Physiological and structural changes
Involution of the uterus
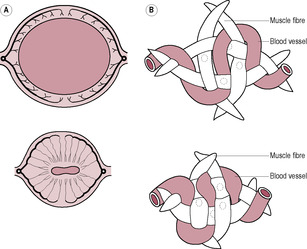
Fig. 14.1
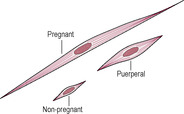
Fig. 14.2
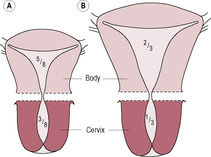
Fig. 14.3
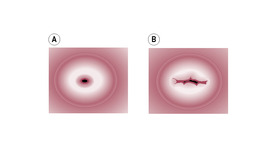
Fig. 14.4
Soft tissue damage and repair
0–3 days
1 week later
6 months later
Lochia
Blood loss
Hormonal changes
The haematological system and cardiovascular changes
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


The puerperium
Zak was born 2 days after his expected date of delivery and was the first son born to his proud parents, Zara and James. Zak was delivered at around 04:30 hours and Zara had laboured in a birthing pool attended by her named midwife. As labour had progressed quickly without complications, the midwife followed Zara’s request for no active management in the third stage of labour. The midwife was unable to accurately estimate Zara’s blood loss due to the waterbirth. Once out of the bath, the midwife assessed Zara’s perineal trauma, which appeared to be a first-degree tear that was not bleeding and did not require suturing.
• How can the midwife effectively assess Zara’s well-being following the delivery, given that she has been unable to estimate Zara’s total blood loss?
• What factors that have occurred during Zak’s delivery will optimize Zara’s transition to parenthood?
The puerperium is sometimes considered to be the ‘Cinderella’ of midwifery and obstetrics as the excitement of the birth is over and after delivery the effects of pregnancy on maternal physiology receive little emphasis. There is not very much research into the timing or mechanisms of the changes in the puerperium. However, the puerperal woman can be very vulnerable to physiological stress, which can become pathological. The midwife’s role is to observe and monitor the early changes and to be able to differentiate between those which are normal and abnormal.
A woman adapts to pregnancy progressively over a period of months, but after childbirth she suddenly no longer needs the physiological changes. During the puerperium, there is a marked decrease in the levels of oestrogen and progesterone within the maternal system. Although the placenta is the main source of progesterone in pregnancy, the corpus luteum continues to produce progesterone for several days into the puerperal period. The fall in concentrations of steroid hormones facilitates the initiation of lactation (see Chapter 16) and allows the physiological systems to return to their prepregnant state. In reality, the puerperium should be described as a transitional phase. It begins at the birth of a child and it ends with the return of fertility. Women do not return to the same physiological and anatomical state, however. The puerperium also, within a social context, represents many transitions for the parents, child and other members of the family. Many of the physiological changes within the puerperium, such as the establishment of parenting skills, lactation and feeding, are modified by the past and present social interactions of the individuals within the new family situation.
Clinical observation and management of the puerperium is essentially based on the return of the uterus to its ‘normal’ size. The puerperium begins as soon as the placenta and membranes are expelled from the uterus together with a substantial proportion of the endometrium. Oxytocin released from the posterior pituitary gland induces strong intermittent myometrial contractions, and as the uterine cavity is empty the whole uterus contracts down fully and the uterine walls become realigned in apposition to each other. The myometrial spiral fibres that occlude the uterine blood vessels (see Chapter 13) constrict the blood supply to the placental site (Fig. 14.1). Uterine vascular resistance increases soon after delivery (Tekay and Jouppila, 1993).
(Reproduced with permission from Sweet and Tiran, 1996.)
About an hour after delivery, the myometrium relaxes slightly but further active bleeding is prevented by the activation of the blood-clotting mechanisms, which are altered greatly during pregnancy to facilitate a swift clotting response. Haemostasis is achieved in three ways:
• Ischaemia
• Pressure: apposition of the uterine walls forming the T-shaped cavity
• Clotting mechanisms.
The midwife has the responsibility to inspect the placenta and membranes to assess that they are complete and that no tissue remains within the uterine cavity. Retained products impede the contraction of the uterus and may be the source of abnormal bleeding and cause secondary postpartum haemorrhage (secondary PPH) as they become the focus of infection. Retained products are often spontaneously voided usually associated with the passing of a blood clot, which facilitates the cleansing of the uterine cavity. Blood clots should always be checked for the presence of placental and membranous tissue.
Immediately after delivery, the uterus weighs about 900–1000 g and the fundus is palpable about 11–12 cm above the symphysis pubis (Howie, 1995) at around the level of the umbilicus. The placental site is raw and exposed. Initially, the uterus is continuous with the vagina with the cervix draping from the body of the uterus. Uterine involution is rapid so 50% of the total mass of the tissue is lost within a week (Howie, 1995). This physiological destruction of most of the uterine tissue is unique in adult life and the mechanisms are not clearly understood. It is suggested that 90% of uterine protein is degraded in the first 10 days of the puerperium (Hytten, 1995). There are rapid and marked changes in collagen and elastin content (Stone and Franzblau, 1995), and water and protein are lost. Involution results from a withdrawal of placental hormones and is thought to be mediated by hydrolytic and proteolytic enzymes released from myometrial cells, endothelial cells of blood vessels and macrophages. Cytoplasmic organelles are autodigested, and intracellular cytoplasm and extracellular collagen are reduced (Howie, 1995). The breakdown of protein from the myometrial cells releases the amino acid components into the circulation and thence into the urine; thus, a puerperal woman is in a state of negative nitrogen balance (see Chapter 12). The myometrial cells are thought to reduce in size (Fig. 14.2) rather than being destroyed and replaced, although there may be an ‘overshoot’ in uterine involution with rebuilding to the resting non-pregnant state. The uterus ultimately returns almost to its prepregnant size (Fig. 14.3), although the proportion of fibrous tissue present in the uterus is progressively increased with successive pregnancies.
(Reproduced with permission from Miller and Hanretty, 1998.)
(Reproduced with permission from Miller and Hanretty, 1998.)
Initially, the cervix is soft, bruised and lacerated following a vaginal delivery (particularly so in primiparae and with premature labour). The cervix rapidly reforms and closes; by the end of the first puerperal week it will admit one finger. However, the cervix never returns to its original state and always shows evidence of parturition. The external os reforms with a slit rather than the nulliparous dimple (Fig. 14.4).
(Reproduced with permission from Miller and Hanretty, 1998.)
The uterus involutes quite quickly, initially at about 1 cm/day; thus by the tenth day it should no longer be palpable above the symphysis pubis. Involution is slower in women who have undergone lower-segment caesarean section (LSCS), but it can be judged by a detectable decrease in fundal height. Subinvolution (a slow rate of uterine involution) may indicate retained products of conception (ERPC) and/or a secondary infection, which is usually found in conjunction with continued lochia rubra that may have an offensive odour. The uterus should be well contracted, hard and central; if it is higher than the umbilicus and soft on palpation (often described as ‘boggy’) then this may also indicate the presence of infection. The endometrial cavity is a potential space in non-pregnant women but gas is commonly detected in the endometrial cavity puerperally (Hytten, 1995).
The initiation of breastfeeding and the infant suckling in the early puerperium augments stimulation of oxytocin release. The oxytocin stimulates further contraction of the myometrium and so uterine evacuation. Involution of the uterus in breastfeeding mothers is more efficient. ‘After-pains’ associated with lactation are often experienced, particularly, by multiparous women who often complain of increased vaginal loss while feeding. Initially, oral analgesia such as paracetamol may be offered but the intensity of the pain usually subsides after about 24 h because expression of myometrial oxytocin receptors is reduced as a result of oestrogen withdrawal.
The superficial layer of the decidua becomes necrotic and is shed in the lochia in the first few days of the puerperium. The epithelium rapidly regenerates, re-forming an intact layer over most of the surface within 7–10 days of delivery. The placental site takes 3 weeks or longer to recover. The endometrium regenerates from the basal layers and grows in from the margins of the placental wound site and from glandular remnants within it (Howie, 1995).
It is not uncommon for soft tissue damage to occur during the delivery of a baby. Trauma to the female genital tract is described as follows:
• Superficial—this usually describes grazes to the skin where the epidermis has split owing to pressure of distension. These require no treatment; however, they often cause discomfort through stinging because of the disruption of the many nerve endings found within the superficial layer of the tissue. Voiding of urine can also be uncomfortable as the urine comes in contact with the grazes.
• First degree—this describes a tear in the skin and underlying superficial tissues (not including the muscle). Often the wound will heal spontaneously as the skin edges are usually in apposition. Ragged tears may result in the formation of excess scar tissue which can cause dyspareunia (pain during intercourse). Tears on the labia minora, a well-innervated area, can cause a lot of discomfort. If bilateral tears are present, suturing needs to be considered as the labia may fuse together if the tears are in close apposition forming a band of tissue over the vaginal opening.
• Second degree—when a tear involves perineal muscle damage it is described as being second degree. Usually these wounds are sutured to aid healing. Simple second degree tears are usually in the midline and involve one line of tearing. Some second degree tears can be complex with more than one tear line radiating in both lateral and downward directions involving larger amounts of muscle trauma.
• Episiotomy—this is a surgical incision to enlarge the introitus to facilitate the delivery of the baby which used to be thought to be beneficial and so done routinely. It falls into the same category as the second-degree tear. Although episiotomies can be performed in the midline, because of the increased risk of extension to a third degree tear and anal sphincter rupture they are usually performed to the side (mediolateral).
• Third degree—here the muscle of the anal sphincter is involved. Obstetric repair is essential so that the sphincter activity of the muscle is restored thus avoiding complications of faecal incontinence at a later time.
• Fourth degree—when the tear is extensive, the anal sphincter may become completely divided and the tear continues through the rectal mucosa. Specialist surgical repair is required to ensure the resumption of normal anal function.
Repair to the perineum involves the clinician suturing the perineum. There is a wide variety of suture materials and techniques for repair; however, suturing aims to achieve the following:
• Haemostasis—this is to ensure that any active bleeding points are ligated to minimize blood loss and the postnatal complication of a haematoma (formation of a blood clot within the wound) which can be extremely painful.
• Alignment—this is to bring the tissues back into alignment to optimize healing and to achieve a near pre-tear condition. If wounds are left gaping, alignment may not occur and as healing is by granulation this can result in the formation of scar tissue. This can result in a rigid misshapen perineum, which can cause dyspareunia (pain on intercourse).
The majority of perineal traumas can be described as being deep wounds as the tissue trauma involves layers below the epidermis and the dermis. Wound healing occurs in three phases: inflammation, tissue formation and tissue remodelling (see also Box 14.1). Some features of wound healing are common to all tissues; others are specific to the tissue involved. For instance, granulation tissue does not develop in the endometrium and the wounds do not heal with scarring. In this respect, there are similarities with fetal wounds which also heal without scarring suggesting that the process of endometrial remodelling is more a developmental mechanism than merely repair.
1. First is the inflammatory response; inflammation is a normal reaction to tissue trauma. Perineal inflammation can initially cause great discomfort for women in the very early postnatal period. An analgesic such as diclofenac sodium is useful as it acts as an anti-inflammatory agent (though it should be used with caution if the woman is asthmatic). Paracetamol and codeine-based products can also be used but codeine can cause or exacerbate bowel constipation so appropriate advice is required. However, a degree of inflammation is vital to ensure tissue healing, so analgesics should be used only when the response is severe and perineal pain restricts normal activity. The inflammation acts to isolate the damaged tissues, reducing the spread of infection. White blood cells, such as neutrophils and macrophages, invade the tissue owing to the increased vasodilatation in the surrounding blood vessels. These cells ingest any invading bacteria and break down any necrotic tissue within the wound.
2. The migratory phase involves the infiltration of the wound by mesenchymal cells that form fibroblasts, initially creating a scab over the open wound site. Following this, blood vessels grow into the wound and the wound is gradually filled from the bottom up by new tissue growth called granulation tissue.
3. There then follows a proliferative phase where epithelial cells grow under the scab. It concludes with the maturation of the new cells and the shedding of the scab.
Box 14.1
• Blood clot forms, reinforced with fibrin fibres.
• Acute inflammatory response occurs: polymorphs and macrophages migrate to site; high-protein exudate leads to local oedema.
• Eschar dries out, hardens and eventually becomes detached.
• Wound contracts.
• Mitotic activity occurs in epidermal cells, which migrate over living tissue.
• New blood capillaries form from endothelial buds, bringing nutrients to healing tissue.
• New connective tissue, formed by fibroblasts, supports capillary loops.
• Surface depression may still be visible at wound site; scar tissue becomes paler.
• Epithelialization is complete.
• Connective tissue is reorganized, less vascular and stronger.
The initial vaginal loss is termed the lochia rubra and consists of blood that has collected within the reproductive tract together with autolytic products of degenerated necrotic decidua from the placental site and any trophoblastic remains. The outward flow of blood lost at delivery and the subsequent discharge of lochia are important in removal of potential sources of ascending infection and, thus, protection of the placental wound site. The alkalinity of the lochia is also important in protecting the vulnerable site. Lochia is the normal discharge in the puerperium; it has a characteristic sweetish smell unless there is an infection.
Lochia may be described by its visual appearance (Box 14.2); normally, the lochia lightens progressively in both volume and colour. However, at about day 7 after delivery, the fibrinous mesh deposited over the placental site may be shed as part of the normal healing process so the vaginal loss may be transiently heavier and flushed with fresh blood. By day 10, the lochia is normally scant and pink in colour although discharge of lochia may persist for up to 6 weeks. Prolonged duration of lochia discharge suggests the placental wound site is not completely epithelialized or that the woman has some retained debris which is still disintegrating (Hytten, 1995). The duration of lochia discharge tends to be longer with the first pregnancy and is also related to birth weight.
Box 14.2
• Lochia rubra (red)
– decidua and frank blood loss from placental site
– initially sterile then uterus begins to be colonized by vaginal flora
– red colour persists for about 3 days
• Lochia serosa (pink/brown)
– contains leukocytes, mucus, vaginal epithelial cells, necrotic decidua, non-pathological bacteria
– may be blood stained for 3–4 weeks
– characteristic sweetish odour
• Lochia alba (yellow–white)
– mostly serous fluid and leukocytes
– plus some cervical mucus and microorganisms
Heavy discharge of lochia with an offensive odour, maternal pyrexia and/or a feeling of general malaise can all indicate possible intrauterine infection. If the lochia remains abnormally heavy and further bleeding occurs, dilatation and curettage (D&C) to empty the uterine cavity may be necessary. The procedure is also termed evacuation of ERPC. The cervix is dilated and the retained products are scraped from the decidua. This procedure is not without complications, however. Excessive scraping can damage or remove the entire endometrium. If the basal layer of the endometrium is removed (see Chapter 2) then proliferation during the menstrual cycle fails to occur, affecting fertility; this is termed Asherman’s syndrome.
Excessive blood loss, that is, more than 500 mL or any amount that jeopardizes the well-being of the mother, at and within 24 h of delivery is termed a primary postpartum haemorrhage (primary PPH). It is usually caused by failure of the myometrium to contract completely, or failure of the blood-clotting mechanisms, or both (see Chapter 13); PPH may be very serious (Box 14.3, Case study 14.1). Women may also loose significant amounts of blood from trauma to the genital tract and perineum. If there is excessive bleeding but the uterus is well contracted, examination of the genital tract and perineum should not be delayed to identify bleeding points and any trauma repaired as quickly as possible to minimize blood loss.
Box 14.3
Disseminated intravascular coagulation (DIC) is a condition caused by abnormal activation of the clotting mechanisms. The blood-clotting factors are induced on a wide basis resulting in fibrin deposits being produced that line the major part of the vascular bed. Once this has occurred, bleeding continues owing to the absence of clotting factors, which were exhausted during the DIC phase and the activation of fibrinolysis. Liver dysfunction occurs in pre-eclampsia and may be associated with DIC and microangiopathic haemolysis (erythrocyte breakdown in small blood vessels). The acronym HELLP refers to Haemolysis, Elevated Liver enzymes and Low Platelet counts.
DIC is an extremely severe condition. Although such a life-threatening case would normally be managed by intensive care staff rather than by the midwifery unit, it is important that midwives are able to recognize the symptoms and implications of DIC, for example, the appearance of bruising on the skin not associated with trauma. In advanced cases, the observation of the failure blood to clot is significant of advanced DIC and warrants immediate intervention as this indicates a critical life-threatening situation.
Lucy is a 35-year-old primigravida who is delivered by emergency LSCS at 30 weeks’ gestation owing to fulminating pre-eclampsia. Following delivery, the blood loss per vaginum is noted to be quite brisk and a Syntocinon (oxytocin) infusion is commenced in an attempt to control the bleeding. On investigation, it is discovered that Lucy’s platelet count is extremely low and that the clotting time for her blood is greatly prolonged. A provisional diagnosis of DIC secondary to HELLP syndrome is made.
• What predisposing factors may have contributed to Lucy’s condition?
• What intervention would Lucy require and what care would she need following this diagnosis?
Occasionally, there may be concealed bleeding, either into the peritoneum from ruptured blood vessels in the broad ligament or into the tissues forming large collections of blood clots called haematomas. Therefore, even in the absence of visible blood loss, women can still be physically in shock if concealed bleeding is present.
The risk of primary PPH is lower 24–72 h following delivery, but until involution of the uterus is complete there is a risk of a secondary PPH if there is an infection within the uterine cavity. The bleeding is usually due to the fibrinolytic action of bacteria such as haemolytic streptococcus. These bacteria are usually anaerobes (able to thrive in the absence of oxygen) and so specific antibiotic treatment with antibiotics such as metronidazole may be required.
In late pregnancy, most of the steroid hormones are derived from the placenta, although the corpus luteum and ovary continue to contribute some progesterone. Levels of progesterone and oestrogen fall to non-pregnant levels within 72 h of delivery. The placental protein hormones have a longer half-life so plasma levels fall more slowly. During pregnancy, production of the gonadotrophins is suppressed. Follicle-stimulating hormone (FSH) levels are restored to prepregnant concentrations within 3 weeks of delivery, but restoration of luteinizing hormone (LH) secretion takes longer, depending on the duration of lactation. Levels of oxytocin and prolactin also depend on lactational performance.
The blood lost at delivery, accepted to be about 300–500 mL normally and about 1000 mL in caesarean sections, is adequately compensated for by the increase in blood volume acquired during pregnancy (see Chapter 11). Women can lose about 1000 mL of their predelivery blood volume before postnatal haemoglobin concentration is compromised (Letsky, 1998; Case study 14.2). Erythropoiesis isstimulated before and after delivery (Richter et al., 1995). Diuresis further decreases plasma volume in the first days, although as interstitial fluid is mobilized subsequently the plasma volume tends to increase transiently causing haemodilution of both haemoglobin and plasma proteins, such as clotting factors. It is this variability in blood lost at delivery and restoration of normal water balance that may result in raised concentrations of clotting factor and hypercoagulability. The tendency to coagulate is also affected by the loss of placental and fetal factors affecting clotting and water regulation (Blackburn, 2007).
Prior to delivery, Megan had a haemoglobin (Hb) concentration of 10.1 g/dL. At delivery, her blood loss is estimated at around 1000 mL. The midwife is quite concerned over this although Megan was asymptomatic. Prior to discharge on day 3, Megan’s Hb concentration is rechecked and is estimated at being 9.8 g/dL.
• How can you account for the Megan’s Hb concentration being relatively stable despite her suffering a postpartum haemorrhage?
• What advice/treatment would you give to Megan following her discharge?
Haemoglobin levels return to normal prepregnant levels within 4–6 weeks and white blood cell numbers fall to normal within a week of delivery (Blackburn, 2007). Platelet number increases in the first few days following delivery, thereafter falling gradually to prepregnant levels. Fibrinolytic activity is maximal for about 48 h after delivery in response to the removal of the placenta, which produces fibrinolytic inhibitors (Lanir et al., 2003). Clotting factors, which peaked in labour, gradually decrease. The net result is that the hypercoagulable state of pregnancy is increased in the early puerperium and then slowly returns to a prepregnant state over a few weeks. This period of prolonged hypercoagulability is why women are at significantly increased risk of thromboembolic episodes in the postnatal period.
In previous eras, wealthy women were advised to rest in bed after childbirth and received indulgent cosseting (Hytten, 1995). However, trials of early ambulation led to early mobility being highly recommended as it facilitates improved vena caval blood flow and rapid disposal of oedema and thus optimizes cardiovascular health. Mobilization is essential to optimize venous return and avoid stasis within the vascular bed, in order to minimize the risk of deep vein thrombosis (DVT) formation (see p. 373). Women who are unable to mobilize owing to obstetric complications, such as an LSCS, are given prophylactic treatment as the risks of DVT and complications are much increased. Women are advised to report any discomfort or swelling in the lower legs as this may indicate DVT formation (especially if one leg appears more swollen than the other although bilateral DVTs are possible); the risks of DVT progressively diminish.
The cardiovascular system is rendered transiently unstable by delivery owing to the blood loss and the ensuing compensatory mechanisms. During the brief period of instability of fluid balance in the first week after delivery, many women experience headaches. Initially, there is a marked increase in cardiac output as the uteroplacental flow is returned to the venous system and the gravid uterus no longer impedes the vena cava blood flow. This is augmented by the mobilization of extracellular fluid. Although pregnant women are normally able to tolerate normal blood lost at delivery, those women who had decreased vascular expansion during pregnancy, such as those with pre-eclampsia (see Chapter 11), may be less able to tolerate blood loss. Vaginal delivery is associated with a higher haemoglobin concentration than operative deliveries because vaginal delivery tends to have less blood loss and to promote diuresis more markedly (Blackburn, 2007).
Get Clinical Tree app for offline access
