The urinary system
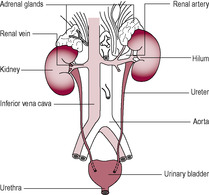
Fig. 2.1
The kidneys
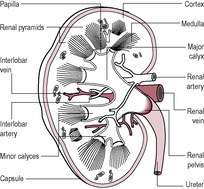
Fig. 2.2
The nephron
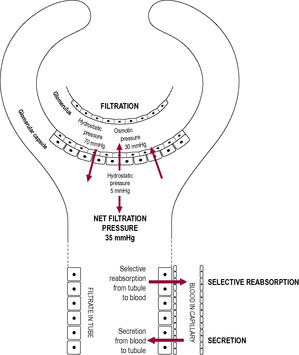
Fig. 2.4
Filtration
Selective reabsorption
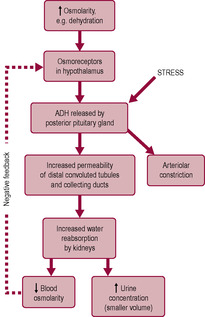
Fig. 2.5
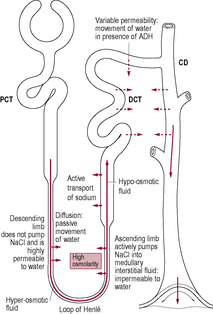
Fig. 2.6
Secretion
The ureters
The bladder
The urethra
Urine
Control of micturition
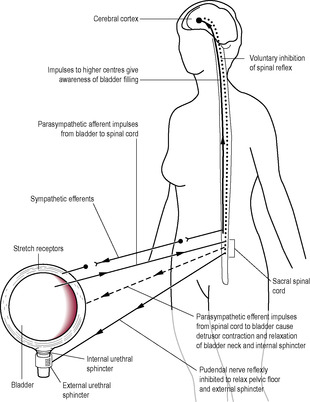
Fig. 2.8
The female reproductive tract
The ovaries
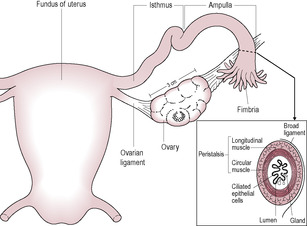
Fig. 2.9
The uterine (fallopian) tubes
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



The reproductive and urinary systems
Zara, during the booking appointment, is asked by the midwife to provide a mid stream specimen of urine to screen for infection. The midwife notes that the specimen appears cloudy although Zara does not have any other signs of a urinary tract infection.
• Are there any reasons apart from infection that could explain the cloudiness of the specimen?
• If the culture of the specimen proves positive, how will this be managed and what advice should the midwife give to Zara?
• If the analysis of the specimen showed a bacteraemia of group B haemolytic streptococcus what would be the significance of this, how should it be managed and what are the possible future consequences for Zara and her baby?
The urinary system is composed of two kidneys, which produce urine, two ureters running from the kidneys to the bladder, which collects and stores the urine, and a urethra from which urine is discharged to the exterior (Fig. 2.1). The uroepithelium which lines the renal pelvis, ureters and bladder is not just a passive impermeable barrier; it can modulate the composition of urine and also transmit information about pressure and composition of the urine to the underlying nervous and muscular tissue (Khandelwal et al., 2009).
(Reproduced with permission from Brooker, 1998.)
The kidneys have a broad range of other functions (see Box 2.1) as well as producing urine. The kidneys are situated upon the posterior wall of the abdominal cavity, one on either side of the vertebral column at the level of the thoracic and lumbar vertebrae (just below the rib cage). The right kidney is slightly lower than the left owing to its relationship to the liver. Each kidney is about 10 cm long, 6.5 cm wide and about 3 cm thick (about the size of a clenched fist). Each kidney weighs about 100 grams (a small proportion of the total body mass), but they receive about 25% of the cardiac output (which per unit of tissue is about eight times higher than the blood flow to muscles undergoing heavy exercise). The renal blood supply arises from the aorta via the renal arteries and returns to the inferior vena cava via the renal veins. Each kidney is enclosed by a thick fibrous capsule and has two distinct layers: the reddish-brown cortex, which has a rich blood supply, and the inner medulla, within which the structural and functional units of the kidney, the nephrons, are found (Fig. 2.2).
Box 2.1
• Regulation of water balance
• Regulation of pH (acid-base balance) and inorganic ion balance (sodium, potassium and calcium)
• Excretion of metabolic and nitrogenous waste products (urea from protein, uric acid from nucleic acids, creatinine from muscle creatine and haemoglobin breakdown products)
• Hormone secretion (erythropoietin, renin, 1,25-dihydroxyvitamin D3 (1,25-dihydroxycholecalciferol, also called calcitriol) and prostaglandins)
• Removal of toxic chemicals (drugs, pesticides and food additives)
• Regulation of blood pressure (renin–angiotensin system)
• Control of formation of red blood cells (via erythropoietin)
• Vitamin D activation and calcium balance
• Gluconeogenesis (formation of glucose from amino acids and other precursors)
(Reproduced with permission from Brooker, 1998.)
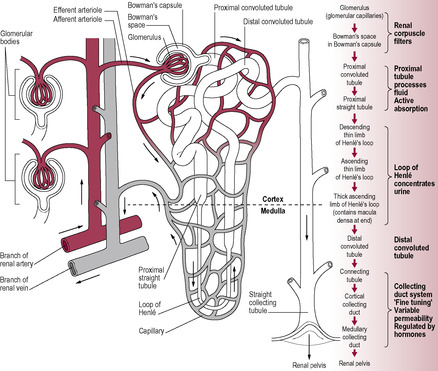
Each kidney has approximately a million nephrons (though the number declines with increasing age), each of which is about 3 cm long. The nephron is a tubule that is closed at one end and opens into the collecting duct at the other. The nephron has six distinct regions, each of which is adapted to a specific function (Fig. 2.3). There are two types of nephron. Most nephrons (85–90%) are cortical nephrons; these have short loops of Henle and are mainly concerned with the control of plasma volume during normal conditions. The juxtamedullary nephrons, which have longer loops of Henle extending into the renal medulla, facilitate increased water retention (and thus the production of hyperosmotic or concentrated urine) when the availability of water is restricted.
The renal corpuscle comprises the Bowman’s capsule, a blind-ended tube, and the glomerulus, a coiled arrangement of capillaries around which the Bowman’s capsule is invaginated. The glomerulus provides a large area of capillary vessels from which substances can leave, crossing the specialized flattened epithelial cells to enter the capsule of the nephron. There is a double capillary arrangement (see Fig. 2.3) whereby afferent arterioles supply the glomerular capillaries and efferent arterioles lead from the glomerulus to a second capillary bed supplying the rest of the nephron. Differential vasoconstriction of the afferent and efferent arterioles maintains a constant blood pressure within the glomerulus, which results in a constant rate of filtration. Urine production relies on three steps: simple filtration, selective reabsorption and secretion (Fig. 2.4).
Filtration is a non-selective passive process that occurs through the semipermeable walls of the glomerulus and glomerular capsule. All substances with a molecular mass of less than 68 kilodaltons (kDa) are forced out of the glomerular capillaries into the Bowman’s capsule. Therefore, water and small molecules such as glucose, amino acids and vitamins enter the nephron whereas blood cells, plasma proteins and other large molecules are usually retained in the blood. The content of the Bowman’s capsule is referred to as the ‘glomerular filtrate’ and the rate at which this is formed is referred to as the ‘glomerular filtration rate’ (GFR). The kidneys form about 180 L of dilute filtrate each day (a GFR of about 125 mL/min). Most of it is selectively reabsorbed so the final volume of urine produced is about 1–1.5 L/day.
Box 2.2 describes an example of disrupted renal function in pregnancy that is detected by abnormal urine composition.
Box 2.2
Hypertensive disorders in pregnancy can disrupt renal function. The detectable presence of protein within the urine (proteinuria) may indicate that larger molecules than normal are being forced into the Bowman’s capsule. This is caused by the increased blood pressure resulting in abnormal ultrafiltration. Women who have a degree of renal damage prior to pregnancy are less likely to be able to adapt to the pregnancy-induced physiological changes as effectively as women with normal renal function. These women tend to develop high blood pressure during early pregnancy and so do not normally demonstrate such a marked physiological reduction in blood pressure parameters, putting both the mother and fetus at risk.
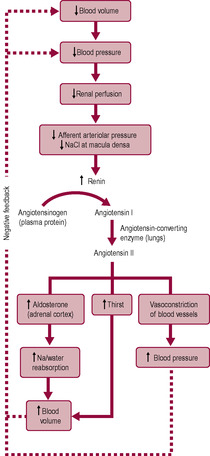
Substances from the glomerular filtrate are reabsorbed from the rest of the nephron into the surrounding capillaries. The proximal convoluted tubule (PCT in Fig. 2.6) is the widest and longest part of the whole nephron (approximately 1.4 cm long). The epithelial cells lining the nephron contain a large number of mitochondria to provide energy for facilitating active transport as most of the reabsorption of the glomerular filtrate takes place here. Some substances, such as glucose and amino acids, are completely reabsorbed and are not normally present in urine. Reabsorption of waste products is largely incomplete, so, for instance, a large proportion of urea is excreted. The reabsorption of other substances is under the regulation of several hormones. Antidiuretic hormone (ADH) controls the insertion of aquaporins, pore-forming membrane proteins, into the walls of the distal convoluted tubule (DCT in Fig. 2.6) and collecting ducts (CD in Fig. 2.6), which allows water to leave the filtrate, thus producing less urine (Fig. 2.5). The formation of concentrated urine is facilitated by the physical arrangement of the loop of Henle and its surrounding capillaries, which create and maintain the conditions for the reabsorption of water by osmosis (Fig. 2.6, Box 2.3). Calcitonin increases calcium excretion and parathyroid hormone enhances reabsorption of calcium from renal tubules. Aldosterone affects the reabsorption of sodium (Fig. 2.7). Atrial natriuretic peptide (ANP) inhibits NaCl reabsorption in the DCT and cortical collecting duct of the nephron. ANP also increases the GFR by dilating the afferent glomerular arterioles and constricting the efferent glomerular arteriole, thus increasing NaCl excretion.
Box 2.3
The evolution of the mammalian kidney has enabled mammals to become highly adapted to terrestrial living. The kidney aids water conservation by producing urine that is able to be concentrated far more than the internal body fluid environment. The scarcer water is within the environment, the longer the nephron to conserve water.
Some waste products may be actively transported directly into the tubules from the surrounding blood capillaries. These include hydrogen and potassium ions, creatinine, toxins and drugs. The cells of the renal tubules synthesize some substances, such as ammonia ions and peptides, which can be secreted into the filtrate.
The ureters, which are tubes about 25–30 cm long and 3 mm in diameter, transport the urine from the kidneys to the bladder. From each kidney the collecting ducts open into the renal pelvis, which leads to the ureter. The walls of the renal pelvis have smooth muscle, which has intrinsic activity (i.e. not controlled by nerves), generating peristaltic waves of contraction every 10 s. These waves of contraction propel urine along the ureters to the bladder. Each ureter is also lined with smooth muscle and transitional epithelium; the lumen has a star-shaped cross-section.
The ureters lie upon the posterior abdominal wall outside the peritoneal cavity, entering the bladder at an oblique angle, one at each side of the base of the specialized muscle area called the trigone which has its apex at the urethral opening. As urine accumulates in the bladder, the ureters are compressed, effectively forming a valve (the vesicoureteral valve), which prevents urinary reflux.
The bladder is a distensible hollow organ, also composed of smooth muscle, which acts as a reservoir for urine. It is intermittently emptied under conscious control. Stretch receptors within the muscle and trigone provide the signals that indicate that the bladder is full. The normal capacity of the bladder is approximately 700–800 mL; however, the natural desire to void urine becomes conscious when the level of urine in the bladder reaches approximately 300 mL. Inflammation in the trigone region caused by infection and or trauma often results in a frequent and urgent desire to void urine but on voiding only small amounts of urine are passed.
As the bladder lies below the uterus, its capacity is compromised by the growing uterus in early pregnancy. Later on, once the pregnant uterus has become an abdominal organ, the pressure on the bladder is relieved. Finally, at the end of pregnancy, bladder capacity is again compromised as the presenting part of the fetus engages, occupying space within the true pelvic cavity and thus restricting the space available to the bladder.
Urine is voided via the urethra. The female urethra is considerably shorter and straighter than the male urethra: only 4 cm in length compared with about 20 cm. This anatomical difference predisposes women towards an increased incidence of ascending urinary tract infections (UTIs). Thus, a colony count of more than 100000 bacterial cells per millilitre of urine is considered to be pathologically significant and is often referred to as bacteraemia. There are small mucus-secreting glands in the urethra that help to protect the epithelium from the corrosive urine. The upper internal sphincter, at the exit from the bladder, is composed of smooth muscle and is under autonomic control. The external sphincter is composed of skeletal muscle and is under voluntary control. The urethra in the man has a dual role as the route for urine and the delivery of spermatozoa, via coitus. Structural differences related to the development of the external genitalia are covered in Chapter 5. Trauma to the pelvic floor during childbirth may result in neurological damage affecting the function of the internal sphincter resulting in urgency of micturition. Urgency to void is increased by the degree of weakness in the sphincter and the amount of urine held in the bladder. Treatment options range from a variety of surgical procedures to drug treatment such as anti-cholinergic drugs (NCCWCH, 2006).
Urine has a specific gravity of 1.010–1.030 and is usually acidic. The volume and final concentration of urea and solutes depend on fluid intake. Sleep and muscular activity also inhibit urine production. The amber colour is due to urobilin, the bile pigment. Urine has a characteristic smell, which is not unpleasant when fresh. Odour or cloudiness generally indicates a bacterial infection (Box 2.4).
Box 2.4
Pregnancy further increases the risk of UTIs in pregnancy and so routine culture and sensitivity test to detect bacteraemia is a common practice. Some women with bacteraemia may be asymptomatic, for example, group B haemolytic streptococcus. If group B streptococcus is present within the urine, antibiotic therapy is recommended (Royal College of Obstetricians and Gynaecologists, 2006) as this represents a high bacterial load which could put the neonate at risk of infection following birth (see Chapter 10).
Micturition (urination) is a coordinated response that is due to the contraction of the muscular wall of the bladder, reflex relaxation of the internal sphincter of the urethra and voluntary relaxation of the external sphincter (Fig. 2.8). It is assisted by increased pressure in the pelvic cavity as the diaphragm is lowered and the abdominal muscles contract. Over-distension of the bladder is painful and can cause involuntary relaxation of the external sphincter resulting in urgency of micturition, incontinence and overflow. The tone of this sphincter is also affected by psychological stimuli (such as waking or getting ready to leave the house) and external stimuli (such as the sound of water or the feel of the lavatory seat). Any factor that raises the intraabdominal and intravesicular pressures (such as laughter or coughing) in excess of the urethral closing pressure can result in stress incontinence.
(Reproduced with permission from Brooker, 1998.)
Accumulation of urine increases bladder wall tension, stimulating the stretch receptors of the bladder, which relay parasympathetic sensory impulses to the brain, generating awareness. However, there is conscious descending inhibition of the reflex bladder contraction and relaxation of the external sphincter. Entry of urine into the urethra irritates and stimulates stretch receptors, augmenting the sensory pathways as the bladder fills. Micturition is postponed until a socially acceptable time and place. This inhibition of the spinal reflex and contraction of the external sphincter is learned. Infants tend to develop bladder control when they are about 2 years old. Irritation of the bladder or urethra, for instance as a result of infection, can also initiate the desire to urinate regardless of the bladder capacity.
Normal physiological control of micturition requires an intact nerve supply to the urinary tract, normal muscle tone (of bladder, urethral sphincters and pelvic floor muscles), absence of any obstruction to flow, normal bladder capacity and, finally, the absence of psychological factors that may inhibit the micturition cycle (such as embarrassment and discomfort).
The main features of the female reproductive tract distinguishing it from the male are that the female reproductive organs are internal and in the non-pregnant state are situated within the true pelvic cavity. The female reproductive tract consists of two ovaries, two uterine (fallopian) tubes, the uterus and cervix, the vagina and external genitalia. The female reproductive system undergoes considerable changes throughout life from childhood through reproductive life (see Box 2.5) to the menopause. Superimposed on these changes are the effects of the menstrual cycle (see Chapter 3). Prevention of infection in the female reproductive tract is essential; the cervix, endometrium and uterine tubes all produce natural antimicrobial secretions with production peaking about the time when implantation would occur (King et al., 2007).
Box 2.5
• Hair appears on mons veneris and subcutaneous fat accumulates
• Secretory glands mature and become active
• Labia majora and minora become pigmented with melanin
• Enlargement of the clitoris occurs
• Vaginal epithelium thickens and becomes responsive to oestrogen
• Vaginal pH decreases as lactobacilli metabolize glycogen from cell secretions
• Uterus grows and cervix doubles in length
The ovaries are dull-white almond-shaped bodies, approximately 4 cm long. They lie posteriorly and laterally relative to the body of the uterus and below the uterine tubes. They are anchored by the ovarian ligaments and attached to the posterior layer of the broad ligament, a fold within the peritoneum that extends from the uterus (Fig. 2.9). The blood supply to the ovary is via the ovarian artery, which runs alongside the ovarian ligament, and the ovarian branch of the uterine artery (see Fig. 2.12). This dual blood supply is important in maintaining reproductive function; if the ovary becomes twisted, for instance because it is displaced by a tumour or cystic growth, the ovarian ligament may occlude the blood supply from the ovarian artery. This torsion of the ovary can cause ischaemia of the tissues and intense pain.
(Reproduced with permission from Sweet, 1996.)
The ovaries are composed of two distinct layers: the outer layer is the cortex and the inner section is referred to as the medulla. The ovary is contained within a sheath of connective tissue, the tunica albuginea. The cortex contains the developing follicles that contain the primary oocytes and is also responsible for the production of the female steroid hormones oestrogen and progesterone (see Chapters 4 and 5). The medulla is composed primarily of connective tissue and blood vessels and provides precursors to facilitate steroid production within the cortex. The ovary has two main functions: to produce fertilizable oocytes which can undergo full development and to secrete the steroid hormones which prepare the reproductive tract for fertilization and to establish and support the pregnancy.
The long-held belief that all oocytes in adults are formed in the fetal and perinatal period (prenatal ‘total endowment’) has been challenged (Bukovsky et al., 2009) as studies of oogenesis have demonstrated that follicular renewal continues through much of female reproductive life and oocyte renewal probably only totally ceases at the onset of natural menopause. This has important clinical significance particularly for the many young women rendered infertile by chemotherapy.
The uterine tubes (also known as the fallopian tubes or oviducts) are approximately 12 cm long and have walls of smooth muscle lined with ciliated epithelial and secretory cells. The uterine tubes are mobile and not fixed to the ovaries. The distal end of the uterine tube has specialized structures called fimbriae, which surround the opening into the tube. The fimbriae lie in close proximity to the ovary and, at ovulation, assist the entry of the ovum into the uterine tube by a wafting action, which facilitates movement of the interperitoneal fluid. The lining of the uterine tubes lies in many folds (called plicae) and is composed of ciliated columnar epithelial cells interspersed with goblet cells that secrete pyruvate to nourish the ovum. The cilia facilitate the movement of the ovum down the uterine tube; this is augmented by coordinated peristaltic contractions of the smooth muscle. The distal end of the uterine tube has a slightly wider area, called the ampulla, where fertilization of the ovum by the sperm usually occurs.
If both uterine tubes are completely blocked, fertilization is prevented as the sperm are unable to access the ovum. If one uterine tube is patent or only partially blocked then sperm may encounter and fertilize an ovum within the peritoneal cavity. However, if a fertilized ovum enters a partially or totally blocked uterine tube its passage to the uterus will be impeded and so the pregnancy may develop within the uterine tube or peritoneal cavity (see Case Study 2.1 and Box 2.6). Infection of the genital tract with Chlamydia trachomatis is becoming more common and can often be asymptomatic (Carey and Beagley, 2010). Undetected or multiple infections can lead to pelvic inflammatory disease which is the main cause of ectopic pregnancy and tubal infertility. Although the prime site of chlamydial infection is the columnar epithelial cells of the cervix, the infection can quickly ascend to the upper reproductive tract probably by attaching to sperm or by being transported in the flow of fluids. Infection leads to production of proinflammatory cytokines which interact with the infected woman’s immune system (see Chapter 10) causing inflammation and tissue destruction. The incidence of infection is increasing because the organism is developing antibiotic resistance and the development of effective vaccines in very early stages.
Julie, during the booking appointment, informs the midwife that she had previously suffered an ectopic pregnancy with her last pregnancy, which was treated conservatively with methotrexate. What does the midwife need to do to ensure that Julie’s pregnancy is progressing normally? What are the signs and symptoms of an ectopic pregnancy; and when are they most likely to become apparent?
Box 2.6
An ectopic pregnancy is one that implants in the uterine tubes or, more rarely, the cervix, ovaries or abdomen. It is relatively common as it occurs in about 1% of all pregnancies and although the fatality rate is much reduced, ectopic pregnancy still remains a significant cause of maternal morbidity and mortality (Raine-Fenning and Hopkisson, 2009). It is usually confirmed by an ultrasound scan revealing an empty uterine cavity and a positive pregnancy test. Raised human chorionic gonadotrophin (hCG) levels confirm pregnancy but levels are lower in ectopic pregnancy than in uterine pregnancy. The term ‘pregnancy of unknown location’ (PUL) is used to describe a pregnancy where there is a positive pregnancy test but no intra- or extra-uterine pregnancy can be visualized on an ultrasound scan (Kirk and Bourne, 2009). In these cases, it is recommended that serial hCG measurements be made to monitor whether the PUL is failing (the hCG ratio is used to compare initial hCG levels with those 48 h later) and also that serum progesterone measurements are made to predict the likely outcome (higher levels are associated with pregnancies subsequently demonstrated to be viable).
The usual first warning sign of an ectopic pregnancy is abdominal pain at around 8 weeks’ gestation which may present with symptoms mimicking gastrointestinal disease (misdiagnosis of ectopic pregnancy as gastroenteritis is associated with maternal mortality). Ectopic pregnancy should be suspected in all women of childbearing age who present with fainting or sudden unexpected collapse (Neilson, 2007); no form of contraception is 100% effective, so pregnancy should not be excluded in women who use contraception. If the uterine tube ruptures, the woman may become clinically shocked owing to excessive bleeding into the peritoneal cavity. The growing fetus can be surgically removed together with the damaged uterine tube if necessary (this is referred to as a salpingectomy). Occasionally, an abdominal pregnancy may ensue if implantation occurs on the peritoneum. The pregnancies rarely go to term; however, delivery of live infants via abdominal surgery has been documented. This phenomenon underpins scientific interest enabling men to have babies through a process of peritoneal implantation. The main causes of tubal blockage are infection (usually due to pelvic inflammatory disease), the formation of scar tissue from surgery or trauma and congenital malformation. High levels of steroid hormones can also affect cilia movement. If a tubal pregnancy is diagnosed early before trauma occurs, it can be treated by the intramuscular administration of the drug methotrexate. Methotrexate is a chemotherapeutic drug which inhibits folic acid metabolism (by inhibiting difolate reductase so DNA synthesis ceases) thus targeting rapidly dividing tissue such as the trophoblast; the embryo is eventually reabsorbed. Methotrexate has side effects on mucosal surfaces as they also have a fast rate of cell division and can cause conjunctivitis, gastrointestinal disturbances and stomatitis (inflammation of the mucous membranes in the mouth). Women may experience some degree of abdominal pain because of tubal miscarriage; it can be difficult to distinguish this from tubal rupture. Although the uterine tube is saved, the risk of another ectopic pregnancy is high. As the rate of sexually-transmitted disease is increasing and there are more assisted conceptions, the rate of ectopic pregnancy is expected to increase (Raine-Fenning and Hopkisson, 2009).
Get Clinical Tree app for offline access