essential to primary bone healing. By 5–6 weeks after the fracture, osteoblasts will fill the canals with osteons, which are the basic structure of the new bone.47 Although the bone is now formed, the strength and shape continues to develop over coming weeks.
In contrast to primary healing, secondary healing is characterised by an intermediate phase, where a callus of connective tissue is first formed and then replaced by bone.47,49 The secondary healing phase begins with an inflammatory phase in which the haematoma clots and provides initial support, then inflammatory cells invade the haematoma to remove necrosed bone and debris. The reparative phase begins 1–2 weeks after the fracture and consists of immature woven bone being laid down and strengthened through a process known as mineralisation. The final remodelling stage consists of replacement of the woven bone by lamellar bone, through osteoblasts secreting osteoid that is mineralised and forms interstitial lamellae. The remodelling of these structures occurs in response to appropriate levels of mechanical loading during this phase.47,48
Fat embolism
Fat embolism syndrome (FES) may occur in patients who have experienced a fracture of a long bone, particularly if multiple fractures or fractures to the middle or proximal parts of the femur are experienced. Fractures to the pelvis can also lead to a fat embolism. Incidence of FES is low (<1%). FES consists of fat in the blood circulation associated with an identifiable pattern of clinical signs and symptoms that include hypoxaemia, neurological symptoms and a petechial rash.49 Patients generally present 12–48 hours after they have experienced a relevant fracture and often require admission to a critical care unit for assessment and treatment, including mechanical ventilation.
Internationally, there continues to be disagreement regarding the pathophysiological changes associated with FES, although there is general consensus on the following principles. It has been accepted that there is a mechanical component to the changes that take place in FES, where fat is physically forced into the venous system and causes physical obstruction of the vasculature. Although marrow pressure is normally 30–50 mmHg, it can be increased up to 600 mmHg during intramedullary reaming (the process where the medullary cavity of the bone is surgically enlarged to fit a surgical implant such as a tibial nail), consequently reaching a pressure significantly above pressures throughout the vasculature.49 A second theory, associated with the biochemical changes that occur during trauma, proposes that trauma is associated with a higher level of circulating free fatty acids, which cause destabilisation of circulating fats and/or direct toxicity to specific tissues, including pulmonary and vascular endothelium.49
Rhabdomyolysis
Rhabdomyolysis is the breakdown of muscle fibres resulting in the distribution of the cellular contents of the affected muscle throughout the circulation, and occurs during the reperfusion of injured muscle. The cellular contents that are circulated include potassium, phosphate, organic acids, myoglobin, creatine kinase and thromboplastin.44 Two phases of injury are essential for the development of rhabdomyolysis: the first is when muscle ischaemia occurs, and the second is with reperfusion of the injured muscle. The length of time that muscle is ischaemic affects the development of rhabdomyolysis, with periods of less than 2 hours generally not producing permanent damage, but periods above this time resulting in irreversible anatomical and functional changes.44 The clinical sequelae of rhabdomyolysis include electrolyte abnormalities such as hypocalcaemia, hyperkalaemia and acidosis, hypovolaemia, acute renal failure and multiorgan failure.
Clinical Manifestations
Common forms of skeletal trauma include the following:
• Long bone fractures. The long bones are the humerus, radius, ulna, femur, tibia and fibula. Fractures of these bones are serious and can carry a high level of morbidity, especially if they involve a joint such as a trimalleolar fracture of the ankle (distal tibia and fibula). In many cases definitive surgical management is required, with internal fixation.
• Dislocations. All joints are at risk of traumatic dislocation, depending on the mechanism of injury. Dislocations can be limb-threatening if they cause neurovascular compromise. Reduction of traumatic dislocation is a medical emergency.
• Open fractures (compound). Any break in the skin that communicates directly with the fracture is classified as an open fracture. Open fractures carry a higher infection risk and require surgical treatment within 8 hours.50,51
• Traumatic amputation. Amputation refers to an avulsion in which the affected limb or body appendage is completely separated from the body. This can occur when a digit or extremity is sheared off by either mechanical or severing forces, for example amputation of a thumb by a bandsaw. Traumatic amputations vary in severity and ongoing compromise, with a cleancut amputation more likely to be successfully reattached than a crushed extremity. Criteria that inform the surgical decision-making process include the amount of tissue loss, location on the body at the connection site, damage to underlying and surrounding tissues, bones, nerves, tendons/muscles and vessels, and condition of the amputated part.
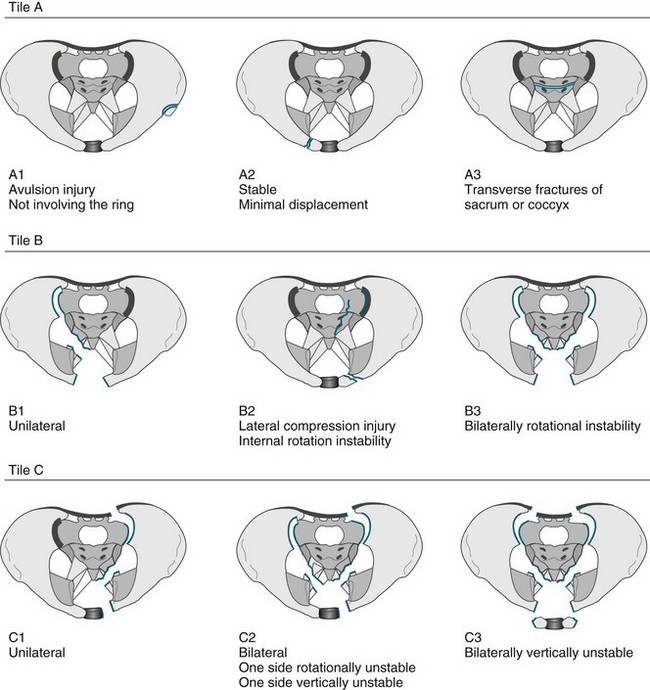
• Fractures of the pelvis. The pelvis is the largest combined bony structure in the body and serves to provide an essential supporting framework for ambulation and protection of pelvic organs. Major blood vessels and nerves traverse the pelvic bones, supplying the lower limbs and pelvic organs. Therefore, injury to any part of the pelvis is serious. The three bones that comprise the pelvic ring are the two innominate bones (ilium and pubic rami) and the sacrum. Due to its reinforced structure, the amount of force required to fracture the pelvis is substantial. Fractures of the pelvis can affect one or both sides of the pelvis, and be stable or unstable. A variety of classification systems exist to describe the severity of pelvic fractures, the most common being the Tile classification (see Figure 23.2).
• Fractures of the spinal column. (see also Chapter 17). The spinal column includes all of the bony components in the cervical, thoracic and lumbar vertebral regions. Fractures of the vertebra are common in trauma patients, but the actual incidence of fracture without spinal cord injury in multitrauma patients is not well described. Not all fractures cause vertebral column instability with the subsequent risk of spinal cord damage. A spine column fracture will be diagnosed as mechanically stable or unstable and this will affect the positioning and possible activity of the patient.
• Discoligamentous injuries of the spinal columns (see also Chapter 17). The soft tissue components of the spinal column include the spinal cord, the inter-vertebral discs and the spinal ligaments. An injury to the spinal column can disrupt one or more of these structures with or without fracture. These injuries can be highly unstable and the nurse must be vigilant with spinal precautions and the fitting and management of the patient requiring a spine orthoses (refer to Figure 23.1).
Nursing Practice
Independent practice
Bones are very vascular structures and can be the cause of substantial blood loss in the trauma patient. The critical care nurse should therefore be cognisant of the potential for extensive blood loss in common fractures (see Table 23.3).
TABLE 23.3 Potential blood loss caused by fractures76
| Fracture | Blood loss (mL) |
|---|---|
| Humerus | 500–1500 |
| Elbow | 250–750 |
| Radius/ulna | 250–500 |
| Pelvis | 500–3000 |
| Femur | 500–3000 |
| Tibia/fibula | 250–2000 |
| Ankle | 250–1000 |
Given the potential for extensive blood loss, as well as the frequent close proximity of nerves and blood vessels to bones, neurovascular assessment of the patient with skeletal trauma is essential (see Table 23.4).
TABLE 23.4 Neurovascular observations of the skeletal trauma patient
Should be undertaken on all injured limbs both pre- and postoperatively as required
| Observation | Process | Comments |
|---|---|---|
| Skin colour | State the skin colour of the area inspected as it compares with the unaffected part. NB: Distal limb pulses may be difficult to palpate in the injured limb; a warm pink limb is a perfused limb. | Pink: normal perfusion |
| Pale: reduced perfusion | ||
| Dusky, purple or cyanotic discolouration: usually indicating significantly reduced perfusion | ||
| Demarcated: a distinct line where the skin colour changes to dusky (usually follows the vessel path) | ||
| Skin temperature to touch | State the ambient temperature of the skin to touch as it compares with normally perfused skin at room temperature. | Normal: not discernibly cold to touch. Reduced skin temperature indicates reduced perfusion. |
| Voluntary movement | The patient should be able to move the non-immobilised distal part of any injured limb (i.e. fingers and toes of a plastered limb). | It is important to assess range of motion where that is possible, provided this will not aggravate the injury. Reduced movement may indicate compromise to either the nerve or blood supply to the limb. |
| Sensation | The patient should be able to report normal sensation to touch. | Sensation should be assessed in nerve distributions (i.e. all fingers and toes). Reduced sensation may indicate compromise to either the nerve or blood supply to the limb. |
Collaborative practice: splinting
• Positioning of injured limbs. All patients who have any form of splint in situ should not have the affected limb below the level of the patient’s body, and may need to have it elevated to promote venous return and minimise tissue oedema. In the ICU the trauma patient will often be nursed flat, with the bed on tilt for a head-elevation position. In these circumstances, the injured dependent limb must be elevated on pillows so that it is no longer dependent. Care must be taken to ensure that elevation does not place pressure on any part of the limb: for example, a hand sack made from a pillowcase tied to an IV pole should not be used, as it places direct pressure on the path of the median nerve and can cause an iatrogenic neurapraxia.
• Wooden/air splints. These are padded appliances that are strapped to the injured limb. Ideally, no patient should remain in wooden splints for longer than 4 hours, as pressure may build up on pressure points.
• Plaster backslab. Limbs with fractures will often swell as a physiological response to injury; a plaster backslab composed of layered Plaster of Paris is the preferred treatment, as it accommodates swelling and can easily be loosened by nursing staff at any time of day. It is imperative that this be adequately padded within the limitations of providing structural support to the limb. Poorly made or ill-fitting backslabs can cause major complications, such as pressure sores or displacement of fractures.
• Traction. Traction may be required as part of fracture management, and involves the application of a pulling force to fractured or dislocated bones. There are three types of traction:
1. The grip or hold on the body must be adequate and secure.
2. Provision for countertraction must be made.
3. There must be minimal friction.
4. The line and magnitude of the pull, once correctly established, must be maintained.
5. There must be frequent checks of the apparatus and of the patient to ensure that: (a) the traction set-up is functioning as planned; and (b) the patient is not suffering any injury as a result of the traction treatment.
Collaborative practice: traumatic amputations
• appropriate positioning of the affected limb, usually based on surgical orders
• frequent neurovascular observations, particularly observing for reperfusion injury, which manifests as an acute compartment syndrome or vascular trashing of distal vessels from a clot
• implementing changes in treatment initiated in response to altered perfusion in a timely manner
• psychological support to assist the patient in dealing with the injury.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree