The cardiovascular system
Blood
Before birth
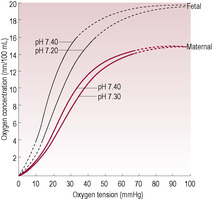
Note: the theoretical value is the amount of oxygen the blood can be saturated with whereas, in practice, the blood is saturated to a lesser degree because the transfer of oxygen across the placenta is less efficient than the transfer of oxygen across the alveoli.
Fetal/Neonatal
Adult
Blood volume
80–100 mL/kg
75 mL/kg
90–105 mL/kg (preterm)
Red blood cell number
6–7 ∞ 106/mL
Female: 4.8 ∞ 106/mL
Male: 5.4 ∞ 106/mL
Haemoglobin content
20.7 g/dL
Female: 14 g/dL
Male: 16 g/dL
Oxygen content of 100 mL saturated blood
21 mL (theory)
16 mL (theory)
13 mL (practice)
15.7 mL (practice)
Red blood cell lifespan
80–100 days
120 days
60–80 days (preterm)
Haemoglobin type
HbF: α2γ2
HbA: α2β2
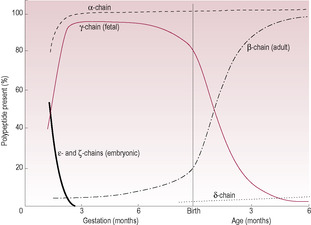
Fig. 15.2
After birth
Haemostasis
The circulation
Before birth
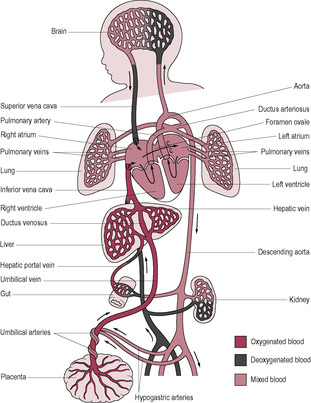
Fig. 15.3
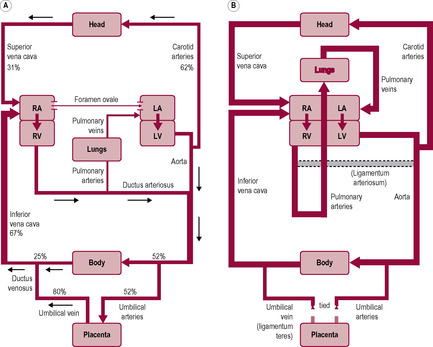
Fig. 15.4
After birth
The respiratory system
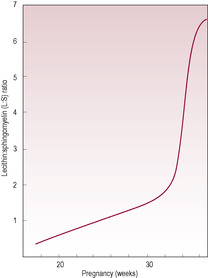
Fig. 15.6
The lungs
Before birth
After birth
Respiratory distress syndrome
Temperature regulation
Before birth
After birth
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


The transition to neonatal life
The midwife officially recorded Zak as having an Apgar score of 9 at approximately 1 min of age. Following the delivery James was surprised how alert his son Zak was, that he seemed to be aware of his surroundings and appeared to be actively looking around.
• What factors could have contributed to Zak’s behaviour and what are the possible explanations for Zak being so alert following his delivery?
Zara asks the midwife if she can attempt to breastfeed.
• What could the midwife do to encourage Zak to suckle?
The process of birth is physiologically stressful with fluctuations in placental blood flow resulting in a degree of hypoxia and respiratory acidosis. Increased secretion of adrenal catecholamines, stimulation of the sympathetic nervous system and the subsequent mobilization of glycogen and lipid stores are fundamental in the activation of essential physiological mechanisms that result in an alert and active baby at birth. However, a prolonged or difficult delivery and marked hypoxia/anoxia and acidosis can result in an overstressed or seriously asphyxiated baby (Box 15.1).
Box 15.1
Fetal asphyxia is due to a significant reduction in the amount of oxygen available via the placenta and the maternal circulation. There are many possible causes for example placental abruption, rapid deterioration in the maternal condition, such as eclampsia, uterine hyperstimulation in response to syntocinon augmentation. Whatever the reason, prolonged fetal hypoxia will result in asphyxia (low oxygen levels and raised carbon dioxide levels). Fetal hypoxia, if suspected during labour, can be assessed by obtaining a small sample of blood from the fetal scalp, from which the pH and base excess can be measured. Acidaemia is diagnosed if the pH of the fetal blood is below 7.2, however many babies can tolerate moderate acidaemia without long term problems. A base excess above 12 mmol indicates chronic or prolonged acidaemia. The risk of asphyxia rises with lower pH and higher base excess. Babies born with asphyxia require active resuscitation to restore pH and base excess. In extreme cases, severe asphyxia results in irreversible brain damage.
Both the fetus and the neonate can tolerate degrees of hypoxia and anoxia that would result in serious morbidity or mortality in an adult. The neonate retains the capability to divert a considerate proportion of its cardiac output to the brain thus protecting it. Although the brain is vulnerable to hypoxia, the compensatory mechanisms can increase tolerance to hypoxic states (Parer, 1998). However, severe asphyxia can cause cerebral microhaemorrhages resulting in a spectrum of damage from impaired intellectual development to spasticity and irreversible brain damage (Box 15.2). Neonates are also vulnerable to infection.
Box 15.2
In extreme cases of neonatal asphyxia, brain cell distruction triggers apotosis in surrounding cells. The number of apoptotic neural cells can be reduced by neonatal head cooling thus potentially reducing the severity of brain damage (Wyatt et al., 2007 and Polderman, 2008).
Although there is usually a good correlation between gestational length and degree of maturity, infants affected by intrauterine growth retardation (IUGR) may have precocious organ development because undernutrition and the resulting fetal stress promote increased fetal cortisol secretion thus enhancing fetal organ maturation. In humans, fetal cortisol appears not to have a role in inducing labour as has been demonstrated in other species (see Chapter 13). The major problems of premature infants can be attributed to a shorter duration of glucocorticoid exposure (even though fetal stress results in actual levels being higher); this results in an increased risk of persistent fetal circulation, increased likelihood of lung immaturity and respiratory diseases syndrome and immaturity of thermoregulatory responses, the gastrointestinal system and enzymes involved in maintaining glucose homeostasis.
Fetal preparation for birth includes storing glycogen, producing catecholamines and laying brown and white fat. The glucocorticoid system is pivotal in the fetal preparation for birth. Fetal cortisol levels rise from about 35 weeks. Glucocorticoids cause the natural decrease in growth that occurs towards term and are also thought to be responsible for the growth retardation associated with physiological stress in utero such as that due to hypoxia and undernutrition. Glucocorticoids bring about functional and morphological changes in many biochemical pathways and fetal tissues including lungs, liver, gut, adipose tissue and skeletal muscle (Fowden and Forhead, 2009). Glucocorticoids influence surfactant production and maturation of the alveoli and other respiratory tissues thus promoting lung maturation. They stimulate glycogen deposition in the fetal liver and skeletal muscle and also induce hepatic gluconeogenesis enzymes by promoting adrenaline synthesis (and potential effective response to stress), inducing hormone receptors and affecting thyroid hormone synthesis. They also enhance proteolysis so fetal protein accretion is reduced. Fetal corticotrophin-releasing factor (CRF) and antidiuretic hormone (ADH) and placental CRF orchestrate the increase in fetal cortisol production, which influence adrenocorticotrophin (ACTH) production by the maturing fetal pituitary gland; increased ACTH stimulates cortisol production.
At birth, there are also changes in the regulation of growth. Fetal growth is substrate-limited and actively constrained to optimize successful delivery (see Chapter 9). The rise in glucocorticoids towards term suppresses growth and is responsible for the natural decrease in growth that occurs at this time (Fowden and Forhead, 2009); the increase in glucocorticoids also induces growth hormone receptors and changes in expression of insulin-like growth factor I (IGF-I).
Fetal blood (Table 15.1) is structurally and functionally different to adult blood; it contains larger and more numerous erythrocytes (red blood cells) with a higher haemoglobin content which maximizes their uptake of oxygen (Palis and Segel, 1998). Fetal haemoglobin with its two α-chains and two γ-chains has a higher affinity for oxygen in the slightly more acid fetal environment. Less-effective binding of 2,3-bisphosphoglycerate (or 2,3-diphosphoglycerate) to the γ-chains means that the oxygen–haemoglobin dissociation curve of the fetus and neonate is shifted to the left (Fig. 15.1). Shifts of pH in the placenta further increase both dissociation of oxygen from maternal haemoglobin and its uptake by fetal haemoglobin. This means that, although fetal haemoglobin has an increased oxygen uptake, it is less efficient at releasing oxygen to the tissues.
At term, the ratio of fetal haemoglobin to adult haemoglobin (HbF:HbA) is 80:20; by 6 months production of the β-chain replaces the γ-chain so the ratio is 1:99 (Fig. 15.2). Preterm infants tend to have an even higher HbF level and a decreased 2,3-bisphosphoglycerate concentration therefore oxygen unloading at the tissue level is even less efficient. The raised levels of HbF in the neonate mean that haemoglobinopathies caused by altered synthesis of β-chains (such as β-thalassaemia) or altered structure of β-chains (such as sickle-cell anaemia) are not evident immediately at birth but become evident when the infant is at least 2 months old. It is possible to detect fetal blood cells in the maternal circulation, an observation that is utilized in the management of Rhesus incompatibility (see Chapter 10) (Lamvu and Kuller, 1997).
(Reproduced with permission from Begley et al., 1978.)
At birth, fetal blood has an increased population of nucleated erythrocytes (even more so if the baby has been subjected to increased stress, is immature or has Down’s syndrome). For the first 3 months of life, the erythrocytes are more fragile, have an increased metabolism and a shorter half-life and erythropoietin production is suppressed (Box 15.3). Once respiration in the neonate is established, the excess red blood cells compensating for the lower oxygen saturation within the uterine environment are no longer needed. These red blood cells are broken down and physiological jaundice may result, usually around day 3 of life as the neonatal liver is immature and initially cannot keep up with the rate of bilirubin production from red blood cell breakdown.
Box 15.3
Haemopoiesis (red blood cell production) is controlled by the hormone erythropoietin, which increases when oxygen delivery to the kidney is reduced; it stimulates red blood cell production by the bone marrow. The increased oxygen levels inhibit erythropoietin levels in the neonate postnatally (Strauss, 1994). Levels remain low for 2–3 months (longer in preterm infants) and then increase, resulting in increased bone marrow activity and red blood cell production. As the neonate appears to tolerate the fall in haemoglobin concentration without ill-effects, it is deemed to be physiological. The haemodilution effects are increased by rapid growth being matched by total blood volume, which precedes any change in red blood cell number.
Neonates, particularly those born prematurely but those who are healthy as well, have an increased risk of haemostatic problems because they are born with a deficiency of plasma coagulation factors, inhibitors of haemostasis and other components of the fibrinolytic system (Aronis-Vournas, 2006). However, despite this, because the deficiencies in components of the coagulation and fibrinolytic system tend to be balanced, the healthy term neonate does not usually have thrombotic or haemorrhagic problems. The most common example of neonatal haemostatic problem is (Kuehl, 1997) disseminated intravascular coagulation (DIC) due to accelerated and inappropriate coagulation, which depletes the body’s supply of platelets and clotting factors and paradoxically increases the risk of haemorrhage. Susceptibility is increased because, first, the immature neonatal reticuloendothelial system has a decreased capacity to remove intermediary products of coagulation so they can further stimulate coagulation and consumption of clotting factors, and, second, synthesis of clotting factors by the immature liver is inefficient. Vitamin K levels in the neonate are about 50% of adult values, which affect the efficiency of the clotting cascade. Vitamin K levels are low because placental transport of the vitamin is poor and colonization of the gut by bacteria that synthesize vitamin K is not immediate. The consequent reduced level of all vitamin K-dependent clotting factors is associated with an increased bleeding tendency, which can predispose to haemorrhagic disease of the newborn (HDNB) (Box 15.4). Neonatal platelets exhibit decreased aggregation and adhesiveness because their production of thromboxane A2 (TxA2) is impaired. This appears to protect the term neonate against thrombosis but to increase the vulnerability to bleeding of the preterm or sick baby. Placental transfer of maternal drugs such as aspirin can affect coagulation in the neonate.
Box 15.4
• Bleeding from the gut, umbilicus, circumcision wounds and oozing from puncture sites
• Evident 2–3 days after birth
• Associated with antibiotics, which affect colonization of the gut with vitamin K-synthesizing bacteria
• Associated with anticonvulsant drugs (e.g. phenobarbital, diphenylhydantoin), which concentrate in the fetal liver and antagonize the effect of vitamin K
• Associated with maternal warfarin treatment, which decreases levels of vitamin K-dependent clotting factors and prolongs prolonged clotting times
• Prophylactic vitamin K is routinely administered to all babies born in the UK (Paediatric Formulary Committee, 2010)
• Term babies respond well to vitamin K therapy but synthesis of clotting factors is further limited in preterm babies by inadequate hepatic synthesis of precursor proteins
As the fetal oxygen source is the placenta rather than the lungs, blood in the fetal circulation flows in a circuit that perfuses the placenta and largely bypasses the lungs (Fig. 15.3). In order to do this, the fetal circulation has several additional structures: the umbilical vein, which carries blood rich in oxygen and nutrients to the underside of the liver, the ductus venosus (a venosus is a shunt that connects a vein to a vein), which bypasses the liver taking blood from the umbilical vein to the inferior umbilical vein en route to the right side of the heart. With increasing gestational age, more blood goes to the liver rather than bypassing it (Askin, 2009a). The hypogastric arteries, which branch off the internal iliac arteries, are contiguous with the umbilical arteries of the umbilical cord, returning blood to the placenta. The lungs are bypassed by two structures: the foramen ovale, which allows blood to move directly from the right atrium to the left atrium, and the ductus arteriosus, which connects the pulmonary arterial trunk to the descending aorta (an arteriosus is a vascular shunt that connects an artery to an artery).
(Reproduced with permission from Goodwin, 1997.)
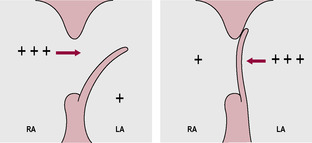
The oxygenated and nutrient-enriched blood is taken from the placenta in the umbilical vein that goes through the abdominal wall to the underside of the liver. This is the only unmixed blood and is about 80% saturated with oxygen; the blood goes through the ductus venosus to the inferior vena cava where it mixes with oxygen-depleted blood returning to the heart from the lower body (Fig. 15.4 A). The inflows of blood from the inferior and superior venae cavae do not mix thoroughly because of their angle of entry and the shape of the right atrium. Because the entry of the inferior vena cava is aligned with the foramen ovale, most (about 60%) of the blood from the inferior vena cava travels from the right atrium through the foramen ovale into the left atrium and thence to the left ventricle and the ascending aorta. The foramen ovale is kept open because the high pulmonary vascular resistance (PVR) means that the pressure in the right atrium is also high.
(Reproduced with permission from Chamberlain et al., 1991.)
Most blood entering the right atrium from the superior vena cava passes through the tricuspid valve into the right ventricle and to the pulmonary arterial trunk. The ductus arteriosus is inserted into the vessel at the bifurcation of the right and left pulmonary artery (taking blood to the right and left lung, respectively); it shunts blood from the pulmonary arterial route into the descending aorta. The pulmonary circulation is vasoconstricted and has a high PVR, because the pulmonary environment is relatively hypoxic. Systemic vascular resistance (SVR) is low. Only about 10% of the output of the right ventricle continues into the pulmonary circulation for the growth and metabolic needs of the lungs; the rest is diverted through the ductus arteriosus which has a low resistance; its patency is maintained by the low fetal PO2 and by high levels of prostaglandins produced by the placenta. Towards the end of gestation, the proportion of blood perfusing the lungs tends to increase. From the descending aorta, the blood supplies the remaining organs and the lower body. The hypogastric arteries branch off the internal iliac arteries and return to the placenta via the umbilical arteries.
The upper body and head are fed from arteries which branch off from the aortic arch before the insertion of the ductus arteriosus and the subsequent mixing of slightly less-well-oxygenated blood. The early branching of the coronary and carotid arteries means the heart and brain receive slightly better oxygenated blood. The advantages conferred by the early branching of the subclavian arteries which supply the upper limbs can be illustrated by the enhanced development of arms compared to the legs.
One of the most important transitional stages in the adaptation to extrauterine life is the establishment of the neonatal circulation. In fetal life, the source of oxygen is the placenta so most of the blood flow bypasses the fetal lungs. At birth, blood fully perfuses the lungs and flow through the fetal vascular structures ceases. At birth, these changes that mark the transfer of the fetal into adult-type circulation (see Fig. 15.4B) are not rapid or immediate. They are initiated within 60 s of delivery but may not be fully completed for a few weeks. The two determining events that initiate the closure of the fetal shunts are the arrest of the umbilical circulation, and therefore placental perfusion, and lung inflation and expansion, which results in increased pulmonary blood flow. The first breath results in lung expansion and vasodilatation of the pulmonary vessels in response to increased partial pressure of oxygen so blood flow to the lungs increases. The tortuosity of the capillaries is reduced and the pulmonary circulation changes from a high-resistance to a low-resistance pathway so 90% of the blood flows through the pulmonary vascular bed. There is a brief reversal of flow through the ductus arteriosus, which vasoconstricts in response to the change in oxygen level, mediated by prostaglandins, especially decreased prostaglandin PGE2 (Thorburn, 1992). The placenta no longer contributes to prostaglandin production and prostaglandin breakdown is increased because more blood flows to the lungs where significant prostaglandin metabolism and breakdown occur.
The smooth muscle of the umbilical artery walls is not innervated but is irritable. Vasoconstriction is stimulated by stretching and handling the cord, by cooling and in response to stress-related catecholamine release. The thicker walls of the umbilical arteries are able to generate high intraluminal pressure, which arrests the placental circulation, preventing flow from the infant to the placenta. This is augmented by the increased synthesis of prostaglandins and thromboxanes in response to the raised oxygen level due to breathing, which increases vessel irritability and vasoconstriction. The umbilical vein remains dilated; blood flow from the placenta to the infant can continue via gravity. Thus, initial neonatal blood volume is affected by the timing of clamping of the umbilical cord and by the relative positions of the infant and placenta at the time of clamping. The usual practice is to clamp the umbilical cord earlier if the baby is subject to fluid overload (hydropic), or if the baby is polycythaemic (e.g. infants of diabetic mothers or growth retarded), to limit the transfer of maternal analgesic agents or antibodies or to avoid possible baby-to-baby transfusions in the cases of multiple births (Kinmond et al., 1993).
The flap of the foramen ovale (Fig. 15.5) is pushed closed because the decreased umbilical flow results in a decreased venous return from the inferior vena cava so the pressure in the right atrium and PVR falls in response to changes in oxygenation. The increased pulmonary blood flow results in an increased return to the left atrium and consequent increase in pressure. Thus, the pressure gradient across the foramen ovale is reversed. So at birth, PVR falls and SVR increases. The relatively thick layer of smooth muscle in the pulmonary blood vessels begins to thin from birth.
The closure of the fetal structures may not be immediate or permanent and may never be completed. The closure of the foramen ovale is reversible at first; interruption of ventilation or a drop in alveolar oxygenation results in constriction of the pulmonary capillaries and consequent reversal of pressure across the atria and reversion to fetal circulation. The incomplete closure can result in intermittent and reversible cyanotic episodes. After a few days of functional closure, the tissue associated with the foramen ovale fuses and closure becomes permanent. In many adults, a patent foramen ovale can be demonstrated (a probe can be passed through) although the pressure gradient maintains effective functional closure. Intermittent flow through the ductus arteriosus may initially occur during each cardiac cycle when aortic pressure is maximal following ventricular contraction. Bradykinin released from the newly inflated lungs mediates the constriction of the ductus arteriosus. Production of prostaglandins, which had maintained the open ductus arteriosus in the fetus, is decreased as oxygenation increases. Most neonates have some degree of patency of the ductus arteriosus in the first 8 h of life but it becomes functionally closed within the first or second day (Askin, 2009a). Fibrolysis and obliteration of the lumen of the ductus arteriosus are usually complete within 3 weeks; continued patency is very serious and can result in left ventricular failure. The ductus venosus constricts when umbilical flow is halted. The obliterated vessels remain as anatomical ligaments; the slow closure of the umbilical vein and its degeneration into ligamentum teres are utilized as a route for neonatal blood transfusions if required.
The nervous control of the cardiovascular system is well developed in the neonate with mature physiological control of blood pressure and cardiac output demonstrable. The systemic arterial blood pressure is relatively low in the first few weeks as vascular tone develops which increases vascular resistance. Pulmonary arterial blood pressure is initially high but falls to mature values as pulmonary resistance falls. The neonate’s heart rate is fast, as in the fetus. As in the fetus, control of cardiac output is largely achieved by changing heart rate because the heart is small and non-compliant and has a relatively thick wall. At birth, the wall of the right ventricle is thicker than the left, which hypertrophies in response to the changed postnatal circulation.
The primitive air sacs are developed by the 20th week of gestation, and by 26 weeks respiratory bronchioles with a rich capillary supply are evident. Although the enzymes for synthesis of phospholipid/lipoprotein components of surfactant are present from week 18, the type II pneumocytes secrete surfactant only from week 26 with a surge in production after week 30. Surfactant, a detergent-like wetting agent, allows increased compliance, so the force required to inflate the alveoli is reduced thus increasing compliance. A lack of surfactant causes respiratory distress syndrome (RDS) (see below). The lecithin:sphingomyelin (L:S) ratio of the surfactant can be determined by amniocentesis indicating the maturity of the respiratory system (Fig. 15.6). By week 35, the L:S ratio in a healthily developing fetus is 2:1. This ratio is decreased in pre-eclampsia, prematurity, narcotic addiction, maternal diabetes and other problems in pregnancy. Administration of cortisol (dexamethasone) to the mother prior to delivery of a baby born from 24 to 34 weeks’ gestation increases fetal surfactant production within 24 h and can be used to decrease the risk of RDS (Hutchison, 1994).Variations (polymorphisms) in the genes for components of surfactant are associated with a range of inherited neonatal respiratory problems including bronchopulmonary dysplasia (BPD), (RDS) and respiratory syncytial virus (RSV) bronchiolitis and may influence susceptibility to influenza virus (Hallman and Haataja, 2006). Premature infants, particularly those born before 28 weeks, have immature alveoli with fewer type II cells and may require instillation of exogenous surfactant down endotracheal tubes to alleviate respiratory distress. Poor ventilation suppresses surfactant secretion so the severity of hypoxia, hypercapnia and acidosis is worsened and respiratory muscle activity is compromised which further compromises surfactant production.
(Reproduced with permission from Chamberlain et al., 1991.)
In fetal life, the lungs are filled by fluid secreted by the lung epithelium; the lung fluid is essential for growth and development of the lungs and this fluid exchanges with amniotic fluid. At birth, the neonate has to rapidly clear fluid from its air-spaces; 10–25 mL/kg fluid will be expelled or resorbed. Fetal breathing movements (FBM) are observed on ultrasound from the first trimester. Initially, they are intermittent, rapid and irregular. As gestation progresses, FBM increase in strength and frequency, occurring up to 80% of the time in an organized episodic pattern (Nijhuis, 2003). The lung fluid is ‘breathed’ out by the fetus into the amniotic fluid. Patterns of FBM dominate during the daytime and are correlated with fetal behavioural states. Fetal wakefulness and arousal are associated with sustained vigorous respiratory patterns. Quiet sleep is associated with an absence of FBM. Adrenergic and cholinergic compounds, prostaglandin synthesis inhibitors and raised maternal carbon dioxide levels stimulate FBM. They are inhibited by hypoglycaemia, cigarette smoking, alcohol consumption and accelerated labour. Despite the relatively low partial pressure of oxygen and high partial pressure of carbon dioxide, the fetus makes only shallow respiratory movements although severe hypoxia and acidosis may stimulate gasping. Mild hypoxia leads to quiet sleep and reduced energy expenditure and oxygen consumption, which may be protective. The movement of the diaphragm generates about 25 mmHg pressure for between 1 and 4 h per day in a pattern that coincides with rapid eye movement (REM) sleep but not during slow-wave sleep or fetal ‘wakefulness’. FBM are important in lung development (Harding and Hooper, 1996), promoting growth and allowing rehearsal of the respiratory actions. Lung development is retarded in conditions where FBM are limited such as congenital disorders of the diaphragm or nervous system. The fluid volume in the fetal airways correlates with the functional residual capacity in postnatal life (Strang, 1991).
The most urgent need after delivery is the initiation of ventilation; the neonate has to clear its lungs of fluid, establish regular breathing and increase pulmonary blood flow to match pulmonary perfusion to ventilation. Many factors interact to stimulate the first breath, including changes in temperature and state. The mild asphyxia (decreased oxygen concentration, raised carbon dioxide concentration) and acidosis (decreased pH) due to flow in the cord ceasing sensitize the fetal aortic, carotid and central (medullary) chemoreceptors that increase ventilatory drive. Tactile stimulation, such as that which occurs during delivery, also promotes respiration. In additional, it is thought that the placental prostaglandins may inhibit breathing (decreased oxygen concentration, raised carbon dioxide concentration). The surge of endogenous steroids and catecholamines associated with labour also contributes; infants who do not experience labour are more likely to retain residual fluid in their lungs and have less efficient respiratory performance.
The fluid-filled lung with collapsed alveoli and undispersed surfactant proffers a high resistance to inflation and the first breath requires considerable effort. The diaphragm contracts strongly and the complaint flexible ribs and sternum of the newborn baby are pulled concave in the effort of the first breath. Once the lungs are inflated, the lung fluid is forced into the alveoli where it aids dispersal of surfactant and is rapidly resorbed into the pulmonary lymphatic vessels. Subsequent breaths require fewer changes in pressure and less mechanical work. The thoracic compression of a vaginal delivery contributes to fluid loss from the upper respiratory tract; the compression of the chest (known as the ‘vaginal squeeze’) creates negative pressure which draws air into the lungs and they re-expand. Most of the fluid clearance is due to a change in the lung epithelium from being a predominantly chloride-secreting membrane at birth to being predominantly a sodium absorbing membrane after birth (Jain and Eaton, 2006).
Most babies gasp within 6 s and have patterns of normal breathing and gas exchange within 15 min. Initially the newborn infant has metabolic and respiratory acidosis due to decreased oxygen concentrations (resulting in increased lactic acid) and increased carbon dioxide levels, respectively; this acid–base imbalance is corrected as ventilation improves. The risk of transient tachypnoea of the newborn (TTN) is increased in babies who are delivered by caesarean section or those who experience perinatal hypoxia. It is thought that TTN is due to immaturity of the sodium transport mechanisms of the lung epithelium (Jain and Eaton, 2006).
The rate of ventilation of the newborn is high compared with an adult but is similar when relative size is taken into account. Ventilation is often irregular with the baby exhibiting periods of fetal-like shallow breathing. The reflexes associated with lung inflation also appear to be different. As well as the Hering–Breuer Reflex (where filling of the lungs increases expiratory centre activity), the newborn infant demonstrates Paradoxical Reflex of Head (where filling the lungs excites the inspiratory centre thus stimulating further inspiration) (Givan, 2003). For the first few weeks, babies breathe via the nose and suck via the mouth. Control of ventilation by chemoreceptors is functional but qualitatively different in that hypoxia tends to increase depth of respiration (rather than respiratory rate) and that the response is temperature-dependent and is abolished in cold temperatures. The chemoreceptors seem to be more sensitive to raised carbon dioxide levels.
Babies have a relatively large oxygen consumption, which reflects their heat generation and that their more metabolically active tissues (e.g. liver and brain) are proportionately larger. The high airway resistance means that the energy cost of respiration is higher. PVR drops 6–8 weeks after birth when the diameter of the small arterioles increases. The relatively high requirement for oxygen means that neonates are more susceptible to asphyxia than other age groups. Neonatal resuscitation aims to prevent mortality and morbidity. Hypothermic neonates are predisposed to hypoglycaemia and acidosis. Acidosis compromises respiration because it increases PVR and suppresses both respiratory drive and surfactant production. The aims of neonatal resuscitation are to promote and maintain adequate ventilation and oxygenation, to initiate and maintain adequate cardiac output and perfusion and to maintain body temperature and adequate blood glucose levels.
RDS is caused by a deficiency in surfactant, which results in alveolar collapse and increased airway resistance. Surfactant deficiency is usually inversely related to gestational age and lung maturity. Abnormal pH, stress and inadequate pulmonary perfusion also inhibit surfactant synthesis and recycling. RDS is worsened by asphyxia and is the most common cause of respiratory failure in the preterm infant. The reduced surface tension affects alveoli expansion. Small alveoli tend to collapse and normal alveoli are overdistended. Segments of the lung close and hypoxaemia and carbon dioxide retention progressively increase. The resulting metabolic and respiratory acidosis further limits the production of surfactant from the type II pneumocytes. Hypoxaemia causes vasoconstriction of the pulmonary arteries thus compromising pulmonary perfusion and increasing the likelihood of right-to-left shunting through the foramen ovale and ductus arteriosus. Local ischaemic damage affects the alveolar tissue and capillary endothelium. Changes in pulmonary pressure brought about by the infant attempting to maintain adequate air flow, together with the low plasma protein level common in preterm infants, tend to cause displacement of fluid into the alveoli. Fibrinogen in the exudate is converted into fibrin and lines the alveoli thickening the membrane. The thickened membrane and excess fluid increase the diffusion distance and impair gas transfer.
The infant responds to the respiratory difficulties by increasing respiratory rate and effort. The clinical signs appear early and increase in severity over 2 or 3 days. The infant may grunt and exhibit oedema and cyanosis. Cyanosis tends to be progressive and is due to high levels of deoxygenated haemoglobin in the capillaries. It is enhanced by right-to-left shunting, alveolar hypoventilation and impaired gas diffusion across the alveolar membranes. The baby grunts because expiration is against a partially closed glottis, which increases pressure and retards expiratory flow, therefore increasing gas exchange. RDS risk is increased in prematurity, babies of diabetic mothers (because insulin is antagonistic to cortisol), antepartum haemorrhage and second-born twins. Male babies are twice as susceptible to RDS. Chronic hypertension, maternal heroin addiction, pre-eclampsia and growth retardation appear to protect against RDS.
In utero, the fetus depends on its mother for temperature regulation. It loses heat via the placenta and via conduction (from skin to amniotic fluid to uterus). The fetus is a net heat producer although raised maternal temperature may compromise it (Edwards et al., 1997). Brown fat is actively inhibited and fetal oxygen consumption is about 30% of postnatal levels. Fetal temperature is maintained at about 0.5°C above maternal temperature and the fetus does not expend energy in keeping warm. Research has focused on raised maternal temperature due to fever, exercise and external raised temperature (such as hot baths and saunas). The results are inconclusive. However, maternal fever has effects not only on temperature gradients but also on oxygen consumption and haemodynamics and may be associated with teratogenesis and preterm labour.
The infant is usually born into a wet and relatively cold environment. As environmental temperature is usually lower than maternal temperature, the baby will experience a temperature loss at birth. Heat transfer is affected by two gradients: the internal gradient involving transfer from the core to the surface of the baby and the external gradient involving heat transfer from the body surface to the environment. Cooling is usually rapid at a rate of 0.2–1.0°C per minute depending on the environmental factors and the gestational age of the infant (which affects body composition). Transfer of heat through the internal gradient depends on insulation and blood flow. Neonates are predisposed to heat loss; they have less subcutaneous fat than adults do (about 16% body fat compared with 30%), a higher surface area:mass ratio (about three times the relative surface area of an adult) and a lower ability to shiver. Should the baby be born small, it will not only have an even larger surface area:mass ratio but also the insulation provided by its subcutaneous fat will also be further compromised and skin permeability will be increased. Small-for-dates babies have proportionately bigger heads and higher metabolism and are disadvantaged in that their heat losses are higher. Changes in peripheral circulation affect heat loss via conduction.
Heat loss across the external gradient depends on the temperature difference between the body and the environment. Conduction, convection, evaporation and radiation transfer heat from the baby. Warming objects that will come into contact with the neonate, and increasing insulation by wrapping, limit heat loss by conduction. Evaporation offers the greatest route for heat loss immediately after delivery but drying the baby, especially the head, immediately after delivery is effective at reducing the loss. Skin keratinization is inadequate in immature infants so evaporative heat losses are higher. Evaporative insensible heat loss increases with respiratory problems, activity, the use of radiant heaters or phototherapy and low relative humidity. Convective losses are related to draughts and are affected by ambient temperature and humidity. Higher air temperatures, minimal air circulation, swaddling and baby hats reduce heat loss by convection. Radiation is the major form of heat loss from babies in incubators. It involves the transfer of radiant energy to surrounding objects not directly in contact with the baby. Consideration therefore has to be given to the temperature of objects in the local environment including the incubator, walls and windows. Skin-to-skin contact with the mother immediately following birth is a very efficient way of reducing heat loss from the neonate. The large skin area and the softness of the breasts enable a large amount of maternal skin to come into direct contact with the baby’s skin surface.
The mechanisms of heat conservation and generation mediated by the peripheral nervous system are insufficient in the neonate (Okken, 1991). Infants can produce heat from metabolic processes and by increasing activity. Postural changes are also important in conserving heat. Shivering is not so important in infants but heat generation by non-shivering thermogenesis (NST) is important. NST takes place in brown adipose tissue (BAT), a specialized type of adipose tissue that is well vascularized, particularly by sympathetic nerves, and has cells densely packed with mitochondria (Fig. 15.7). In humans, BAT is mostly replaced by white adipose tissue (WAT); adults have very few BAT cells which are interspersed with WAT (Wolf, 2009). However, BAT has a major role in heat production in the neonate. BAT is formed from about 30 weeks’ gestation until about 4 weeks’ postbirth. Fat mass is significantly altered by maternal nutrition and gestational length; stores of BAT (and white fat) are lower in preterm infants. BAT comprises about 2–7% of birth weight and is predominantly located around the core organs (Fig. 15.8). It generates heat by uncoupling electron transport from oxidative phosphorylation in the mitochondria so the energy released by electron transport will not be used to synthesize ATP but will be liberated as heat instead (Fig. 15.9). Fifty percentage of cellular respiration is uncoupled from ATP formation in BAT (Wolf, 2009). The unique uncoupling protein (UCP1) is a proton transporter located in the inner mitochondrial membrane. UCP1 causes protons to leak across the inner mitochondrial membrane so the electrochemical gradient, that usually drives ATP production, is lost and heat is produced. When UCP1 is maximally activated, it allows the production of at least 100 times as much heat from BAT compared to other tissue. UCP1 is synthesized during the maturation of fetal fat (Symonds et al., 2003). At birth, the activation of BAT is accompanied by mobilization of fat and a marked increase in lipolysis. Thermogenesis by BAT is inhibited in the fetus by PGE2 and prostacyclin (PGI2) produced by the placenta. The placenta also suppresses formation of active T3 (tri-iodothyronine) from T4 (thyroxine) (see below).
Get Clinical Tree app for offline access