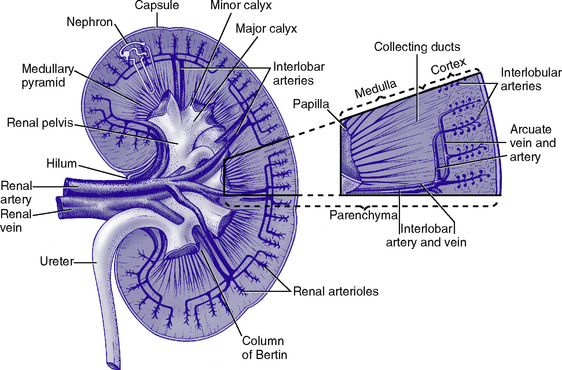
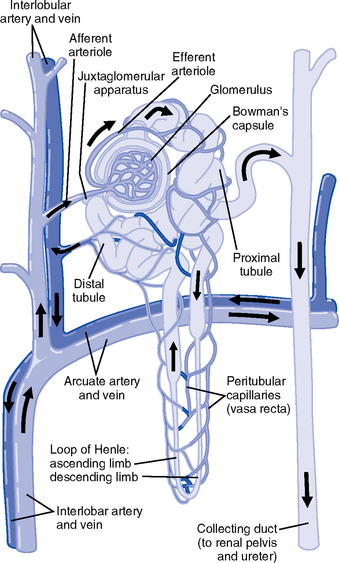
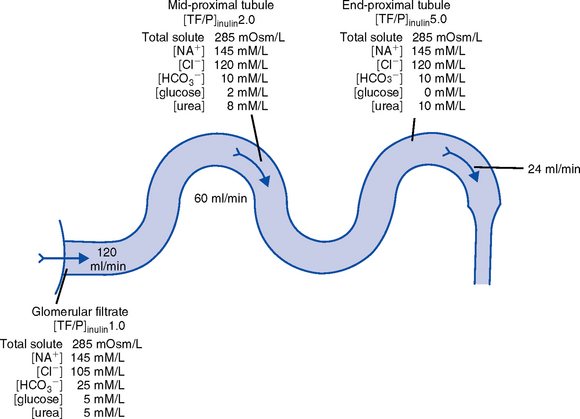
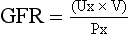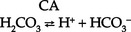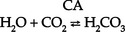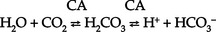CHAPTER 5 a. Anatomic structures of the kidney: Most humans are born with two kidneys; a small number are born with one. The kidneys are located in the retroperitoneal space above the waist (Figure 5-1). (a) Metabolically active portion of the kidney, where aerobic metabolism occurs and where ammonia and glucose are formed (b) Metabolic needs more than satisfactorily met by an abundant oxygen supply (c) Contains all glomeruli and portions of the proximal and distal tubules (a) Region of active glycolytic metabolism; supplies energy for active transport (b) Metabolism demands high oxygen consumption, yet oxygen supply limited (c) Plays role in concentration of urine (d) Composed of 6 to 10 renal pyramids, formed by collecting ducts and extending into the renal pelvis (e) Site of the deepest part of the long loops of Henle and the collecting ducts of the nephron iii. Renal sinus, pelvis, and collecting system (a) Papillae: Rounded projections of renal tissue located at the apical ends of the renal pyramids positioned with the base facing the cortex and the apices facing the renal pelvis; the apical portion opens into the minor calices (b) Corticomedullary junction: Point of division between the cortex and the medulla formed by the base of the pyramids (c) Renal lobe: Composed of a pyramid plus the surrounding cortical tissue iv. Nephron: Anatomic microscopic structure (Figure 5-2) (a) Structural and functional unit of the kidney (b) Approximately 1 million in each kidney (c) Compensates for a significant degree of nephron destruction by (d) Types of nephrons, based on location and function (1) Cortical nephrons located in the outer region of the cortex; contain short loops of Henle with a low capacity for sodium reabsorption (2) Juxtamedullary nephrons located in the inner cortex adjacent to the medulla; have long loops of Henle that penetrate deep into the medulla and have a greater capacity for concentration of urine because they are sodium-retaining nephrons (e) Functional segments of the nephron a) Bowman’s capsule: Specialized portion of the proximal tubule that supports the glomerulus b) Glomerulus: Capillary bed with semipermeable membrane 1) Normally permeable to water, electrolytes, nutrients, wastes; relatively impermeable to large protein molecules, albumin, erythrocytes 2) Composed of three cellular layers: Fenestrated endothelial layer, basement membrane, and epithelium podocyte cells that contribute to characteristic semipermeability of this membrane 3) Characteristics of cellular layers: Endothelial cells contain fenestrations 50 to 100 nm wide, favoring the movement of water and solute; remaining layers are less porous, with openings 1500 nm thick, which may explain the impedance of macromolecules 4) Major factor influencing filtration is molecular size 5) Ionic charge also affects filtration i. Glomerular ultrafiltration is the first step in the formation of urine (a) Characteristics of glomerular filtrate (1) Normal: Protein-free and red blood cell (RBC)–free, plasmalike substance with a specific gravity (SG) of 1.010. Filtrate contains water, electrolytes, glucose, amino acids, acid-base components, wastes, and other solutes. Pharmaceutical agents can also be included in filtrate. (2) Small and middle-sized molecules (up to 60 to 75 kd): Pass freely through the glomerular membrane (i.e., inulin 5 kd, albumin >60 kd) (3) Abnormal: Increased permeability of the glomerular membrane allows erythrocytes and protein to be filtered into urine. SG of urine may artificially increase because of the presence of protein or glucose. (4) Increased osmotically active substances (glucose, urea): Can cause diuresis (b) Filtration is determined by the glomerular pressure and presence of a normal semipermeable glomerular membrane (1) Glomerular hydrostatic pressure is 50 mm Hg and favors filtration; this capillary hydrostatic pressure reflects cardiac output (2) Colloid osmotic pressure of 25 mm Hg and Bowman’s capsule hydrostatic pressure of 10 mm Hg oppose hydrostatic pressure and thus oppose filtration a) Colloid osmotic pressure results from oncotic pressure of plasma proteins in the glomerular blood supply b) Bowman’s capsule pressure reflects renal interstitial pressure (3) Net filtration pressure is derived using the following formula: (c) Glomerular filtration rate (GFR) (1) Clinical assessment tool to determine renal function (2) Definition: Volume of plasma cleared of a given substance per minute (may be determined by using endogenous creatinine) (4) Normal adult GFR: 125 ml/min or 180 L/day (5) Normal adult urine volume: 1 to 2 L/day, reflecting greater than 99% reabsorption of filtrate a) Changes in glomerular hydrostatic pressure 1) Secondary to changes in systemic blood pressure (BP) 2) Caused by variation in afferent or efferent arteriolar tone; increased afferent arteriole resistance decreases GFR; increased efferent arteriole tone increases GFR b) Alterations in oncotic pressure due to dehydration, hypoproteinemia, or hyperproteinemia c) Alterations in Bowman’s capsule pressure due to urinary tract or nephron destruction, or interstitial edema of kidney ii. Tubular functions of reabsorption, secretion, and excretion comprise the following steps in urine formation (Figure 5-3): (a) Conversion of 180 L of plasma filtered per day to 1 to 2 L of excreted urine (b) Absorption and secretion by two processes: (1) Passive mechanisms: Solute moves without the expenditure of metabolic energy a) Diffusion: Solute following either a concentration or an electrical gradient 1) A solute moves from a solution of higher concentration through a semipermeable membrane to a solution of lower concentration 2) Selectivity of the membrane’s permeability and electrical gradient determine diffusion of the solute 3) The electrical gradient causes a solute to passively migrate to the oppositely charged compartment (e.g., Na+, a positive ion, migrates to a negatively charged compartment, whereas Cl−, a negative ion, moves toward a positively charged compartment) b) Osmosis: Water following an osmotic gradient a) Ion transport requires energy; adenosine triphosphate [ATP]) permits ions to move against the concentration gradient b) Maximal tubular transport capacity: Active reabsorption mechanisms in the tubule have limited capacity for reabsorption of certain substances such as glucose. Plasma glucose level of 375 mg/min (transport maximum [Tm]), results in no excretion in urine; plasma glucose level above 375 mg/min results in glucose excretion in urine. Tm for glucose can vary from one nephron to another; as a result, glucose can sometimes spill into the urine at lower serum levels. (c) Proximal convoluted tubule (1) Reabsorbs 60% to 80% of filtrate, which remains isotonic to plasma (2) Major function is active reabsorption of sodium chloride (NaCl) with passive reabsorption of water (3) Also reabsorbs glucose, amino acids, phosphates (PO43−), uric acid, potassium ion (K+) (4) Regulates acid-base balance through reabsorption of carbonic acid (H2CO3) and bicarbonate (HCO3−) and secretion of hydrogen ions (H+) (5) Secretes K+, ammonium ion (NH4+), organic acids, bases, foreign substances (e.g., drugs) (1) Variations in length depend on the type of nephron (i.e., juxtamedullary with long loops or cortical with short loops) a) Descending segment, the thin limb, is permeable to water and impermeable to Na+ b) Ascending segment, the thick limb, has active NaCl pump and is impermeable to water; target site for loop diuretics (3) Major function is concentration or dilution of urine, accomplished by a countercurrent mechanism that maintains hyperosmolar concentration in the interstitium of the renal medulla (1) Receives hyposmotic (or hypotonic) urine from the ascending loop of Henle (3) Water permeability here is controlled by antidiuretic hormone (ADH); Na+ reabsorption is determined by aldosterone (1) Receives urine, which is isotonic to plasma, from the distal convoluted and collecting tubules (2) Functions with the distal convoluted tubule; affected by ADH and aldosterone (3) Final urinary adjustments for composition, tonicity, and volume made here before urine enters the renal pelvis and progresses to the ureter and bladder 2. Renal hemodynamics: Normal blood flow patterns i. Specialized arrangement of renal blood vessels reflects interdependence of blood supply with kidney function (a) Kidney: Aorta→segmented renal arteries→interlobar artery→arcuate artery→interlobular artery→(nephron)→interlobular vein→arcuate vein→interlobar vein→renal vein→inferior vena cava (b) Nephron: Afferent arteriole→glomerular capillary→efferent arteriole→ peritubular capillary→vasa recta adjacent to tubules→interlobular vein→renal vein→inferior vena cava iii. Juxtaglomerular apparatus: Site of renin synthesis (a) Specialized cells composed of juxtaglomerular cells and macula densa (1) Juxtaglomerular granular cells: Smooth muscle cells containing granules of inactive renin (2) Macula densa: Portion of the distal tubule making contact with afferent arterioles of its respective glomerulus (b) Responds to arterial BP in afferent and efferent arterioles and to sodium in distal tubule b. Renal blood flow (RBF) parameters i. Kidney receives 20% to 25% of cardiac output or 1200 ml/min, which translates to flow rate of 4 ml/g/min to the kidney ii. Oxygen extraction from renal cells is high, but the amount is not significant enough to account for flow rate; rather, the flow is required to support normal renal function (a) Cortex: Metabolically active region, receives most (80%) of the blood supply (b) Medulla: Site of anaerobic metabolism, receives 20% of blood supply ii. Nephrons: Receive 600 to 650 ml/min of renal plasma flow d. Intrarenal autoregulation: General principles i. Mean arterial pressure (MAP) is maintained in a range of 80 to 180 mm Hg to prevent large changes in GFR ii. Major site of autoregulation is the afferent arteriole iii. Increase in the renal arterial pressure causes afferent vasoconstriction; decrease causes efferent vasoconstriction, producing an increased GFR/RBF ratio iv. Changes in vascular tone of the efferent arteriole (primarily vasoconstriction) complement efforts to maintain GFR by compensating for reduced blood flow v. Autoregulation is essentially absent at an MAP of 70 mm Hg or below i. Route of nerve supply is along renal blood vessels; renal neurologic intervention is vasoconstrictive ii. Hypotension decreases systemic arterial pressure, stimulating the carotid sinus and aortic arch baroreceptors to trigger the sympathetic response (release of epinephrine), which decreases RBF and GFR by vasoconstricting both afferent and efferent arterioles iii. Other factors that stimulate increased sympathetic tone are stress, fear, and exercise iv. This neuronal effect is not the primary factor in autoregulation; a denervated kidney can be transplanted and still be able to compensate for changes in BP f. Hormonal modulation of RBF (see Renal Regulation of Blood Pressure) i. Renin-angiotensin system: A mechanism to sustain systemic BP and plasma volume (a) Responds to a decreased afferent arteriolar pressure by increasing angiotensin II levels (b) Angiotensin II vasoconstricts renal blood vessels, particularly the efferent artery, which reduces RBF but increases GFR ii. Renal prostaglandins: Modulate the effects of vasoactive substances, such as angiotensin II, on the kidney by causing vasodilatation i. Epinephrine and norepinephrine: Cause efferent arterioles to vasoconstrict, which leads to a rise in the filtration fraction and a dose-related decrease in RBF ii. Dopamine: Pharmacologic action on RBF is dose-related for renal vasodilatation and increased sodium excretion. Generally has a vasodilatory effect on renal vasculature at dosages between 1 and 4 mcg/kg/min intravenously (IV) (optimal dosage, 3 mcg/kg/min); dosages above 10 mcg/kg/min cause renal vasoconstriction, decreasing RBF and GFR. Dopamine therapy has no impact on the prevention of acute tubular necrosis. iii. Furosemide and mannitol: Increase GFR initially by increasing blood flow to the kidney and later by decreasing intratubular pressure iv. Calcium channel blockers: Relax renal arteriole and ameliorate renal failure related to renal transplantation and nephrotoxicity due to radiocontrast dyes or cyclosporine v. Atrial natriuretic factor (atrial natriuretic peptide, or ANP): Improves function in oliguric acute renal failure (ARF), but not preventive a. Thirst: Regulator of water intake i. Thirst center is located in the anterior hypothalamus ii. Neuronal cells are stimulated by intracellular dehydration, which causes sensation of thirst iii. Role is maintenance of satiety state (i.e., drinking exact amount of fluid to return body to normal hydration state) b. ADH: Sodium osmoreceptor mechanism for control of extracellular fluid (ECF) osmolality and sodium concentration i. ADH is synthesized in the paraventricular and supraoptic nuclei of the hypothalamus and travels along the axons of the supraopticohypophysial tract for storage or release from the posterior pituitary. The supraoptic area of the hypothalamus may overlap with the thirst center, providing integration of the thirst mechanism, osmolality detection, and ADH release. ii. Release of ADH occurs with the following: (a) Increased serum osmolality stimulates osmoreceptor cells in the hypothalamus that transmit along the neurohypophysial tracts, leading to ADH release from the posterior pituitary; normal serum osmolality is 285 to 295 mOsm/L (b) Volume contraction states reverse the inhibitory effect on ADH release; controlled by stretch receptors in the left atrium that activate the ADH mechanism iii. In the presence of ADH, water reabsorption occurs in the distal tubule and collecting ducts, which results in a hypertonic urine, hypotonic medullary interstitium, and eventual correction of contracted ECF iv. ADH secretion is inhibited when serum osmolality decreases (water intoxication). When this occurs, the distal tubule and collecting duct become relatively impermeable to water, so that large volumes of hypotonic filtrate are delivered to the collecting duct; this results in dilute urine and excess water loss (compared to extracellular solute concentration), which returns serum osmolality to normal limits. c. Countercurrent mechanism of the kidney: Mechanism for the concentration and dilution of urine; adjusts urine osmolality from 50 to 1200 mOsm/L i. Isotonic glomerular filtrate leaves the proximal tubule and enters the loop of Henle at 300 mOsm/L ii. Descending limb of the loop of Henle is permeable to water only. Water is gradually drawn into the hypertonic medullary interstitium, which gradually increases the osmolality of the filtrate as it becomes dehydrated. At the hairpin turn of the loop, osmolality is dramatically increased by the removal of water and NaCl pump action; osmolality can reach 1000 to 1200 mOsm/L. Concurrently, the medullary interstitium becomes hypotonic. iii. Thick ascending limb of the loop of Henle is permeable to NaCl but impermeable to water. The medullary interstitium becomes more hypertonic as its sodium concentration is increased by pumping action at the ascending limb. iv. A dilute filtrate reaches the distal tubule. If ADH is absent, dilute filtrate is excreted unchanged, which results in dilute urine with water excretion in excess of solute. If ADH is present, the collecting duct reabsorbs water and concentrated urine is excreted. a. Sodium regulation: Normal serum concentration is 136 to 145 mEq/L solute i. Na+ is the major extracellular cation and osmotically active solute. Because variation in body sodium can be associated with an exchange of water between intracellular and extracellular compartments, sodium affects ECF volume. ii. Renal reabsorption sites: Normal percentages of reabsorbed filtered sodium iii. Major factors that influence Na+ excretion include GFR, the sympathetic nervous system, aldosterone, the renin-angiotensin-aldosterone system, vasopressin (ADH), and ANP (a peptide hormone that plays a role in regulating and monitoring fluid, electrolyte, and cardiovascular balance) iv. Sodium reabsorption increases at the renal tubules under the following conditions: (a) Decreased GFR secondary to renal hypoperfusion (e.g., shock): Less sodium is delivered to the renal tubules, and less is excreted (b) Secretion of aldosterone (a mineralocorticoid secreted by the adrenal cortex) (1) Major effects are to increase renal tubular reabsorption of Na+ and to control selective renal excretion of K+ (2) Increases Na+ in ECF, which promotes water reabsorption; at the same time, K+ is secreted into the distal tubule and collecting duct to be excreted (3) Regulated by K+ concentration in the ECF, the renin-angiotensin-aldosterone mechanism, total body sodium, and adrenocorticotropic hormone (ACTH) (c) ANP action: Causes natriuretic, diuretic, and hypotensive effects secondary to its potent vasodilatory properties; the increased urinary excretion of Na+ is matched by an accompanying loss of K+ and PO43− v. Sodium reabsorption decreases at the renal tubules under the following conditions: (a) Increased GFR (excess ECF volume): Increases renal perfusion and GFR; more sodium is delivered to the renal tubules and more is excreted in urine (b) Inhibition of aldosterone secretion, which results in renal Na+ excretion (c) Secretion of ANP and ADH, administration of diuretics, especially loop-affecting diuretics b. Potassium regulation: Normal serum concentration is 3.5 to 5.5 mEq/L i. Potassium is a major intracellular cation (K+) necessary for the maintenance of osmolality and electroneutrality of cells ii. Renal transport sites: K+ is actively reabsorbed in the proximal tubule (60% to 70%) and thick ascending loop (10%); active and passive secretion in the distal tubule and collecting duct maintain the electroneutrality of urine. This electrical gradient is determined primarily by reabsorption of Na+ from urine. iii. Factors enhancing K+ excretion (a) Increase in cellular potassium via increased exchange with Na+ (K+ excreted in urine whereas Na+ is reabsorbed) or via acute metabolic or respiratory alkalosis (causes movement of K+ ions into cells) (b) High-volume tubular flow rates in the distal portion of the nephron: Increase the number of available K+ ions and thus increase the excretion of potassium (c) Aldosterone (provides feedback mechanism for maintenance of K+ in ECF) (1) Elevation of serum potassium stimulates the secretion of aldosterone (2) Aldosterone acts on the distal nephrons and collecting ducts, enhancing the retention of Na+ and excretion of K+ (3) Excretion of excess K+ eventually returns levels to normal (d) Hydrogen ions: Alkalemia (associated decrease in H+) stimulates K+ secretion (e) Diuretics: Loop and thiazide diuretics block NaCl and waste reabsorption, increasing tubular flow and secretion of K+ c. Calcium regulation: Normal serum concentration is 8.5 to 10.5 mg/dl or 2.20 to 2.60 mmol/L i. Major functions of calcium ions (Ca2+): Generation of cardiac action potential and pacemaker function, contraction of cardiac and vascular smooth muscle, transmission of nerve impulses, blood coagulation, formation of bones and teeth, and maintenance of cellular permeability ii. Total serum Ca2+: 40% bound to protein, 50% ionized, and 10% combined with carbonate, phosphate, citrate, and various ions iii. Renal transport sites: 98% of filtered Ca2+ is reabsorbed. Reabsorptive pathways are similar to those for sodium transport. Most active reabsorption occurs in the proximal tubule. Other sites include the loop (20% to 25%) and the distal tubule (10%). iv. Factors influencing Ca2+ reabsorption: (1) Decrease in serum calcium stimulates secretion of PTH (2) PTH stimulates tubular reabsorption of Ca2+ at the distal portion of the nephron, stimulates increased phosphate excretion, and mobilizes calcium and phosphate from bone (b) Vitamin D: Calcium absorption from the small intestine depends on the presence of activated vitamin D (1,25-dihydroxycholecalciferol) (1) Activation process: Absorption of ultraviolet light converts 7-dehydrocholesterol in skin to cholecalciferol. The liver hydroxylates vitamin D to form 25-hydroxycholecalciferol. The kidney further hydroxylates to the final activated form of vitamin D (1,25-dihydroxycholecalciferol) in the proximal tubule. PTH stimulates this activation process. (2) Decreased serum calcium level reduces urinary Ca2+ excretion, so activated vitamin D must be available to absorb Ca2+ from the small intestine to maintain adequate serum calcium levels (c) Corticosteroid effect: Large doses decrease Ca2+ absorption in the intestines; may influence the activation of vitamin D in the liver (d) Diuretic effect: Diuretics can cause Na+ and Ca2+ excretion. Ultimate effect of reduced serum calcium is decreased excretion. A decrease in total body fluid volume leads to diminished GFR and reduced calcium excretion. d. Phosphate regulation: Normal serum concentration is 3.0 to 4.5 mg/dl i. About 90% of phosphate is found in bone, 10% in intracellular and extracellular fluid spaces. Phosphates (PO43−) play significant role in intracellular energy production and may also influence DNA, RNA, and genetic code information. Phosphates are used by the kidneys to buffer H+. ii. Renal transport sites: Reabsorption of phosphate is an active process that occurs in the proximal tubule and requires Na+. Factors influencing phosphate excretion include the following: e. Magnesium regulation: Normal serum concentration is 1.5 to 2.2 mEq/L i. The magnesium ion (Mg2+) is the second major intracellular cation and is a significant factor in cellular enzyme systems and biochemical reactions ii. Mg2+ may have a role in the management of acute myocardial infarction (MI), because magnesium administration decreases the mortality rate in MI by 24% and improves ventricular function by 25%. Benefits may be attributed to magnesium’s ability to enhance coronary blood flow, conserve potassium, improve cellular function, and diminish dysrhythmias. iii. Renal transport site: The reabsorptive process is similar to that of ca2+ and is linked to Na+ reabsorption along the renal tubules iv. Factors influencing reabsorption include the availability of sodium (Na+ is necessary for reabsorption) and the availability of PTH (has minimal effect on Mg2+ reabsorption) f. Chloride regulation: Normal serum concentration is 96 to 106 mEq/L 5. Excretion of metabolic waste products: Excretion is a primary renal function. The kidney excretes more than 200 metabolic waste products. The products measured for interpretation of renal function are blood urea nitrogen (BUN) and serum creatinine. a. Urea: Nitrogen waste product of protein metabolism filtered and reabsorbed along the entire nephron i. Is an unreliable indicator of GFR, because urea excretion is influenced by (a) Urine flow (decrease in urine flow rate may allow for reabsorption of urea) (b) Extrarenal factors (e.g., hypoperfusion states or drugs such as corticosteroids) (c) Gastrointestinal (GI) bleeding or catabolic states such as fever or infection ii. Elevation in BUN level without an associated rise in creatinine level (>25:1 ratio) suggests (a) Volume depletion, low renal perfusion pressure (b) Severe catabolic process or trauma with massive muscle injury (e.g., burns) iii. Elevated levels of both BUN and creatinine (at a 10:1 ratio) indicate renal disease b. Creatinine: A waste product of muscle metabolism i. Amount produced daily is proportional to muscle mass, and production occurs at a constant rate ii. Normal kidney excretes creatinine at a rate equal to RBF or GFR iii. Creatinine is freely filtered, so its production normally equals its excretion, which makes it a reliable indicator of kidney function iv. Elevated serum creatinine level is directly correlated with deterioration in renal function 6. Renal regulation of acid-base balance: The kidneys regulate acid-base balance by minimizing wide variations in body fluid balance in conjunction with retaining or excreting hydrogen ions. Acid-base balance is also regulated by the lungs and the body buffers (serum bicarbonate, blood, and plasma proteins) a. Bicarbonate (HCO3−) reabsorption i. Primarily occurs in the proximal tubule with less in the distal tubule; occurs with reabsorption of Na+ ii. Occurs if the filtrate contains more than 28 mEq/L (Tm) as in acidemia, volume contraction i. Passive secretion occurs in the proximal tubule; active secretion occurs distally in exchange for Na+ ii. Acid is buffered by ammonia (NH3+) or phosphate (HPO42−) before excretion, which provides for hydrogen (H+) excretion without lowering pH iii. H+ secretion is increased during acidemia and decreased during alkalemia c. Renal buffers of hydrogen ions i. Buffers that are filtered by the glomerulus (a) HCO3− is completely reabsorbed (up to 28 mEq/L) (b) Phosphate (PO43−) is secreted and then reacts with hydrogen ii. Buffers produced by the kidney tubule (a) HCO3− can be synthesized in the distal tubule when H+, excreted into urine as HCO3−, is delivered by ECF with Na+. H+ and HCO3− both come from the distal tubule cell as a result of ionization of carbonic acid (H2CO3); thus where CA is carbonic anhydrase (b) Carbonic acid comes from hydration of carbon dioxide (CO2) via CA: (c) CO2 is derived from either cellular metabolism or dissolved CO2 in venous blood; thus new HCO3− can be made in the distal tubule from extraurinary sources d. Summary of renal responses to acidemia i. H+ secretion is increased at the distal tubule with increased excretion of titratable acids (HPO42−) ii. All HCO3− is reabsorbed in the proximal tubule iii. Ammonium is produced to accommodate H+ excretion: NH3+ + H+ E NH4+ iv. Urinary pH can be as low as 4.5 for excretion of a more acid urine in the presence of acidemia e. Summary of renal responses to alkalemia 7. Renal regulation of blood pressure: Renal regulation of BP involves five mechanisms: a. Maintenance of volume and composition of ECF i. Normal plasma volume is essential for control of BP ii. Alterations in plasma volume eventually affect BP. Reduction of plasma volume lowers arterial BP, leading to compensation by vasoconstriction. Expansion of plasma volume increases cardiac preload and, in accordance with Starling’s curve, raises BP. b. Aldosterone–body sodium balance, which determines ECF volume: Aldosterone stimulates renal tubular reabsorption of Na+ in exchange for excretion of primarily K+ ions c. Renin-angiotensin-aldosterone system: Preserves BP and avoids serious volume reduction i. Juxtaglomerular apparatus: Granular cells contain inactivated renin. Factors that trigger juxtaglomerular cells to release renin reflect diminished GFR (e.g., reduced arterial BP in afferent and efferent arterioles, reduced Na+ content or concentration at distal tubule, sympathetic stimulation of kidneys). ii. Renin, an enzyme, is released from juxtaglomerular cells into the afferent arteriole iii. On entering the circulation, renin acts on angiotensinogen to split away the vasoactive peptide angiotensin I and convert it to angiotensin II. Requires the presence of angiotensin-converting enzyme (ACE), found primarily in the lung and liver but also in the kidney and all blood vessels. Angiotensin II is a potent systemic vasoconstrictor. iv. Circulatory effect of angiotensin II on arterial BP (a) Significant peripheral arteriole constriction with moderate venous constriction occurs, which results in the reduction of vascular volume (b) Renal arteriolar constriction results in the renal retention of sodium and water; this expands ECF volume, thus increasing arterial BP v. Fluid volume response to angiotensin II restores effective circulating volume in the following ways: d. Renal prostaglandins: Modulating effect i. Major renal prostaglandins are prostaglandins E2, D2, I2 (vasodilators) and A2 (vasoconstrictor) ii. Physiologic role is modulation, amplification, and inhibition. Vasoactive substances (angiotensin, norepinephrine, bradykinins) stimulate the synthesis and release of prostaglandins. Prostaglandins modulate the action of the vasoactive substances. iii. Prostaglandins diminish arterial BP and increase RBF by arterial vasodilation and inhibition of the distal tubules’ response to ADH. Suppressed ADH response leads to sodium and water excretion, which ultimately decreases the effective circulatory volume. iv. Pharmacologic prostaglandin inhibitors are the nonsteroidal antiinflammatory drugs (NSAIDs). In cases of compromised renal function avoid the use of NSAIDs (i.e., salicylic acid, ibuprofen [Motrin], indomethacin [Indocin], and naproxen [Naprosyn]). v. Loop diuretics stimulate prostaglandin secretion, which leads to vasodilation and decreased preload e. Kallikrein-kinin system: Renal kallikreins are proteases that release kinins and are excreted in the urine. Kinins stimulate both the renin-angiotensin and prostaglandin systems, appearing to link renal hemodynamics and fluid-electrolyte excretion. 8. Red blood cell synthesis and maturation a. Erythropoietin secretion: Stimulates the production of erythrocytes in the bone marrow and prolongs the life of erythrocytes b. Mechanism of erythropoietin synthesis and secretion i. Renal cortical interstitial cells produce erythropoietin, a glycosylated, 165-amino-acid protein ii. Renal erythropoietin production accounts for 90% of RBC production; the remaining 10% is produced by the liver iii. Hypoxia stimulates renal erythropoietin production; the liver is not as responsive to hypoxia and therefore cannot support erythropoiesis in renal failure c. Erythropoietin deficiency: Primary cause of anemia in chronic renal failure (CRF); bleeding is the second most common cause a. Age-related changes can occur as early as 20 to 40 years of age. Changes include a decrease in tubular length and, at and over age 40 years, a progressive decrease in the percentage of glomeruli. Generally, renal function is diminished by 10% at age 65; may diminish further with aging. b. Renal response in the elderly i. Decreased renal mass associated with a diminished number of nephrons ii. Decreased GFR; diminished RBF secondary to age-related changes in vasculature iii. Diminished creatinine production (10 mL/min/1.73 m2 per decade) and diminished ability to excrete creatinine; therefore, change in serum creatinine level may not be evident. Uric acid levels are slightly increased. iv. Decreased serum renin and aldosterone levels reduce the ability to conserve sodium, impair urinary water excretion, and limit urinary concentration i. Previous health problems: Indicate the presence of or predisposition to renal disease (a) Kidney and/or urinary tract disease (1) Hypertension: BP control and treatment may prevent or halt renal damage; hypertension develops in 70% to 80% of patients with advanced renal failure (2) Heart failure with diminished renal perfusion (c) Diabetes mellitus: Renal disease caused by vascular disease alterations, infection, or neuropathy (d) Immunologic disorders, recent infections (streptococcal) (e) Pulmonary disease (Goodpasture’s syndrome) (f) Allergies, recent blood transfusions (history of incompatibility reaction) (g) Other: Toxemia of pregnancy, renal transplantation, anemia, recent surgery, dialysis, exposure to drugs and toxins, renal calculi, azotemia, hematuria, exposure to chemicals or poisons ii. History of specific signs and symptoms (a) Signs and symptoms of urinary tract disorders (2) Abnormal appearance of urine c) Biliuria or bilirubinuria (orange) d) Myoglobinuria (usually clear; red-brown urine; Hematest positive) (3) Urine frequency, urgency, incontinence, hesitancy; nocturia a) Normal volume: Approximately 1500 ml/24 hr b) Oliguria: Less than 400 ml/24 hr c) Anuria: Less than 50 ml to no output over 24 hours d) Polyuria: Excessive output exceeding 24-hour intake e) Nonoliguria: Normal or excess urine volume in the presence of ARF (7) Pain in costovertebral angle, flank, or groin (8) Pattern of weight gain or loss; dry weight is the ideal weight that minimizes symptomatology for a patient with renal failure as achieved by a dialysis treatment b. Family health history: Genetic renal disease accounts for about 30% of azotemia. Genetically transmitted diseases that can cause or precipitate renal disease include the following: i. Cardiovascular disease, hypertension v. Polycystic kidney disease and medullary cystic disease vi. Hereditary nephritis (Alport’s syndrome)
The Renal System
SYSTEMWIDE ELEMENTS
Glomerular hydrostatic pressure (facilitates):
+50 mm Hg
Colloid osmotic pressure (opposes):
−25 mm Hg
Bowman’s capsule pressure (opposes):
−10 mm Hg
Net pressure favoring filtration:
+15 mm Hg
Patient Assessment

The Renal System
Get Clinical Tree app for offline access