Function
Placental Role
Respiration
Maternal oxyhaemoglobin dissociates in the intervillous spaces. O2 diffuses through the walls of the villi where it binds to fetal haemoglobin forming fetal oxyhaemoglobin. Transfer is increased by the higher affinity of fetal haemoglobin for O2 (see Chapter 15). The lower CO2 level facilitates transfer of CO2 in the reverse direction in pregnancy (see Chapter 11)
Nutrition
Active transport of glucose, iron and some vitamins and passive transport of other nutrients. The placenta can metabolize proteins, fats and carbohydrates into simple molecules. Fats cross the placenta with less ease so the fat-soluble vitamins (A, D, E and K) cross slowly. The placenta stores glycogen, which can be converted to glucose when required
Excretion
Waste products of metabolism, CO2 and heat cross from the fetus to the mother
Protection
The placenta acts as a barrier against most bacteria (such as cocci and bacilli). However, smaller micro-organisms (such as the syphilis bacterium) and viruses (including rubella, varicella-zoster, cytomegalovirus, coxsackie and HIV) can cross the villi. The placenta transfers IgG antibodies (see Chapter 10) and Rhesus antibodies to the fetus. Drugs including teratogens (see Chapter 9), anaesthetics and carbon monoxide (from smoking) can cross the placenta
Endocrine role
Initially, the trophoblast produces hCG, which maintains the corpus luteum and its production of steroid hormones. From the third month onwards, oestrogen and progesterone are produced in large quantities by the placenta. hPL is produced from the syncytiotrophoblast. The placenta also produces a broad range of other hormones including corticosteroids, ACTH, TSH, IGFs, prolactin, relaxin, endothelin and prostaglandins
Immunological role
The trophoblast has unique immunological properties that render it immunologically inert so a maternal antigenic response does not occur (see Chapter 10)
Uterine receptivity
Implantation
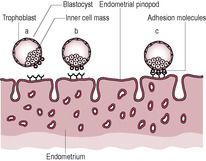
Fig. 8.1
Differentiation into cytotrophoblast and syncytiotrophoblast
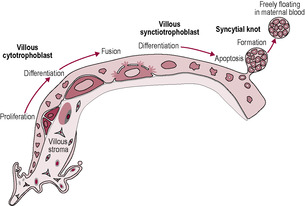
Fig. 8.2
Extravillous cytotrophoblast and remodelling of the uterine vessels
Cytotrophoblast migration and invasion
Spiral artery conversion
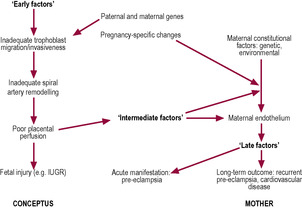
Fig. 8.8
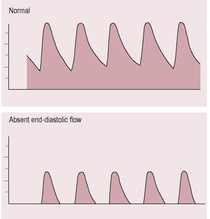
Fig. 8.9
Vascularization of the placental villi
Development of the discoid placenta and chorionic membrane
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


The placenta
At Zara’s 20-week scan, the ultrasonographer documented in her report that the placenta was situated on the anterior wall of the uterus with the majority of the placental body situated in the middle and lower pole of the uterine body with the lateral edge of the placenta approximately 2 cm from the internal cervical os.
• What are the possible complications that could arise from this situation?
• How do you think the midwife should discuss these possible complications with Zara and how they could be recognized?
• Why is it common for women with this situation to have vaginal bleeding around 34 weeks of pregnancy?
• Are there any conditions or factors related to low lying placentas and if so how can these be managed?
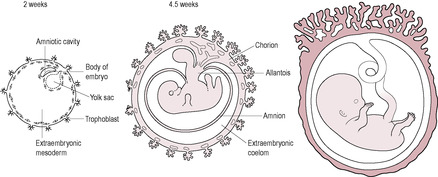
The placenta and the chorion (outer membrane) are derived from the trophoblast layer of blastocyst cells (see Fig. 6.6, p. 128). Other extraembryonic tissues develop from the inner cell mass. These include the amnion (inner membrane), the yolk sac, the allantois (a largely vestigial structure in humans) and the extraembryonic mesoderm. The umbilical cord and the blood vessels of the placenta are derived from the extraembryonic mesoderm.
The human placenta is haemochorial, which means that maternal blood comes into contact with the placental trophoblast cells. The uteroplacental unit is made up of both fetal and maternal components. The placenta as seen at delivery is just the fetal component or chorionic plate. The maternal component or basal plate is the placental bed which underlies the fetal component and the uteroplacental circulation that vascularizes the placental bed. Between the chorionic and basal plates is the intervillous space where the maternal–fetal exchange occurs. Conversion of the maternal spiral arteries to dilated and flaccid vessels is an essential step for successful pregnancy. Abnormal placental function is strongly associated with fetal complications, but study of the human placenta, particularly the maternal component, is not easy. Placentation in the human is unique, which means that observations from other species can be applied to humans only with caution. Placental reserve needs to exceed fetal requirements (otherwise the fetus could be compromised under conditions of hypoxia). It might be expected that placental size would increase in parallel with increased fetal size; however, the placental:fetal weight ratio actually decreases during gestation (Kingdom et al., 1993). Placental efficiency is best described as grams of fetus produced per gram of placenta developed (Wilson and Ford, 2001). Indeed, as there is a relationship between size at birth and life expectancy which is associated with the placental supply of nutrients; lighter placentas are more efficient placentas and associated with better long-term health outcome (Fowden et al., 2009). Placental efficiency is affected by the surface area for exchange, the thickness of the barriers between fetal and maternal circulations and the arrangement of the fetal and maternal blood vessels (‘vascular architecture’). It is the later variable that appears to account for species differences in placental efficiency. The most efficient human placentas are those which are small in diameter and thin; it is thought that these small placentas must functionally adapt to increase nutrient transporter abundance and become more efficient.
Instead, placental efficiency is enhanced by an increase in both the number of carrier proteins involved in the transport of substances across the placenta and the placental perfusion.
The first phase of the development of uterine receptivity is regulated by oestrogen and progesterone which stimulate the presence of microvilli on the columnar epithelial cells of the endometrium. Smooth muscle myosin also increases and the stromal cells proliferate. The second phase is described as the blastocyst response phase (Banerjee and Fazleabas, 2010). The signals involved in the exquisitely sensitive dialogue between the embryo and the endometrium include human chorionic gonadotrophin (hCG), interleukin-1 and insulin-like growth factor (IGF) 2 (see Chapter 3). In the third phase following attachment and implantation, signals between the newly formed embryo and primed endometrium cause endometrial changes, or decidualization, whereby the stromal cells under the endometrial epithelium accumulate lipid and glycogen and become known as decidual cells. The stroma thickens and blood flow increases. Decidualization promotes changes in the endometrium that make it receptive to implantation. Synchronous development and communication between the maternal endometrium and the embryonic tissue are required for successful establishment of pregnancy. Whereas mammalian embryos have intrinsic invasive potential and can initiate implantation-type reactions in many different tissues, the endometrium protects itself from implantation except for the limited duration of the implantation window (Bischof et al., 2000).
During the implantation or nidation window, microvilli on the surface of the uterine endometrial cells fuse together to form single flower-like projections called pinopods or uterodomes (Murphy, 2000). These smooth bleb-like protrusions which lack the typical microvilli form under the influence of progesterone (during the mid-luteal phase) only in the preferred sites of embryo–endometrial interaction and thus act as markers of uterine receptivity (Usadi et al., 2003). The pinopods are only present for 2–3 days during which implantation must occur. The interaction between the endometrium and the developing trophoblast is facilitated by a number of cytokines, metalloproteinases, surface integrins and growth factors, including IGFs and their binding proteins, which create a specific microenvironment which modulates trophoblast function. It is evident that implantation is a rather inefficient process in the human; the probability of conception during a menstrual cycle (defined as fecundity) is about 30% and over three quarters of failed pregnancies are thought to be due to implantation defects (Wilcox et al., 1999).
Implantation is the consequence of a well-organized sequence of events involving synchronized crosstalk between the receptive endometrium and a functional blastocyst (Achache and Revel, 2006). It occurs in three stages: apposition, adhesion and invasion. The trophoblastic cells overlying the inner cell mass are known as the polar trophoblast; it is these cells that initiate the adhesion and implantation processes. The morula enters the uterus about 4 days after fertilization. It may float freely in the uterus before it hatches out of the protective zona pellucida (Kingdom and Sibley, 1996). About 7 days after fertilization, the blastocyst hatches and comes into contact with the endometrium. The blastocyst rolls freely over the endometrium until it reaches a receptive area. This process is thought to be mediated by glycoproteins called selectins which are expressed on the polar trophoblast cells of the newly hatched blastocyst (Vitiello and Patrizio, 2007). The blastocyst then orientates itself so that the embryonic pole implants first. Inappropriate implantation of the blastocyst so there is reduced contact between the polar trophoblast and the uterine endothelium is thought to lead to abnormalities of umbilical cord insertion and even failure of pregnancy (Huppertz, 2008). The endometrium produces MUC1, a mucin-rich glycoprotein, to prevent the blastocyst adhering to areas of the endometrium with poor chances of implantation. The optimally receptive areas of the endometrium secrete chemokines and growth factors to attract the blastocyst to the pinopods. The apposition of the blastocyst to the endometrium triggers the production of adhesion molecules such as integrins and cadherins which firmly anchor the blastocyst to the endometrial pinopods (Fig. 8.1). This process is enhanced because the endometrial surface expresses receptors for selectins. The tethering of the blastocyst to the endometrium stimulates the polar trophoblastic cells to undergo rapid mitosis and proliferate as the invasion of the uterine wall commences. Implantation may be affected by maternal antiphospholipid syndrome hence the high incidence of pregnancy loss associated with this condition (Stone et al., 2006) (Case study 8.1).
(Reproduced with permission from Achache and Revel, 2006.)
Trudy is a 36-year-old, para 1, gravida 11. She attends the midwives clinic at 6 weeks gestation, very distressed as this was not a planned pregnancy. Trudy informs the midwife that her last baby was born at 32 weeks gestation, very small for dates and that Trudy had also developed fulminating pre-eclampsia which was the main reason for the early delivery. Three days after this delivery Trudy developed severe difficulty in breathing which was diagnosed as a pulmonary embolism. Trudy then informs the midwife that all her other pregnancies had been spontaneous miscarriages at around 10 weeks gestation.
The midwife reviews Trudy’s post obstetric notes and discovers that Trudy has antiphospholipid syndrome and that at her last delivery it was documented that the placenta was small and infarcted. As a result of this, the midwife immediately refers Trudy to attend a consultant clinic as an emergency.
• Why did the midwife refer Trudy to the consultant as an emergency?
• What treatment would be offered to Trudy and how will the pregnancy be managed?
• What is the significance of the placental infarcts and what was the most likely cause?
There are two distinct cell layers in the blastocyst (see Chapter 6): the inner cell mass which is surrounded by an outer sphere of a single layer of mononucleated trophoblast cells. The trophoblast is the first cell type to differentiate; this outer layer rapidly proliferates and develops into the placental tissue and fetal membranes.
The trophoblast differentiates into two layers: the outer syncytiotrophoblast and the inner cytotrophoblastic layer. Some of the proliferative cytotrophoblast cells lose their cell membranes and coalesce to form a multinucleated syncytium (a united mass of fused cellular material): the syncytiotrophoblast (Fig. 8.2). This outermost layer of the placenta has little proliferative and transcriptional activity; the maintenance and growth of the syncytiotrophoblast throughout gestation is dependent on the incorporation of cytotrophoblast cells into the layer. Apoptosis (programmed cell death) of trophoblast tissue increases throughout pregnancy as a normal part of trophoblast turnover and syncytiotrophoblast formation. The nuclei of the cells newly incorporated into the syncytiotrophoblast are initially similar to the nuclei of the cytotrophoblast cells but then undergo morphological changes; the chromatin condenses so the nuclei become smaller and denser eventually resembling late apoptotic nuclei. Some of these nuclei aggregate and are packaged into syncytial knots which are shred from the apical surface of the syncytiotrophoblast into the maternal circulation. In a normal pregnancy, these syncytial knots can be identified in maternal blood in the uterine veins but are destroyed in the maternal pulmonary vessels. In pre-eclampsia, syncytiotrophoblast renewal is overactive; there is an increase in apoptosis often complicated by aponecrosis. The syncytial knots are more prominent and are smaller so they can survive the maternal pulmonary vasculature and trigger a maternal systemic inflammatory response including an activated endothelium and increase in proinflammatory markers (Hawfield and Freedman, 2009).
(Reproduced from Huppertz, 2008.)
The surface of the syncytiotrophoblast is covered in microvilli which increase the surface area. The syncytiotrophoblast expresses transporters, enzymes and receptors on its surface. It also produces hCG and hPL which are crucial to the maintenance of the pregnancy. hCG enhances differentiation of cytotrophoblasts into the syncytiotrophoblast. Electron microscopy reveals the syncytiotrophoblast to be a mass of cytoplasm containing remnants of intercellular membrane, evenly dispersed nuclei and a few intermediate cells. This syncytial organization of cells is unusual; other than the trophoblast, multinucleated cells are seen only in some tumour cells and inflammatory giant cells (Chard, 1998). Interestingly, a range of tumour cells appears to secrete hCG (Iles and Chard, 1991) but at lower concentrations than those characteristic of trophoblast cells; these tumours usually affect individuals late in life.
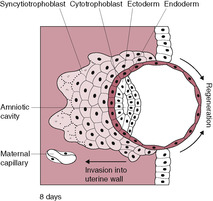
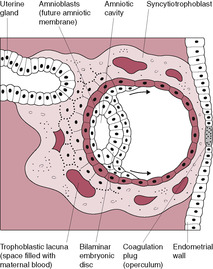
The cytotrophoblasts, which form the inner monolayer of placental stem cells, are large clear discrete cuboidal cells each with a single nucleus, a few organelles and a well-defined cell membrane. These cells have marked mitotic activity and DNA synthesis. The syncytiotrophoblast increases in volume throughout the second week as cells detach from the proliferating layer of cytotrophoblast and fuse with the mass of syncytiotrophoblast. The syncytiotrophoblast has an invasive phenotype, secreting enzymes, which attack the endometrium, and hormones, which sustain the pregnancy. It is also involved in absorption of nutrients. The syncytiotrophoblast invasion is aggressive; between 6 and 9 days postfertilization the embryo becomes completely implanted into the endometrial stroma. The hydrolytic enzymes produced cause breakdown of the extracellular matrix between the cells of the endometrium thus eroding a pathway. The surface of the syncytiotrophoblast has tiny processes extending from it that penetrate between the endometrial cells, pulling the conceptus into the uterine wall. As implantation progresses, the expanding syncytiotrophoblast gradually envelops and encircles the blastocyst. The endometrial epithelium regenerates over the site of implantation, forming the decidua capsularis (Fig. 8.3). By 9 days, the embryo is completely embedded within the endometrial wall with the syncytiotrophoblast forming a complete mantle around the entire conceptus so it is the only embryonic tissue in direct contact with the maternal tissue; this is important in protecting the embryo from rejection, The syncytiotrophoblast has a developmental gradient (Huppertz, 2008); it is thicker and better developed over the embryonic pole. Implantation is complete by about 10–12 days after fertilization. A plug of a cellular material called the coagulation plug or operculum seals the small hole at the point of implantation (Fig. 8.4).
In the first week of development, as the free-floating embryo or conceptus moves towards the uterine cavity propelled by the cilia movement and muscular contraction of the uterine tube, the cells can obtain nutrients from secretions of the uterine tubes and endometrium and eliminate waste products by simple diffusion. It is thought that endometrial secretions of lactate and pyruvate and possibly some amino acids may be important in the nutrition of the embryo for up to 8 weeks (Burton et al., 2001). The nutrients and oxygen are taken up by the syncytiotrophoblast; this is known as histiotrophic nutrition. By the end of the first trimester, there is a transition to haemotrophic nutrition from the uteroplacental circulation which provides a system in which the maternal and fetal circulations come into close contact to facilitate transfer of substances from one system to the other.
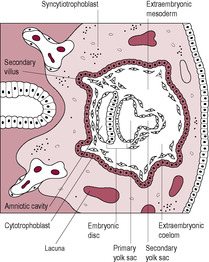
As the syncytiotrophoblast penetrates the uterine wall, it comes into contact with the maternal endometrial capillaries and superficial veins. Fragments of these are engulfed within the syncytiotrophoblast forming fluid-filled trophoblastic lacunae (literally ‘little lakes’); these coalesce to form larger lacunae which are the precursors of the intervillous spaces. As maternal blood vessels are progressively invaded, the lacunae fill with maternal blood. Maternal capillaries near the syncytiotrophoblast expand to form maternal sinusoids which rapidly anastomose with the trophoblastic lacunae. As this development continues, the lacunae become separated by columns of syncytiotrophoblast, or trabeculae, which effectively form a framework on which the villous tree of the placenta develops. The trabecular columns project radially from the blastocyst. The cytotrophoblast at the core of the columns proliferates locally to form extensions, which grow into the columns of syncytiotrophoblast. The growth of these protrusions is induced by the newly formed extraembryonic mesoderm (Fig. 8.5). The result is the primary stem villus, an outgrowth of cytotrophoblast covered by syncytiotrophoblast, which penetrates into the blood-filled lacuna (Fig. 8.6).
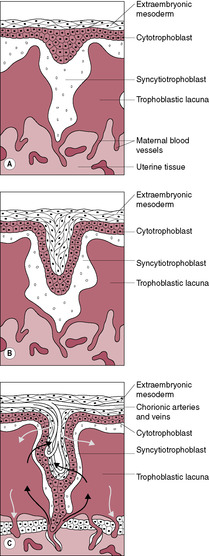
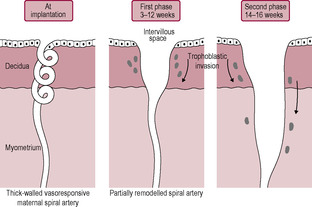
The cytotrophoblast layer has several distinct roles: (1) acting as proliferative progenitor or stem cell layer to generate and construct the developing syncytiotrophoblast which covers the villi; (2) forming the proliferative cell columns of the anchoring villi; (3) detaching from the cell columns and migrating into the maternal stroma to form interstitial cytotrophoblasts; and (4) migrating as non-proliferative extravillous cytotrophoblast cells to remodel the spiral arteries and replace the maternal endothelial cells (Knofler, 2010). These extravillous cytotrophoblast cells migrate from the villi beyond the leading edge of syncytiotrophoblast into the stroma. From about 12 days postfertilization, these cells invade the maternal capillaries and spiral arteries of the decidua. The extravillous cytotrophoblast cells initially plug the lumen of the maternal vessels that have been invaded and subsequently replace the maternal endothelium of these vessels. Plugging of the lumen of the invaded maternal blood vessels prevents bleeding and is achieved by day 14, which coincides with the expected date of the next menstrual period. If the maternal vessels are not plugged adequately during implantation and early development then vaginal bleeding may occur, which is associated with an increased risk of spontaneous miscarriage (sometimes haematomas can be seen on ultrasound investigation). The plugs prevent flow of maternal blood into the intervillous space in early pregnancy but allow a slow seepage of plasma (Jauniaux et al., 2003) so the lacunar spaces enclosed by the syncytiotrophoblast initially contain exudate from maternal vessels rather than blood. The developing placenta forms an effective barrier between the mother and developing embryo that persists up to 10 weeks’ gestation when the trophoblastic plugs are dislodged and intervillous blood flow is established (Jauniaux et al., 1992). It is at this time that peak hCG secretion occurs (Meuris et al., 1995). The increasing oxygen level and concomitant oxidative stress also stimulate cytotrophoblast proliferation and differentiation and the increased expression of antioxidant enzymes. Doppler ultrasound shows there is no intervillous blood flow in normal pregnancies before this period and oxygen electrodes have demonstrated that an oxygen gradient exists across the placenta and decidua. This means that embryogenesis occurs in a relatively hypoxic environment. In fact, maternal–placental blood flow has been observed in the first trimester in a number of non-viable pregnancies but it is not clear whether this is a cause of the pregnancy failure or an effect (Kingdom and Sibley, 1996). The developing embryo is thought to be particularly vulnerable to damaging oxygen-free radicals during the sensitive period of organogenesis and the first-trimester placenta has limited antioxidant capacity; thus, limiting fetal exposure to oxygen may be protective. The differentiation of trophoblast cells is influenced by the local oxygen levels; hypoxia promotes trophoblast proliferation and normoxia inhibits proliferation and induces migration (Knofler, 2010). In most other mammalian species, organogenesis is complete and embryonic development is advanced before placental attachment. But in the human, implantation is highly invasive so the precocious conceptus is embedded in the uterine wall even before the primitive streak is evident (Jauniaux et al., 2006). Hence it is much more sensitive to oxidative stress. Embryonic and placental cells are particularly vulnerable because they are undergoing extensive cell division and DNA replication. The syncytiotrophoblast is exposed to the highest concentration of oxygen as it is closest to the maternal blood but it has low levels of antioxidant enzymes. Maternal metabolic disorders such as diabetes generate more oxidative free radicals and are associated with a higher incidence of miscarriage, vasculopathy and fetal structural defects which is thought to be due to oxidative stress (Jauniaux et al., 2006).
Between the 4th and 16th week of gestation, villus growth and considerable remodelling of the placenta occur, including remarkable changes in the maternal blood vessels underlying the fetal placenta ensuring that the spiral arteries are capable of delivering large volumes of blood to the placental intervillous spaces at an appropriate rate and pressure to protect the delicate fetal villi perfused by the low-pressure developing fetal circulation which are immersed in maternal blood which circulates at a much higher pressure and velocity. Failure of the transformation of the spiral arteries is associated with a number of common complications of pregnancy including pre-eclampsia, fetal growth restriction, recurrent first and second trimester losses, spontaneous preterm labour and premature rupture of the membranes (Burton et al., 2009a). In the early weeks, some of the cytotrophoblastic cells (described as extravillous cytotrophoblast) move from the tips of the anchoring villi to colonize the decidua and myometrium of the placental bed. It is this invasion of extravillous cytotrophoblast cells into the maternal blood vessels that promotes maternal recognition of the fetus and the subsequent production of blocking antibodies (see Chapter 10), which are important for the survival of the pregnancy. The extravillous cytotrophoblast cells are involved in physiologically remodelling the maternal spiral arteries which is completed by the end of the first trimester. After an apparent rest phase of a couple of weeks (weeks 14–16), there is a resurgence of the endovascular trophoblastic migration. The second wave of cytotrophoblast cells moves down the myometrial segments of the spiral arteries to their origin at the branching from the radial arteries. The cytotrophoblast cells are involved with the destruction of the maternal artery musculoelastic tissue and the replacement of the maternal endothelial wall with trophoblast resulting in a change in the vessel wall vasoresponsiveness. The result is conversion of the thick-walled muscular spiral arteries to compliant dilated sac-like uteroplacental vessels that have low impedance to blood flow (Fig. 8.7). The changes in the spiral arteries are augmented by interstitial trophoblast cells which migrate through the endometrial stroma and penetrate the spiral arteries from the outside; they continue to invade the myometrium where they transform into immotile giant cells (Burton et al., 2009a). Insufficient remodelling of the spiral arteries is associated with failure of the fetus to reach its genetic growth potential (intrauterine growth restriction (IUGR)) and pre-eclampsia (Box 8.1). Maternal spiral arteries maintaining a high vasculature resistance because of incomplete or failed remodelling are predisposed to hypoperfusion, hypoxia, reperfusion injury and oxidative stress. Although the trophoblast invasion and remodelling are limited to the spiral arteries, the radial, arcuate and uterine arteries also undergo profound dilation especially close to the site of implantation (Burton et al., 2009a and Burton et al., 2009b). These vessels unusually progressively increase rather than decrease in diameter as they reach their target organ.
Box 8.1
Pre-eclampsia is a placental condition; it can occur in the absence of a fetus in a molar pregnancy. One of the most convincing theories about the aetiology of pre-eclampsia and the associated condition of IUGR (which can occur independently or with pre-eclampsia) is that they are due to placental malperfusion secondary to deficient spiral artery conversion (Burton et al., 2009). In normal pregnancies, all spiral arteries in the placental bed are invaded by cytotrophoblast cells. In pre-eclampsia, it seems that only a proportion of the maternal vessels are invaded and that a significant number of vessels show complete absence of physiological changes. The second wave of arterial invasion may be the stage that is most compromised owing to the endovascular trophoblast failing to reach the intramyometrial portion of the vessels. This means that the spiral arteries are not completely transformed to uteroplacental vessels. Maternal uteroplacental blood flow is therefore restricted, which results in placental abnormalities and fetal complications such as IUGR. The effect is compounded by the persistence of vasoresponsiveness of the spiral arteries, which retain the ability to constrict and limit placental perfusion, like the spiral arteries of a non-pregnant uterus (see Chapter 4). Impaired placental perfusion increases the risk of ischaemia–reperfusion type insult which leads to the generation of reactive oxygen species (oxidative stress). Oxidative stress results in increased generation of oxygen-free radicals which lead to the formation of lipid peroxides which alter cell membranes. The incorporation of cholesterol, oxidized free fatty acids and LDLs into membranes is increased which leads to a biological cascade of leukocyte activation, platelet adhesion and aggregation and the release of vasoconstrictive agents (Jauniaux et al., 2006). Acute atherotic changes can lead to the development of intimal plaques which can project into the vessel lumen and restrict blood flow. In addition, endoplasmic reticulum (ER) stress is also triggered by ischaemia–perfusion and hypoxia. ER stress can lead to inhibition of protein synthesis and reduced expression of amino acid transporters (thus affecting growth) as well as activating apoptosis. There are serious maternal complications of pre-eclampsia which have been attributed to an as-yet unidentified placentally derived ‘factor X’ which is released into the maternal circulation; possible candidates include proinflammatory cytokines from the syncytiotrophoblast, products of placental oxidative stress, anti-angiogenic factors and trophoblastic apoptotic debris such as syncytiotrophoblast micro-fragments all of which could contribute to activation of maternal endothelial cells and cause the peripheral syndrome of pre-eclampsia (Fig. 8.8).
(Reproduced with permission from Lala and Chakraborty, 2003.)
The remodelled vessels can passively dilate and accommodate a greatly increased blood flow (about 30% of maternal cardiac output) but they are not responsive to vasoactive agents. The effect of this interaction between the trophoblastic cells and the maternal blood vessels is that a low-pressure, high-conductance vascular system is established, which provides an adequate maternal blood flow to the placenta and thus a plentiful provision of oxygen and nutrients to the fetus. The maternal uteroplacental circulatory system is mostly complete by mid-gestation. In contrast, the fetal villous tree continues to branch and develop throughout the pregnancy, ensuring that the capacity of the placenta matches the growth of the fetus.
As the maternal cardiac output increases by about 40% (see Chapter 11), the net effect is to increase the uteroplacental blood flow by about 10-fold to over 500 mL/min (Kingdom and Sibley, 1996). Doppler ultrasound flow velocity waveforms provide diagnostic and prognostic information about maternal vessels, placental circulation and fetal vessels together with implications for both mother and fetus (Harman and Baschat, 2003). Flow in the uterine arteries gives a picture of the maternal vascular effects of the invading placenta, predicting likely pre-eclampsia and IUGR. Umbilical artery Doppler ultrasound depicts placental vascular resistance, which correlates with IUGR and effects of placental deficiency. Before pregnancy and in the first trimester, the uterine arterial waveform has a low end-diastolic flow velocity and early dichrotic notch during diastole. By 18–20 weeks’ gestation, successful trophoblastic invasion alters this pattern to one showing a high diastolic flow velocity and loss of the dichrotic notch. If the dichrotic notch and low end-diastolic velocity persist, this indicates that the uterus still has high resistance to blood flow, which is predictive of intrauterine growth retardation (IUGR) and severe pre-eclampsia (Fig. 8.9).
(Reproduced with permission from Miller and Hanretty, 1998.)
A number of pathologies including placental infarction or abruption, pre-eclampsia and recurrent pregnancy loss are associated with defects within the placental vascular bed. Deficiencies of vitamin B12 and folate and hyperhomocysteinaemia are risk factors for these placenta-mediated diseases (Ray and Laskin, 1999). Smoking is associated with poorer outcomes of pregnancy including low birthweight, spontaneous abortion and placental abruption. Nicotine, cadmium and polyaromatic hydrocarbons in cigarette smoke adversely affect fertility, oocyte development and oestrogen synthesis (Shiverick and Salafia, 1999). Effects of these compounds on trophoblastic invasion and proliferation are thought to account for the increased miscarriage rate of women who smoke; however, smoking seems to reduce the incidence of pre-eclampsia, possibly because smoking reduces endothelium sensitivity. Severe placental dysfunction is more common with a male fetus (Edwards et al., 2000); this may be the cause of the male:female ratio decreasing from 164:100 soon after conception to 106:100 at birth. Placental pathologies may be due to defective trophoblast function and/or impaired maternal decidualization. The placental-related disorders of pregnancy, such as miscarriage and pre-eclampsia, are almost unique to humans (Jauniaux et al., 2006) and the incidence in other mammals is extremely low. It is thought that the rate of these disorders is increasing because of recent lifestyle changes such as hypercaloric diets and delayed childbirth; however, current populations of hunter-gatherers are also affected.
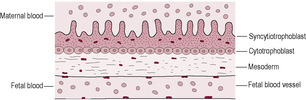
Fetal blood cells are derived from blood islands in the extraembryonic mesoderm surrounding the yolk sac (see Chapter 9). The blood vessels that perfuse the placenta also develop in this tissue. In the third week of postfertilization, the extraembryonic mesoderm associated with the cytotrophoblast penetrates into the core of the primary stem villi transforming them into secondary stem villi. This mesoderm develops into the blood vessels and connective tissue of the villi. It forms at the same time as the embryonic vasculature with which it will eventually connect. Haemangioblast cells (precursors of blood cells) appear and capillaries form. The linking of the blood vessels of the villi with the vessels of the embryo results in a circulating blood system so the villi begin to be perfused by the fetal circulation at about 28 days after fertilization. The fetal red blood cells containing embryonic haemoglobin allow oxygen transfer at low partial pressures of oxygen and low pH. The villi containing differentiated blood vessels are described as tertiary stem villi. By the end of the fourth week after fertilization, these villi cover the entire blastocyst surface forming a spherical shell of villi projecting outwards into the maternal tissue (Fig. 8.10). It is possible to remove a sample of the developing placental villi for genetic testing (Box 8.2). The placental barrier now effectively limits diffusion of gases, nutrients and waste materials. There are four layers: the endothelium lining the villus capillary, the connective tissue in the villus core, a layer of cytotrophoblast cells and a maternal-facing layer of syncytiotrophoblast (Fig. 8.11). By mid-gestation, most of the cytotrophoblast layer of many villi disappears and the placental barrier becomes very thin. The syncytiotrophoblast may even come into direct contact with the fetal capillary so the maternal and fetal blood may only be about 2–4 μm apart.
Box 8.2
In the chorionic villus sampling (CVS) procedure, 20–40 mg of placental tissue can be obtained from a villus for genetic diagnosis of trisomy 21, for instance or of a single-gene abnormality such as cystic fibrosis or β-thalassaemia. After 10 weeks’ gestation, the tissue can be extracted transabdominally by needle aspiration or transcervically using curved biopsy forceps. The collected trophoblast cells, which divide very rapidly, can be cultured for 24 h and then the chromosome number can be determined (see Chapter 7). Because of the problems associated with mosaicism (see Chapter 7), a more accurate determination of chromosome number and structure is obtained by using fibroblast cells taken from the vascular core of the villus. These cells grow more slowly so they have to be cultured for 2 weeks before being stained and examined (which means the results of the test take longer). As fibroblast cells are derived from the mesoderm, they originate from the inner cell mass and are embryonic rather than the trophoblast-derived cells from the outer layers of the villus. Placental mosaicism is associated with increased fetal loss and IUGR. There is a 1–2% procedure-related loss in CVS, although it should be remembered that the procedure is being performed because there is already a concern about the pregnancy. Pregnancies associated with genetic abnormality have a much higher risk of spontaneous failure.
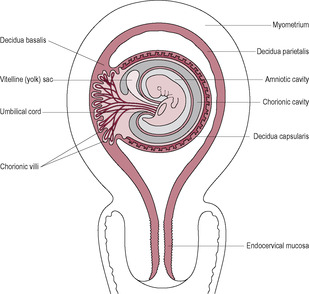
From the 4th week to the 16th week, the villus growth over the entire surface of the blastocyst is remodelled. Most of the villi orientated towards the uterine cavity degenerate and regress, leaving behind an area that develops into the typical placental structure and shape seen at delivery. As the embryo starts to enlarge, the uterine wall where it has implanted starts to protrude into the uterine cavity (Fig. 8.12). The protruding portion of the embryo is covered by the decidua capsularis, a thin layer or capsule of endometrium. The layer of decidua under the embryonic pole of the embryo is the decidua basalis. The remaining areas of the decidua are described as the decidua parietalis.
In the third month, as the fetus enlarges and grows to fill the uterus, the thin rim of decidua capsularis covering the bulge gradually thins and disappears so the chorion comes into contact with the decidua parietalis of the opposite wall of the uterus. Before the trophoblastic shell comes into contact with the uterine wall on the opposite side, cells of fetal origin can enter the uterine cavity and can be collected by flushing or aspiration from the endocervical canal (Kingdom et al., 1995). This is a route of non-invasive prenatal diagnosis, particularly for newer testing procedures that require fewer cells. The size of the chorionic, or embryonic, sac can be used to determine the gestational age of the embryo.