Chapter 20 The physiology of conception and pregnancy
Learning outcomes for this chapter are:
1. To describe the key structures in the female and male reproductive systems and relate these to the processes of reproduction
2. To outline the physiological changes and hormonal control of the ovarian and uterine cycles
3. To describe the process of fertilisation and implantation
4. To list key events in the continuum of development from zygote to embryo to mature fetus
5. To provide examples of ways that teratogens may interfere with normal development
6. To describe the development and functioning of fetal, uteroplacental and feto-placental circulation
7. To describe maternal anatomical and physiological changes associated with pregnancy, and the hormonal control of these changes
8. To discuss the role of hormones in the reproductive process and in the maintenance of pregnancy.
For ease of study, the development of the embryo and placenta and the changes in the woman through pregnancy are presented as separate topics, but the processes are interwoven and simultaneous. The scope of this chapter is limited, but further reading is provided at the end of the chapter for a more in-depth exploration of specific topics. Some of the online resources listed provide animations that can help with the understanding of complex anatomical changes.
FEMALE AND MALE REPRODUCTIVE SYSTEMS
Anatomy of the female reproductive system
Female internal genitalia
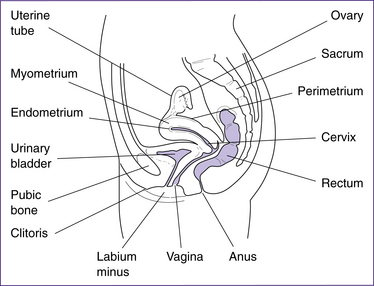
The structures of the female internal genitalia are depicted in Figure 20.1.
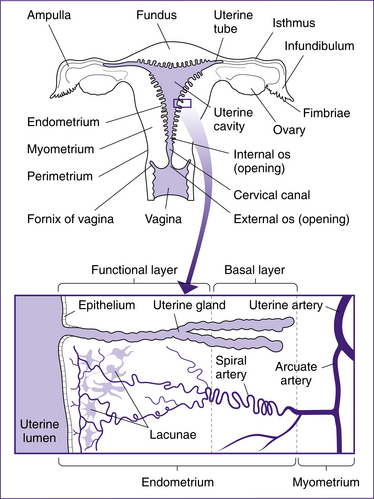
The uterus is a hollow cavity consisting of three regions: the fundus, the body and the cervix. The fundus is the region superior to the uterine tubes. The body is the major portion of the uterus and is the site where implantation occurs. The cervix is the region that connects the uterus with the vagina and consists of the cervical canal, the internal os and external os. The uterus is a thick-walled organ with three layers: the perimetrium (outer layer), the myometrium (thick muscle layer) and the endometrium (the inner mucosal lining). The endometrium consists of two layers: the stratum basalis, which lies next to the myometrium, and the stratum functionalis, the outer mucosa that is shed during menstruation (Fig 20.2).
The vagina is often referred to as the ‘birth canal’, and is the region of the internal female genitalia that connects to the external female genitalia. The vagina is a passage for semen during intercourse and for menstrual blood during menstruation. The epithelial cells that line the vagina produce glycogen, which is metabolised to lactic acid by the vaginal microflora. This results in the vagina having an acidic pH, which protects against foreign microorganisms as well as destroying many sperm cells that enter the vagina.
Female external genitalia
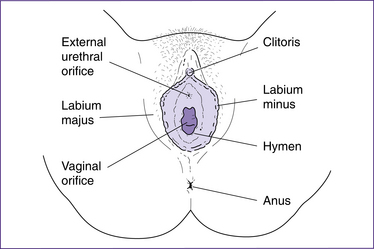
The female external genitalia or vulva—mons pubis, labia majora, labia minora, clitoris and vestibule—are shown in figure 20.3).
The female pelvis
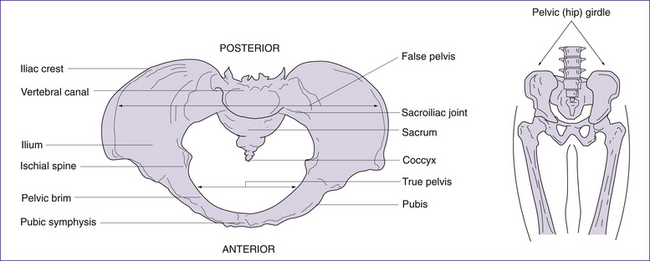
The pelvic (hip) girdle
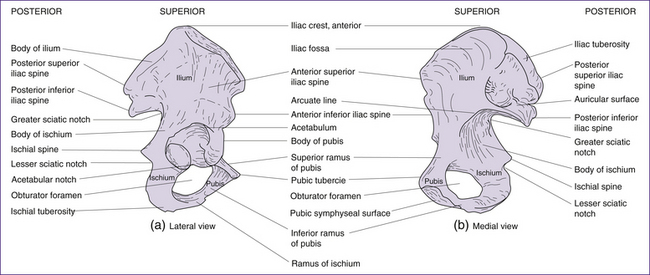
The pelvic girdle consists of a pair of hip (innominate) bones. They join anteriorly at the symphysis pubis and meet posteriorly with the sacrum to form the sacroiliac joint. Together, the innominate bones, sacrum and coccyx, form the deep basin-like structure known as the bony pelvis. Each innominate bone actually consists of three separate bones, the ilium, the ischium and the pubis (Figs 20.4 and 20.5). In infancy these three bones are separate. By puberty most growth is completed, and the three bones fuse at the acetabulum (cup-shaped hollow in the bone). Growth continues in other areas of the pelvis (i.e. the iliac crests, the ischial tuberosities—bumps or protuberances on the ischium) until the late teens or early twenties (Rissech et al 2001).
Ligaments associated with the pelvic girdle
Ligaments are dense bands of fibrous tissue which serve to reinforce joints and to maintain bones in their correct anatomical arrangement. They contain collagen and elastic fibres. The elastic fibres enable ligaments to stretch, and this assists in the softening of the pubic symphysis and sacroiliac joints during pregnancy. This softening is stimulated by hormones such as relaxin, oestrogen and progesterone. Joint laxity may lead to backache and joint instability (Stables & Rankin 2005).
The following ligaments support the joints of the pelvis.
• Sacroiliac ligaments—span the sacroiliac joints. These are very strong ligaments which transmit the weight of the trunk, head and arms to the legs. There is normally only very slight movement at these joints, but movement increases during pregnancy under the influence of relaxin (Stables & Rankin 2005).
• Symphysis pubis ligaments—bridge anteriorly and posteriorly from one pubic bone to the other.
• Sacrotuberous ligaments—from the sides of the sacrum to the ischial tuberosities (across the greater and lesser sciatic notches).
• Sacrospinous ligaments—from the sides of the sacrum to the ischial spines (across the greater sciatic notch).
• Sacrococcygeal ligaments—from the sacrum to the coccyx.
• Inguinal ligament—from the anterior superior iliac spine to the lateral edge of the pubic bone, forming the groin.
Bones of the pelvic girdle
Ilium
The ilium is a large flaring bone that forms the major part of the hip bone. It consists of a body and ala (wings). Important landmarks include the iliac crests, which are the thickened, superior portions of the alae, and the iliac spines: the anterior superior, the posterior superior, the anterior inferior and the posterior inferior iliac spines. The iliac spines provide important points of muscle attachment for muscles of the hip, trunk and thigh. The anterior superior iliac spine provides an important anatomical landmark. The greater sciatic notch provides for passage of the sciatic nerve and the iliac fossa is found on the medial surface of iliac alae. The auricular surface is the point of articulation between ilium and sacrum, and the arcuate (iliopectineal) line runs anteriorly and inferiorly from the auricular surface. Together with the sacral promontory, the iliopectineal line defines the pelvic brim, i.e. the superior margin of the true pelvis (Fig 20.6) (Stables & Rankin 2005).
Ischium
The ischium forms the posterior inferior part of the hip bone and appears L- or arc-shaped. It is composed of a superior portion, the body; and an inferior portion, the ramus (or the branch). Important landmarks include the ischial spines, which project medially into the pelvic cavity and are the points of attachment for the sacrospinous ligament. The lesser sciatic notch allows nerves and blood vessels to pass through on their way from the pelvis to the thigh. The ischial tuberosities bear our upper bodyweight when we sit. They also provide attachment for hamstring muscles and the sacrotuberous ligament (Stables & Rankin 2005).
Pubic bones
The pubic bones form the anterior part of the innominate bone and are V-shaped. Important landmarks include the pubic crests, which are formed by the anterior border of the pubic bones, and the pubic tubercle which provides attachment for the inguinal ligament. The obturator foramen is an opening covered by a membrane through which the obturator nerve and blood vessels pass. The pubic arch is formed from inferior borders of pubic rami. This structure is wider in females, serving to increase the anteroposterior diameter of the pelvic outlet (Stables & Rankin 2005).
Pelvis, true or false
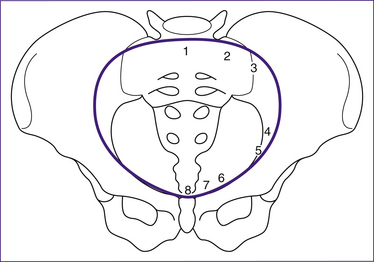
The pelvis is described in terms of a true and a false pelvis. The true or lesser pelvis is almost entirely surrounded by bone, including the inferior region of the ilium, ischium, pubis and sacrum, and is separated from the false pelvis by the pelvic brim. The false or greater pelvis is superior to the pelvic brim. The false pelvis serves only to support the abdominal viscera and so, unlike the true pelvis, does not have a potentially restrictive role in childbirth (Stables & Rankin 2005). The true pelvis is considered in three planes:
• the pelvic brim can be used in pelvimetry to establish if the infant’s head and the pelvis share compatible dimensions. The infant’s head passes through the pelvic brim when it engages in late pregnancy or during the onset of labour.
• the pelvic cavity. The dimensions of this area are normally enhanced by the curvature of the sacrum. The rounded shape of the cavity encourages rotation of the baby’s head; the ischial spines can reduce the dimensions of the pelvic cavity. The infant’s head is considered engaged when it has reached the level of the ischial spines (Stables & Rankin 2005).
• the pelvic outlet can be described as the anatomical or the obstetric outlet. The anatomical outlet refers to the lower border of the pelvis and is not significant in terms of birth, but the obstetric outlet, the constricted lower portion of the pelvis, is more relevant. It is bound by the subpubic arch, ischial spines, the sacrospinous ligaments and the lower border of the sacrum (Stables & Rankin 2005). The sacro-coccygeal joint is flexible to a degree which allows the coccyx to move posteriorly out of the way during childbirth.
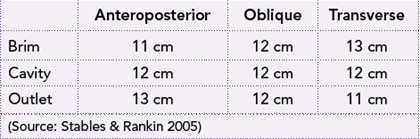
The maximum diameter of the pelvis through which the infant’s head can pass differs for all three planes. Initially the largest diameter is in the transverse plane (pelvic brim), but the anteroposterior diameter becomes the largest at the pelvic outlet, particularly in females who possess a classical gynaecoid pelvis where the angle of the subpubic arch is equal to or greater than 90 degrees. Measurements of the diameter of the pelvis at the brim, cavity and outlet are presented in Table 20.1.
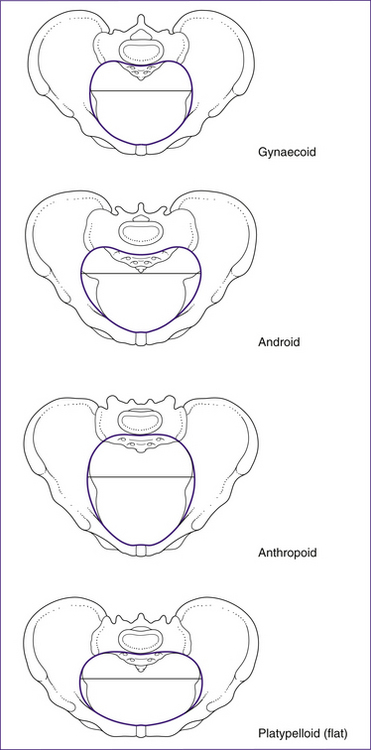
The shape of the pelvis can vary in individuals. While the gynaecoid is most common in women, three other types have been described: the android, anthropoid and platypelloid pelvis. These are illustrated in Figure 20.7.
• The android pelvis has male type features. It is characterised by a small inlet that is somewhat heart-shaped. The side walls converge, causing the pelvis to narrow as the fetus descends. The ischial spines are prominent and the angle of the subpubic arch is less than 90 degrees. This type of pelvis is least suited to childbearing.
• In the anthropoid pelvis the anteroposterior diameter is greater than the transverse diameter, but the pelvis is generally large all over. The side walls are parallel or flare outward, ischial spines are not prominent and the subpubic angle may be normal or wide. The pelvis is so large that delivery may occur without rotation.
• In the platypelloid pelvis there is a reduced anteroposterior diameter. The side walls diverge, the cavity is shallow and the ischial spines are blunt with a wide subpubic angle. The fetus may have difficulties entering the brim, but once through here there should be no further difficulty.
The shape of the pelvis does not necessarily predict whether a successful vaginal delivery is likely; the size of the fetal head in relation to the pelvis is more important (Stables & Rankin 2005).
Anatomy of the male reproductive system
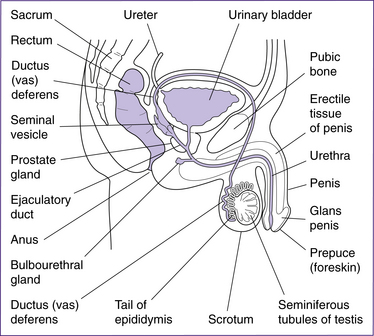
The structures of the male reproductive system (Fig 20.8) include the testes, penis, accessory ducts (epididymis, vas deferens or ductus deferens, ejaculatory ducts and urethra) and the accessory glands (prostate, seminal vesicles and bulbourethral glands).
The paired testes each contain coiled seminiferous tubules, which are the site of sperm production. The sperm are moved from the seminiferous tubules to the coils of the epididymis by peristalsis. Secretions from the Sertoli cells, adjacent to the seminiferous tubules, build up pressure and contribute to the movement of sperm (Fig 20.9).
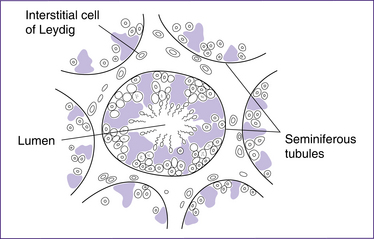
Figure 20.9 The testis in cross-section, illustrating the seminiferous tubules and interstitial cells of Leydig
(based on Bray et al 1994)
Accessory sex glands and semen
Semen is the alkaline fluid that protects, nourishes and transports sperm during ejaculation. Two to five millilitres is ejaculated during orgasm, containing 50–100 million sperm/mL. At least 20 million sperm/mL are necessary for fertility. However, 90% of the fluid is made up of secretions from the seminal vesicles, prostate gland and bulbourethral glands.
Gametogenesis
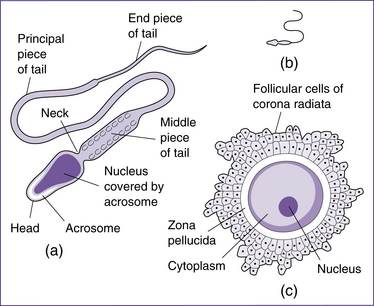
Fertilisation involves the union of two gametes—an oocyte from the female and a sperm from the male. Gametes are highly specialised sex cells (Fig 20.10). Gametogenesis (formation of the sex cells) necessarily involves halving the number of chromosomes in each cell, by a type of cell division called meiosis, and by altering the shape of the cells. In females this process is termed oogenesis and in males it is spermatogenesis. When the two gametes join at fertilisation, the full number of chromosomes is restored.
Oogenesis
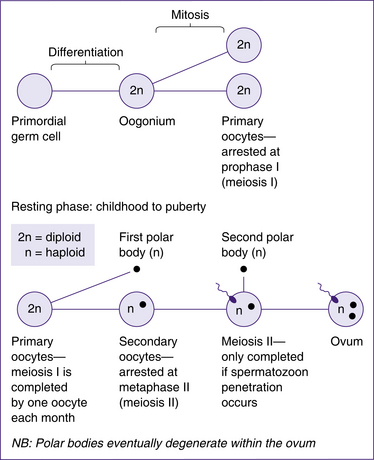
Oogenesis is the process by which mature female sex cells are formed. Much of this process occurs in the fetus prior to birth. Oogonia in the fetus multiply by mitotic division and develop into primary oocytes (containing 46 chromosomes). The first meiotic division also begins before birth, but is arrested until after puberty. Completion of the first meiotic division is linked to the ovarian cycle. Each month, following puberty, several primary oocytes begin to develop but only one matures to complete the first meiotic division 36–48 hours before ovulation (Blackburn 2007). This division results in a large secondary oocyte containing most of the cytoplasm, and a smaller non-functional polar body that soon degenerates. Each has 23 chromosomes (22 autosomes and 1 sex chromosome). At ovulation the secondary oocyte begins the second meiotic division, but the division is only completed if fertilisation occurs. Once again, almost all the cytoplasm goes to one cell, the mature oocyte; a second polar body is non-functional and degenerates. The cellular divisions of oogenesis are outlined in Figure 20.11.
Spermatogenesis
The mature sperm has relatively little cytoplasm. It is divided into a head, midpiece and tail, and is motile. The head forms most of the bulk, contains the nucleus with the chromosomes and is covered anteriorly by the acrosome, a structure containing enzymes that facilitate penetration of the ovum at fertilisation. The midpiece is rich in energy-producing mitochondria, which fuel the lashing movements of the tail. Spermatogenesis takes about two months to complete and continues throughout the reproductive life of a male (Moore & Persaud 2008). The sperm move to the epididymis, where they are stored and become functionally mature.
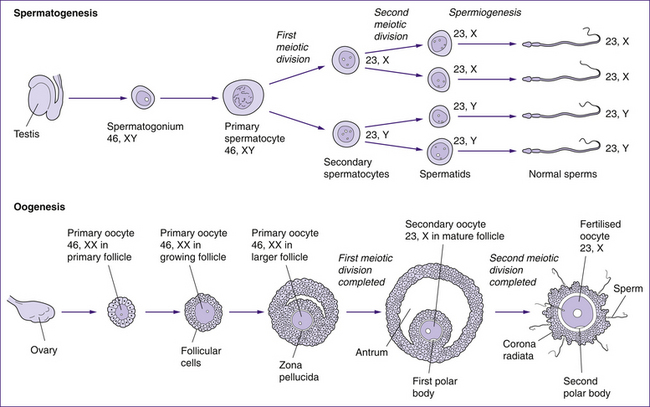
A comparison of oogenesis and spermatogenesis is shown in Figure 20.12.
Female reproductive cycles
Females undergo monthly reproductive cycles starting at puberty and continuing through the reproductive years. These cycles are controlled by the hypothalamic–pituitary–ovarian hormones and result in changes in the ovary that lead to release of one secondary oocyte per month, and in changes in the uterus in preparation for implantation of the fertilised ovum. If fertilisation does not occur, the cycle begins again under the influence of the hypothalamic–pituitary hormones. For simplicity, the reproductive cycle is described as an average 28-day cycle. There is considerable individual variation, but the cycle length of most women is between 21 and 35 days (Blackburn 2007).
Ovarian cycle
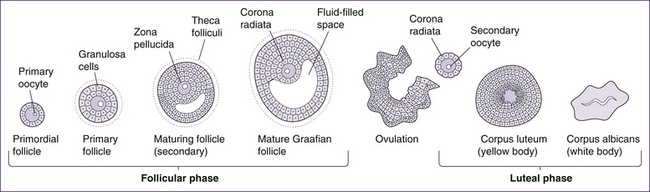
The ovarian cycle (Fig 20.13) includes three phases: the follicular or pre-ovulatory phase, the ovulatory phase or ovulation, and the post-ovulatory or luteal phase.
Follicular phase
• Primordial follicles contain primary oocytes and are the type of follicle present at birth. The first meiotic division has already begun in utero, but the process is arrested partway and does not continue again until puberty, when levels of gonadotrophin-releasing hormone (GnRH), luteinising hormone (LH) and follicle-stimulating hormone (FSH) increase.
• The primary follicle is formed in response to the increased level of the hormones mentioned above. The hormones signal the cells surrounding the oocyte to proliferate and form a layer of granulosa cells. These cells allow nutrients and signalling molecules to reach the oocyte.
• The secondary follicle is formed when a layer of connective tissue, called the theca folliculi, develops around the follicle and an antrum begins to develop with it. The thecal cells, together with the granulosa cells, produce steroid hormones (mainly oestrogens with small quantities of progesterone). The secondary follicle is also characterised by the zona pellucida, which surrounds the oocyte and is released along with the oocyte at ovulation.
• Graafian or vesicular follicles are the final and most mature form of ovarian follicle. This stage of follicular development is characterised by a large antrum and a follicular size of approximately 2.5 cm diameter. Due to its size it bulges from the ovary midway through the ovarian cycle.
The completion of meiosis I, in which a secondary oocyte and the first polar body are formed, occurs 36–48 hours before ovulation (Blackburn 2007). Meiosis II continues but is not completed until fertilisation occurs. The process of meiosis results in the genetic material in each oocyte being halved from 46 chromosomes (2n) to 23 chromosomes (n).
Ovulation
The ovulatory phase begins when oestrogen levels peak and ends when the vesicular follicle ruptures, releasing the secondary oocyte surrounded by the zona pellucida and corona radiata (the crown of granulosa cells around the zona pellucida). Ovulation is under hormonal control, as follows: LH interacts with LH receptors on the granulosa cells, increasing the production of oestrogen by the dominant follicle. A rise in oestrogen levels 12–24 hours before ovulation triggers the LH surge. The high level of LH stimulates production of prostaglandins (PGF2α and PGE2), and allows the primary oocyte to complete the first meiotic division and become a secondary oocyte. Simultaneously, levels of oestrogen drop and proteolytic enzymes are synthesised, which break down the thecal cells and assist rupture of the vesicular follicle (Blackburn 2007).
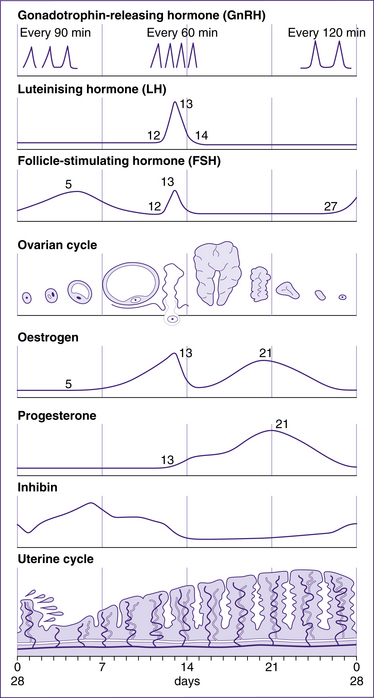
Uterine cycle
The uterine cycle occurs in the same timeframe as the ovarian cycle and is similarly controlled by changes in hormonal levels (Fig 20.14). It consists of three stages: the menstrual phase (days 1–5), the proliferative phase (days 6–14) and the secretory phase (days 15–28). The following is a review of each stage.
Menstrual phase
Menstruation is triggered by several factors. The first is a drop in levels of progesterone and oestradiol (one form of oestrogen). The second is a change in the ratio of prostaglandins and prostacyclins (hormone-like compounds) that cause vasoconstriction and increased coiling of the spiral arterioles, leading to a decrease in blood flow to the stratum functionalis of the uterus. The cells in the stratum functionalis become ischaemic and necrosed, and slough away from the stratum basalis. The straight arterioles dilate, contributing to menstrual flow (Blackburn 2007).
Proliferative phase
In the proliferative phase, the stratum functionalis of the endometrium begins to thicken as endometrial cells proliferate. Arterioles regenerate and endometrial glands develop. These changes occur in response to increased levels of oestradiol. The mucus produced by the cervix also responds to these hormones. Its consistency becomes less viscous and less stretchy, showing little or no ferning (spinnbarkeit test), thus becoming more receptive to sperm. Variation in the length of the proliferative phase accounts for most of the variation in uterine cycles (Blackburn 2007).
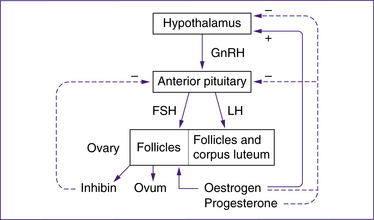
Hypothalamic–pituitary–ovarian (HPO) axis
The female reproductive system is regulated by a variety of hormones produced by the brain (the hypothalamus and the pituitary gland) and the ovaries (Fig 20.15).
Hypothalamus
The hypothalamus produces a peptide hormone called gonadotrophin-releasing hormone (GnRH) within its neurons. This hormone stimulates the release of several hormones from the anterior pituitary gland, specifically FSH and LH.
Pituitary gland
FSH, along with oestrogen produced by the ovaries, acts on granulosa cells of the follicles to stimulate growth and produce FSH receptors and LH receptors. The LH receptors combine with LH during the LH surge to inhibit the growth of granulosa cells and initiate production of progesterone from the follicle. The granulosa cells of a primordial follicle undergo limited growth without FSH. Secretion of FSH peaks mid-cycle at a lower level than the LH surge (Blackburn 2007).
Research activity
A review publication in the journal Drug Safety by Al-Shawaf et al (2005) suggests that as many as 15% of couples may experience problems with fertility and that pharmacological agents are now routinely used with the aim of retrieving multiple oocytes to increase the chance of pregnancy. They describe the side-effects associated with fertility drugs as changes to the ovarian cycle, ovarian hyperstimulation syndrome and congenital malformations, although they suggest this may relate to the high-risk populations studied rather than the drugs themselves. High-order multiple pregnancies are also identified as being commonly associated with the use of fertility drugs—due to multiple ovulations or more than one embryo replacement. Gonadotrophin-releasing hormone (GnRH) agonists are widely used in assisted reproduction, and while there is considerable research into the possible link of GnRH with increasing risk of ovarian cancer and possibly breast cancer, these authors suggest that this has not been substantiated.
Ovaries
The ovaries produce the oestrogens (oestradiol, oestrone and oestriol), progesterone and inhibin.
Oestrogens are produced in the ovaries by granulosa cells within the follicles and the corpus luteum. The most abundant oestrogen during a woman’s reproductive years is oestradiol, which is responsible for most of the effects of oestrogen in the female reproductive system. However, during pregnancy, oestriol becomes the main oestrogen secreted (Blackburn 2007). The stimulus for oestrogen release is primarily FSH and, to a lesser extent, LH. Oestrogens are responsible for various functions, including:
• development and maintenance of the female reproductive structures such as the endometrial lining of the uterus, and secondary sexual characteristics such as fat content of the breasts, abdomen, mons pubis and hips, voice pitch, broadness of the pelvis, distribution of underarm and pubic hair, and the development of breasts
• fluid and electrolyte balance—by influencing the action of aldosterone, sodium reabsorption in the renal tubules is stimulated and diuresis is reduced
• increasing the rate at which amino acids enter cells, thereby altering protein metabolism, allowing cells to grow and multiply. This action is supported by the influence of growth hormone.
• regulation of oxytocin and adrenergic receptors (Henderson & Macdonald 2004)
• stimulation of uterine tube contractility to assist sperm motility and to retain the ovum (Blackburn 2007).
Progesterone is produced in the ovaries by granulosa cells, primarily of the corpus luteum. Therefore progesterone levels peak in the post-ovulatory phase when the corpus luteum is active, and fall if pregnancy does not occur and the corpus luteum degenerates. LH is the stimulus for progesterone release. Progesterone has various functions, including:
• preparation of the endometrium of the uterus to receive a fertilised ovum
• prevention of sperm entry into the uterus by promoting changes in the cervical mucus
• increasing basal body temperature by influencing the thermostat located in the hypothalamus
• relaxation of the muscle of the uterine tubes during the luteal phase to assist passage of the fertilised ovum to the uterus (Blackburn 2007).
Clinical points
1. Body temperature rises after ovulation, in response to progesterone, and is used in natural family planning to indicate the fertile phases of the reproductive cycle.
2. Hormonal contraceptives contain levels of oestrogen and/or progesterone that cause feedback control on the HPO axis to prevent follicle development and ovulation. Some contraceptives use the cervical mucus-changing properties of progesterone (e.g. the mini-pill).
Summary: homeostatic control of the reproductive cycle
Research activity
Hormone replacement therapy (HRT) has previously been suggested to reduce the risk of cardiovascular disease. A study published in the Journal of the American Medical Association in 2002 in fact identified that HRT increased the risk of cardiovascular disease in healthy postmenopausal women. The study was halted early because it was decided that these results presented an unacceptable risk for the participating women (Rossouw et al 2002). HRT has also been used to treat the symptoms of menopause.
Hypothalamic–pituitary–testicular control in the male
Testosterone is produced by the Leydig (interstitial) cells in the seminiferous tubules (Fig 20.9) and diffuses across the basal membrane to directly influence the spermatogonia and Sertoli cells. Testosterone levels are maintained by negative feedback to the hypothalamus and anterior pituitary, via the HPT axis.
Summary points
• The structures of the female and male reproductive systems are designed to produce and transport gametes: ova and sperm.
• Gametogenesis involves cell divisions that halve the chromosome number: oogenesis produces ova in the ovaries, and spermatogenesis produces sperm in the testes.
• Following puberty, a single oocyte is released from the ovary each month under the control of the hormones of the HPO axis.
• Ovarian hormones stimulate monthly vascular and glandular development of the uterus in preparation for pregnancy.
• Hormones of the HPT axis stimulate sperm production from puberty onwards.
• Sperm are produced in the seminiferous tubules and stored in the epididymis until ejaculation.
• Semen released at ejaculation includes about 300 million sperm and 2–5 mL of secretions from the accessory glands to aid nourishment and support of the sperm.
EMBRYOLOGY
Embryology is described as the study of ‘the origin and development of a human being from a zygote to the birth’. (Moore & Persaud 2008, p 2). Moore and Persaud (2008) emphasise that development is a continuum that starts at fertilisation, includes birth—a dramatic event resulting in a change in environment—and continues after birth, with development of teeth, bones, reproductive structures and so on. The process finishes by about the age of 25 years.
This section is limited to antenatal development, which begins with conception. Conception refers to fertilisation that results in pregnancy (Blackburn 2007). The probability of a viable conception has been calculated at only 30% per menstrual cycle (Blackburn 2007), with spontaneous abortions resulting from a range of abnormalities. The processes needed to produce a viable oocyte and a viable sperm have been described earlier in this chapter. This section examines fertilisation and the factors that contribute to successful fertilisation, implantation, development of the embryo and fetus, and development of the placenta.