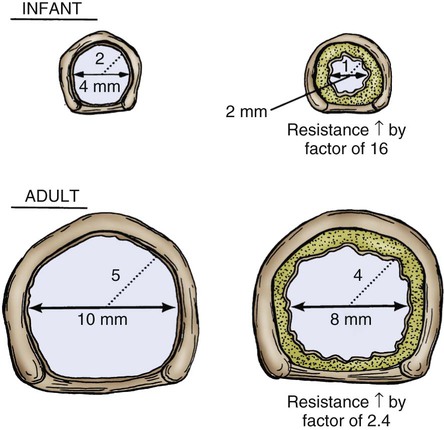
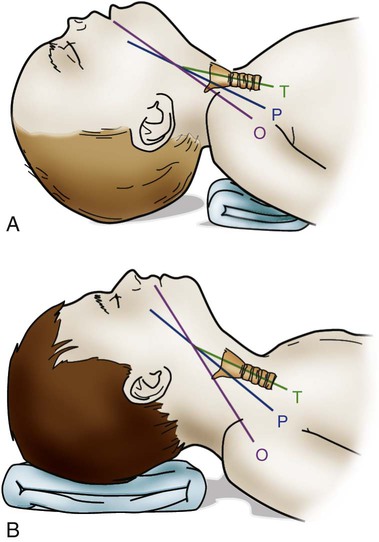
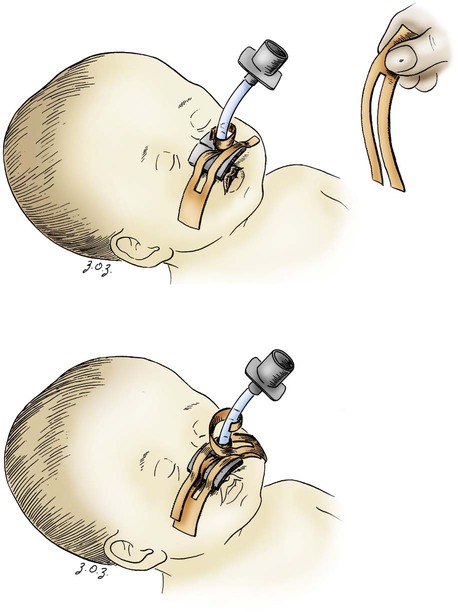
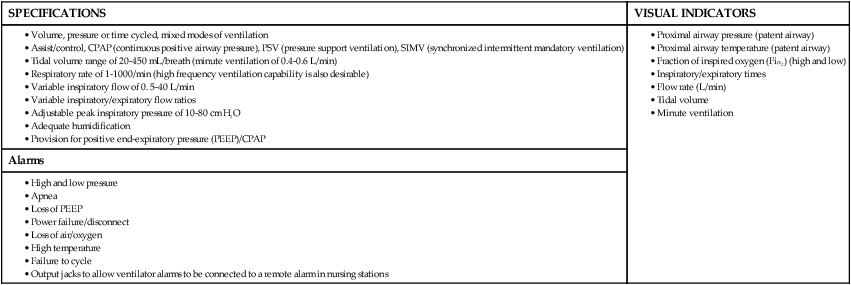
Some of the developmental and physiologic differences between adults and children, older than 1 month, are discussed in this chapter. Although children may experience medical conditions similar to those of adults, they are assessed and managed differently. During periods of stress, children may maintain physiologic stability for a period, but they may then decompensate quickly. Children are not small adults. Box 40-1 describes the differences between children and adults. Many of the laboratory values, medications, blood product dosages and methods of administration, and other therapeutic modalities for children are different from those used with adults. Regardless of the anticipated outcome, admission to a critical care unit is stressful for families. Critical care nurses who successfully deal with pediatric patients see the child and the family as an integral unit and are perceptive to the needs of the entire family.1 Knowledge of normal growth and development and the ability to assess the child’s developmental level are important for working with children and their parents. Nurses who take care of pediatric patients can conceptualize using a developmental perspective as the ideal norm.1 The developmental stages include the different age groups: infancy (0 to 12 months), toddlers (1 to 3 years), preschoolers (3 to 5 years), school-age children (6 to 12 years), and adolescents (12 to 18 years). Even though some critically ill children may be managed in adult critical care units, in certain situations, children need the services of various pediatric subspecialists or pediatric intensivists, and they must be transferred to a tertiary care pediatric critical care unit (PICU). This also is true for pediatric trauma patients. The risk of death is significantly lower for patients receiving care in a facility with a designated trauma center.2 Conditions that may require transfer to a hospital with a PICU include the need for high-frequency ventilation, extracorporeal membrane oxygenation (ECMO), or cardiac surgery and treatment for some neurologic conditions that require intracranial pressure monitoring. Transfer is also considered for children who do not respond to treatment. The infant’s epiglottis is large and floppy, and because of its high placement, it may press against the pharyngeal soft palate on inspiration. The infant’s tongue is large relative to its head size. The tongue fills most of the oral cavity. Because of this anatomy, the infant usually is an obligate nose breather until 4 to 6 months of age, after which the larynx descends with growth.1 Oral breathing is a very complex process for an infant, and it never occurs alone. Oronasal breathing is possible, but only up to 30% to 40% of ventilation may be provided orally. During sleep, oronasal breathing may occur spontaneously and last for about 20 seconds. The larynx of the infant and young child, unlike that of the adult, is a funnel-shaped structure, with the narrowest portion at the cricoid ring.3 The larynx is pliable because the cartilage is less developed, making it easier to collapse on inspiration or expiration. With changes in intrathoracic pressure, collapse may occur even with crying.3 By age 8 to 10 years, the larynx has grown cylindrical, has assumed the narrowest portion at the glottic opening, and has increased in length, width, and internal diameter. By age 12 years, the diameter has grown to 1.8 cm. The submucosal layer of the larynx is also looser in the infant and young child, and fluid accumulates more easily in that space. Within the airway’s relatively rigid confines, any accumulation of fluid encroaches into the airway space. Along with a shorter and narrower airway, any decrease in airway radius leads to an exponential increase in airflow resistance, which increases the work of breathing. Turbulent airflow, as occurs with crying, doubles the already increased airflow resistance.4 Figure 40-1 illustrates the changes in airway diameter and airflow resistance with obstruction from edema in an adult and in an infant. The infant or child with an abnormally small jaw and low-set ears should be considered as having a potentially difficult airway to manage and must have a consultation with an anesthesiologist if airway management is required. The respiratory structure and mechanics of infants and young children are very different from those of mature adults. In the infant and young child, the chest wall is more compliant because the bones are smaller and more cartilaginous. The ribs are more horizontally placed, providing less of a bellowing action on inspiration. Accessory muscles are less developed, and the external intercostals do not contribute to pulling the ribs up on inspiration. The diaphragm is the principal muscle for inspiration. The diaphragm is more horizontal in the chest of the infant and tends to pull the lower ribs inward on inspiration.5 Because of these mechanics, the infant and the toddler depend almost totally on diaphragmatic contraction for lung expansion. Anything that impedes diaphragmatic contractions may result in respiratory compromise. The intercostal muscles are inadequately developed before school age, and they are unlikely to help with effective ventilation if the diaphragm is impaired.3 With any decrease in lung compliance, as with lung disease, diaphragmatic contractions, which cause decreased intrathoracic pressure, produce intercostal and substernal retractions rather than inflation of the lungs.5 The greater the chest wall retractions, the more the diaphragm must contract to offset the changes in intrathoracic pressure to generate an adequate tidal volume for the child. The compliant chest wall of the infant or the young child should expand easily outward with positive-pressure ventilation. If the chest wall does not expand bilaterally during the positive-pressure ventilation, either the ventilation effort is inadequate or the airway is obstructed.3 The infant or child usually experiences respiratory failure more often than primary heart failure. Unlike the older adult, who may have underlying cardiovascular disease, the infant and the child tend to demonstrate bradycardia and apnea in cardiopulmonary failure and not ventricular dysrythmias.4 For the infant or child who is conscious and needs supplemental oxygen, the device of comfort must be selected.3 Minimizing anxiety and fear in the child is paramount to decrease the work of breathing. Table 40-1 outlines the assessment areas for the pediatric patient at risk for respiratory failure.3 TABLE 40-1 CLINICAL INDICATORS FOR THE INFANT OR CHILD AT RISK FOR RESPIRATORY FAILURE PEEP, Positive end-expiratory pressure. Based on data from Kline-Tilford A, et al. Pulmonary disorders. In: Hazinski MF, ed. Nursing Care of the Critically Ill Child. 3rd ed. St. Louis: Elsevier; 2013. Knowledge of childhood anatomy is necessary to establish a patent airway. The infant or toddler younger than 2 years of age, because of the large occiput, needs to have a small roll or towel placed under the upper shoulders, with the jaw slightly extended into a “sniffing” position.4 Optimal positioning of the head should assist in maintenance of a patent airway or help when bag-mask ventilation is required.4 This head positioning displaces the tongue and lines up the posterior pharynx and tracheal opening for a clear airway. For the infant younger than 6 months, correct head positioning still may not prevent the large tongue from falling back into the posterior pharynx. Oral airways must not be used unless the infant is unconscious because the airway tip may stimulate laryngospasm as a result of the higher placement of the larynx. Side-lying placement, with the neck in a neutral position, should be attempted.3 The older child needs to have a folded towel placed under the head, with the neck in an extended position to maintain a patent airway.3 Figure 40-2 illustrates proper head positioning for the infant and the child. The conscious child must be allowed to assume a position of choice for airway maintenance. Many of the oxygen devices used for adults also are used for children. Some additions include oxygen hoods for infants up to 1 year old. The hoods are clear plastic boxes that envelop the head and allow full vision of the head and access to the body. Other oxygen devices include oxygen tents, which are used less often, and oxygen “blow-by,” which uses oxygen tubing to blow oxygen approximately 2 to 3 inches from the child’s face. Because of the unpredictability of the actual oxygen percentage that is delivered, the child should never be left unattended with blow-by as an oxygen delivery method. Oxygen masks may aggravate the child and often are not well tolerated because of the snug fit of the mask to ensure adequate delivery of oxygen.6 One option in older infants and children is to use a nasal prong or cannula.6 This method allows the child to talk and eat without a facial obstruction. The appropriate device for oxygen delivery is determined by the patient’s age, size, and inspiratory flow rate (tidal volume milliliter per second [mL/s]), and the fraction of inspired oxygen (Fio2) needed.7 Oxygen delivery devices may be divided into two different classes: low-flow devices and high-flow devices. Low-flow (variable performance) devices are unable to deliver an oxygen flow rate sufficient to supply the patient’s inspiratory flow rate. This allows the child to entrain room air with supplemental oxygen on inspiration. The Fio2 the child receives depends on the patient’s respiratory rate and tidal volume. High-flow (fixed performance) devices may deliver an oxygen flow rate that meets or exceeds the child’s inspiratory flow rate. This allows a higher Fio2 to be consistently delivered.7 Nursing care for a child receiving supplemental oxygen delivery should include monitoring and recording the type of oxygen delivery device, the liter flow (liters per minute [L/min]), the Fio2, and the patient’s response to the oxygen therapy. An adult-sized, self-inflating resuscitation bag may be carefully used on an infant, provided only the force needed to cause appropriate chest expansion is used.3 In general, two types of manual resuscitation bags are used: the self-inflating bag and the flow-dependent bag. Self-inflating bags do not require a gas source to provide ventilation, but flow-dependent bags do require a gas flow.7 Resuscitation bag sizes, along with other supplemental oxygen devices and oxygen administration, are summarized in Table 40-2. Leaf-flap outlet valves should be avoided when a self-inflating bag is used to assist spontaneous ventilation in an infant because the infant cannot generate enough negative inspiratory pressure to open the valve.3 Resuscitation bags equipped with spring-loaded, positive end-expiratory pressure (PEEP) valves to provide continuous positive airway pressure (CPAP) must not be used with the spontaneously breathing child for the same reason previously discussed.3 Flow-inflating bags have no flow valves that require opening on inspiration and, therefore, may be used to provide supplemental oxygen, PEEP, or CPAP to the spontaneously breathing infant or child.3 Pressure manometers may be attached to these bags to measure peak inspiratory pressure. Ventilatory masks are measured in the child, as in the adult, from the bridge of the nose to the point before the end of the chin. Using a correctly sized mask for the infant or child is critical for adequate oxygenation and ventilation of the patient. TABLE 40-2 SUPPLEMENTAL OXYGEN DEVICES AND OXYGEN ADMINISTRATION IN INFANTS AND CHILDREN PEEP, Positive end-expiratory pressure. Based on data from Kuch B. Respiratory monitoring and support. In: Hazinski MF, ed. Nursing Care of the Critically Ill Child. 3rd ed. St. Louis: Elsevier; 2013:1007. Endotracheal tube (ETT) placement and management for the pediatric patient is an important intervention to maintain airway in a patient with respiratory failure.4 Preoxygenation of the child before intubation is very important. Bag-mask ventilation is an effective way to assist the child’s ventilation. Attempts for intubation should to be no longer than approximately 30 seconds per attempt to prevent deterioration in heart rate or the child’s appearance. Box 40-2 provides formulas as a guideline for ETT measurements in the pediatric patient. It is important to ensure that pediatric emergency equipment of the correct size is used. Many facilities are using length-based or color-coded resuscitation tapes such as the Broselow system.4,8 This tape gives an estimate of the child’s body weight based on the crown-to-heel length and may be used to determine the appropriate size of resuscitation equipment and medication dosages for the child. Figure 40-3 provides an illustration of a Broselow tape. When correctly placed, the tip of the ETT should be 1 to 2 cm above the carina, no higher than the first rib.3 After the child is intubated, bilateral breath sounds should be assessed high in the axillae along with bilateral chest expansion. The easy transmission of sounds in the chest of the child may be mistaken for breath sounds in the event of accidental esophageal intubation. Initial confirmation of the tube placement after assessment of bilateral breath sounds and adequate chest expansion is made with the use of the colorimetric carbon dioxide (CO2) detector. This device detects the delivery of CO2 after six breaths. Additional confirmation of the correct placement of the endotracheal tube must be obtained by using a capnography waveform.4 This is a very reliable indicator of exhaled CO2 and tracheal tube placement because a perfusing cardiac rhythm is needed to deliver CO2 to the lungs. During a cardiac arrest, CO2 may not be present, and CO2 may not be detected even if the ETT is in the correct location. Re-expansion of the bulb of the esophageal detector device indicates tracheal tube placement.9 A chest radiograph is obtained once the endotracheal tube is taped in place to confirm proper tube depth and the position. The nurse and the respiratory therapist must ensure that the child’s ETT remains patent and in correct placement to maintain correct oxygenation and ventilation. Causes of acute deterioration in the intubated pediatric patient may be recalled by using the mnemonic DOPE3: The small infant or child has less facial area for tape adherence for securing the tubes. A method with a low incidence of accidental extubation uses two pieces of cloth tape, split halfway down the middle, creating a “Y” shape (Figure 40-4). The skin of the child is more fragile than that of an adult. Cloth tape may be irritating to the child’s skin. A soft foam dressing (i.e., Mepilex) may be applied to the cheeks, with the securing tape attached on top of the dressing. Breath sounds should be auscultated before and after taping or re-taping of the tubing to ensure that the ETT position has not changed.3 Commercially manufactured devices to secure ETTs are available, but these are limited in infant and pediatric sizes. No single method of securing a tube has been identified as superior for minimizing ETT dislodgement.9 Many types of unconventional mechanical ventilation (e.g., high-frequency, oscillation, jet ventilation) are used, but for most infants and children, standard means of positive-pressure ventilation support use volume or pressure-controlled ventilators. The type of ventilation chosen depends on the child’s size, minute ventilation requirements, and lung compliance. Newer ventilators have flow and pressure triggers that are sensitive enough to ventilate infants and children.10 For the older child, noncontinuous-flow, volume-limited ventilation in synchronized intermittent mandatory ventilation (SIMV) mode is used most often. Pressure support ventilation is also used in the child in conjunction with other modes to assist with spontaneous breathing, especially during the weaning process. Currently used ventilators have flow triggering designed for infants. Some type of synchronized ventilation mode is used almost exclusively for the ventilation of infants and children. Box 40-3 outlines what the characteristics are for the ideal pediatric ventilator for the infant and child.11 Asynchrony may lead to poor oxygenation or volutrauma. Significant asynchrony may require sedation, alone or with neuromuscular blockade. Criteria for weaning and extubation are much more extensive for the adult than for the child, but some guidelines exist for these procedures. SIMV with pressure support ventilation (PSV) is used to wean from positive-pressure ventilation. PSV allows the child to have greater control over breathing, and asynchrony is not a problem. Table 40-3 outlines the indicators for initiating weaning and extubation.9,10 Supplemental oxygen may be supplied after extubation by a nasal cannula, ventilation mask, or oxygen hood. Noninvasive ventilation devices such as nasal or facial CPAP may be an option for the patient, but if the child cannot be managed on these options, reintubation may be necessary. TABLE 40-3 INDICATORS FOR INITIATING WEANING OF THE PEDIATRIC PATIENT From Kline-Tilford A, et al. Pulmonary disorders. In: Hazinski MF, ed. Nursing Care of the Critically Ill Child. 3rd ed. St. Louis: Elsevier; 2013:483. Over the past three decades, tracheostomy has become an increasingly common procedure in children. The primary reasons for having a tracheotomy performed include upper airway obstruction caused by anatomic abnormalities, the anticipated need for prolonged ventilation, or the need for effective pulmonary toilet.12 Most children who require a tracheostomy are younger than 1 year, and a higher incidence is seen among boys.12 A tracheotomy is recommended if an older child is to remain intubated for longer than 2 to 3 weeks. Several types of tracheostomy tubes are available for the child, with the plastic, single-cannula type being the most popular for in-hospital care because it has few complications. Silastic tubes have been recommended for the infant and the child because they are pliable and bend with tracheal movement. Uncuffed tubes usually are preferred for the pediatric patient to prevent subglottic stenosis.13 The diameter of the tracheostomy tube should be carefully selected to avoid any damage to the tracheal wall, to minimize the work of breathing, and to promote translaryngeal airflow, when possible. Table 40-4 describes tracheostomy tube sizes for infants and children. TABLE 40-4 GUIDELINES FOR INTUBATION EQUIPMENT AND TRACHEOSTOMY TUBES FOR PEDIATRICS *Catheter size twice the internal diameter size of any tracheal tube. Based on data from Kuch B. Respiratory monitoring and support. In: Hazinski MF, ed. Nursing Care of the Critically Ill Child. 3rd ed. St. Louis: Elsevier; 2013:1007. Complications with a tracheostomy may be categorized as those occurring early or late. Early complications may occur intraoperatively and up to the first tracheostomy tube change. Studies have shown that a cannula obstruction is the most common early complication.12 Late complications may occur after the first tube change. Some of the most significant late complications include recurrent tracheitis, accidental decannulation, cannula obstruction, subglottic stenosis, suprastomal obstruction, and tracheal ulceration.13 Bronchiolitis is one of the diseases that primarily affects the very young infant and is the most common disease in infants and children less than 2 years of age.14 Bronchiolitis is a term used to describe a condition that affects the lower respiratory tract and results in obstruction of the small airways. Although this disease has a relatively low mortality rate (200-500 deaths per year), it is the most frequent cause of hospitalization in the infant population. 14 However, bronchiolitis is one of the most common diagnoses in children who present in the critical care unit with respiratory failure.15 The disease is characterized by mucosal edema, inflammation, increased mucus production, and sloughing of epithelial cells. This leads to obstruction of the bronchioles, resulting in hypoxemia and hypercapnia.14 Widespread fine end-inspiratory crackles and an expiratory wheeze are heard on auscultation. This clinical pattern may be seen during the first year of life, with most hospital admissions occurring within the first 6 months of life.14 Respiratory syncytial virus (RSV) is the most common cause of viral bronchiolitis, and it infects almost all children by the age of 2 years.16 The peak incidence for viral bronchiolitis occurs during midwinter and into early spring. RSV is highly contagious and may be spread by close contact through droplets. Meticulous hand washing by clinical staff is the most important step to prevent hospital-acquired infections in other patients or staff members.17 RSV infection has an overall low mortality rate, but the mortality rate is 37% among infected infants with congenital heart disease. In children less than 2 years of age, up to 40% of RSV infections may progress to lower respiratory infections.14 Those with cystic fibrosis or bronchopulmonary dysplasia or who are immunocompromised are also at greater risk for more serious disease and for occurrence beyond 1 year of age. The chance of recovery from RSV may be excellent, but reactive airway disease residual effects may be seen several years after infection.18 RSV involves inflammation of respiratory epithelium, which leads to necrosis. The epithelium is replaced with nonciliated tissue. Submucosal edema forms with lymphocytic infiltrates and other alveolar debris. Obstruction occurs from mucous secretions and debris not being cleared because of the lack of ciliated epithelium. Pathologic pulmonary dynamics involve lung hyperinflation almost two times the normal. Obstruction occurs in a patchy distribution with complete obstruction, leading to atelectasis and partial obstruction, which results in hyperinflation. Inspiratory resistance and expiratory resistance are present, along with ventilation–perfusion mismatch, which leads to hypoxemia and some degree of CO2 retention. The probable mechanism in addition to the ventilation–perfusion mismatch is hypoventilation, which results from a marked increase in the work of breathing in the infant.18 The first symptoms to appear are those of an upper respiratory tract infection—sneezing and rhinorrhea. In many cases, a family member has had a respiratory illness. After 2 to 3 days, respiratory distress ensues with increased respirations, coughing, nasal flaring, chest retractions, wheezing, irritability, and feeding difficulties. Fever and lung rhonchi may or may not occur. After bronchiolar obstruction has occurred, these patients present with increased work of breathing. This may lead to muscle fatigue and respiratory failure if the work of breathing exceeds the capacity of the patient’s respiratory muscles. Infants tolerate respiratory loads poorly and are susceptible to fatigue because of the immature pattern of their muscle fibers.19 The overall treatment goal with bronchiolitis is supportive. Oxygen continues to be the primary therapy to decrease the work of breathing and oxygen demands. Depending on the severity of illness, the infant may receive supplemental humidified oxygen by mask, nasal CPAP (despite lung hyperinflation), or mechanical ventilation.5,20 RSV causes airway obstruction, and no therapy has demonstrated an ability to rapidly reduce this obstruction. Suctioning of the airways will be required to help alleviate the signs and symptoms of airway obstruction from mucous secretions. The mucus will be thick and initially cause the infant to be suctioned frequently. Inhaled beta2-agonists, anticholinergic agents, and corticosteroids during the acute or recovery phase have been tried with various degrees of success.14 The use of a clinical pathway for bronchiolitis has been shown to be effective in the literature. A decrease in the use of therapies such as bronchodilators, glucocorticoids, antibiotics, and chest physiotherapy, and a decrease in admission rates and length of stays have occurred in association with the use of a bronchiolitis clinical pathway.14 The current recommendation from the American Academy of Pediatrics for the prevention of RSV is the administration of the monoclonal antibody palivizumab (Synagis, MedImmune). Clinicians may administer palivizumab prophylactically to selected infants and children with chronic lung disease or a history of prematurity (<35 weeks’ gestation) or with congenital heart disease.17 Palivizumab is administered in 5 monthly doses during the RSV season, usually beginning in November or December, at a dose of 15 milligrams per kilogram (mg/kg) per dose administered intramuscularly.17 Asthma is a chronic inflammatory disorder of the airways, in which many cells and cellular elements, including mast cells, eosinophils, T lymphocytes, neutrophils, and epithelial cells, play a role.21 An acute asthma exacerbation is an event of progressive wheezing, cough, chest tightness, shortness of breath, or a combination of all these symptoms. Asthma is identified as the disease with a triad of physiologic processes, airway inflammation, edema, and airway hyperactivity. Inflammation has been recognized as the primary underlying cause in the pathogenesis of this disease.21 The treatment for asthma is aimed at decreasing and reducing airway inflammation. The universal feature of the inflammatory response in asthma includes the activation and infiltration of the airway by cells. The early phase in the inflammation reaction is caused by a trigger, and this may be different with each child. The immediate response to this trigger is bronchospasm and smooth muscle contraction caused by the mediators from the various inflammatory cells in the airway. If this early phase is not responsive to beta2-agonists, a late phase will occur about 6 to 9 hours after the initial exposure to the trigger.21 This will lead to increased release of the mediator cells in the airway and produce cellular infiltration, airway edema, mucus secretions, bronchospasm, and smooth muscle contraction. Without treatment, atelectasis and mucous plugging may occur in the child. Assessing severe asthma in the infant is different compared with that in the older child because of the anatomic and physiologic differences between them. Physiologic changes may progress rapidly to respiratory failure in the infant. Table 40-5 outlines guidelines for classifying the severity of an asthma exacerbation. A moderate asthma episode requires hospitalization with close monitoring. In a severe event, the child may only able to speak in short phrases or not at all. The position of comfort or degree of agitation should be noted; sitting upright and unable to lie down indicates severe distress. 22 A severe episode requires critical care, with intubation and ventilation equipment readily available.5 TABLE 40-5 GUIDELINES FOR ASSESSING SEVERE ASTHMA IN INFANTS AND CHILDREN Based on data from Gomez M, Felauer A. Asthma. In: Reuter-Rice K, et al, eds. Pediatric Acute Care: A Guide for Interprofessional Practice. Burlington, MA: Jones & Bartlett Learning, 2012; Marcoux K: Current management of status asthmaticus in the pediatric ICU. Crit Care Nurs Clin North Am. 2005;17:463. Standard treatment for the pediatric patient who has asthma includes receiving oxygen, beta-adrenergic therapy, corticosteroids, and anticholinergic medications, as indicated.5,21 Pharmacologic therapy is based on the concept of reducing airway inflammation. Beta-adrenergic agonists are the first-line agents in the treatment of asthma. Beta-agonist medications that are selective for beta2-receptors on airway smooth muscle (e.g., albuterol, levalbuterol) are preferred to avoid the stimulation of the beta1-cardiac receptors.21 These medications are given by nebulation intermittently or continuously. Anticholinergic medications in conjunction with beta-agonists improve pulmonary function in children, especially school-age patients.21 These medications may decrease bronchomotor tone and secretions. The most commonly administered inhaled anticholinergic is ipratropium bromide. This medication works synergistically with beta-agonists to improve and prolong bronchodilation. Ipratropium bromide should be administered with albuterol in a nebulizer and not given alone. Corticosteroids are also an important part of the treatment for airway inflammation. Corticosteroids may be administered enterally or parenterally. Both methods are equally efficacious in the treatment of asthma.21 The peak effect for corticosteroids is virtually the same with either route. Treatment for asthma does not have to be held up because of a lack of intravenous access in the pediatric patient. The corticosteroid of choice is a glucocorticoid (e.g., prednisone, prednisolone, methylprednisolone). Intravenous magnesium sulfate is administered to pediatric patients as an adjunct therapy, as it provides smooth muscle relaxation to patients with severe or life-threatening asthma events.22 Magnesium is a physiologic calcium antagonist, which has a direct effect on the calcium uptake in the muscle, causing smooth muscle relaxation.5 Magnesium sulfate (12 to 50 mg/kg) is administered intravenously, usually over 20 minutes.23 Critical care management of the child with status asthmaticus involves humidified oxygen to maintain an oxygen saturation of more than 95%, combined with the pharmacologic therapies. Heliox is a mixture of 80% helium and 20% oxygen, which makes this mixture lighter than air. Administration of heliox is effective in decreasing airway resistance and in decreasing the work of breathing. The use of heliox does not appear to have adverse effects, and its administration may improve the status of the child.21 The use of noninvasive positive-pressure ventilation by means of nasal prongs or a facemask may avoid the need for intubation. Some of the indications for considering mechanical ventilation are respiratory muscle fatigue, markedly diminished or absent breath sounds, pulsus paradoxus greater than 20 to 40 mm Hg, deterioration in mental status, and partial pressure of oxygen (Pao2) less than 70 mm Hg on 100% Fio2.21 The child and family should be informed of the management approach and plan of care. Pediatric patients, who have status asthmaticus, if properly and effectively treated, may return to their usual state of health but will require close follow-up by their pediatrician or pulmonologist.21 An apparent life-threatening event (ALTE) is an episode that is characterized by a combination of apnea, change in skin color, marked changes in muscle tone, and choking or gagging, as defined by the National institutes of Health Consensus Development Conference on infant Apnea in 1986.24 An ALTE is an apneic event, which is combined with pallor or cyanosis and a loss of muscle tone that requires vigorous stimulation all the way to full cardiopulmonary resuscitation (CPR).25 Many abnormalities have been found through detailed investigation and reported in the literature. They include relationships among gastroesophageal reflux disease (GERD), apnea, and sleep-related impairment of respiratory control when the infant sleeps in the prone position.25 In one study, GERD was documented in 55% of the infants with ALTE.25 The next highest documented condition (30%) related to ALTE was chronic gastric volvulus.25 Chronic gastric volvulus is a condition in which all or part of the stomach has rotated over the physiologic range. In infants, this condition may occur easily because of weak ligaments around the stomach. This may cause a large amount of gas to accumulate and push the stomach upward, resulting in worsening of the volvulus and causing vomiting and apnea.25 Vagally induced fainting spells may occur in specific circumstances such as after vomiting, feeding, crying, bathing, and pain. Vagal overstimulation is probably an underestimated condition in ALTE infants.25 Some of the infants with ALTE requiring resuscitation ultimately pass away, and their condition may be classified as sudden infant death syndrome (SIDS), raising the possibility that ALTE and SIDS are the same disease.26 Although some similarities exist in clinical presentations, ALTE and SIDS should not be considered different manifestations of the same disease process. ALTE and SIDS are disorders of the first year of life; each event occurs at different ages, with ALTE manifesting 10 weeks earlier than SIDS on average.26 One of most widely used diagnostic tests for ALTE is the use of a continuous recording of cardiorespiratory patterns, a pneumocardiogram.25 The four channels monitor heart rate, respiratory rate, nasal airflow, and oxygen saturation. Some infants who have normal results may still have subsequent apneic episodes. The critical care nurse is a major source of support for families in terms of education, observation of the infant’s status, and immediate intervention during an apneic episode. The appropriately sized resuscitation equipment should be available at the bedside of these infants. Episodes of apnea must first be treated with gentle shaking of the infant or tapping the bottoms of the infant’s feet while observing for return of effective respirations. Slight extension of the neck to reopen the airway may be attempted. If recovery does not occur, manual ventilation with bag and mask at the infant’s normal respiratory rate for age should be performed. This manual ventilation should continue until the infant’s normal respiratory pattern and heart rate return. For frequent episodes (i.e., more than two to three per hour) or for those who require prolonged manual ventilation, other treatments include prone positioning, rocker beds, recurrent cutaneous stimulation, nasal CPAP, or a switch to continuous gavage feedings to help control symptoms of GERD. Treatment may also include the use of respiratory stimulant drugs such as theophylline or caffeine.25 The therapeutic ranges are 6 to 13 microgram per milliliter (mcg/mL) of theophylline and 10 to 20 mcg/mL of caffeine. These infants are sent home with a continuous home monitor until they have gone at least 3 months without an ALTE requiring intervention. Topics for discharge education for parents need to include the following: 1) Attend a CPR class prior to discharge; 2) ensure that the infant will reside in a smoke-free environment; 3) have a firm mattress for the infant for sleeping; 4) breastfeed the infant, if possible; 5) avoid overheating the infant; 6) do not permit bed linens to cover infant’s face; and 7) if a “blue spell” is noted, seek immediate medical attention.27
The Pediatric Patient
Respiratory System
Anatomy and Physiology
Upper Airway
Chest Mechanics
Assessment and Oxygen Devices
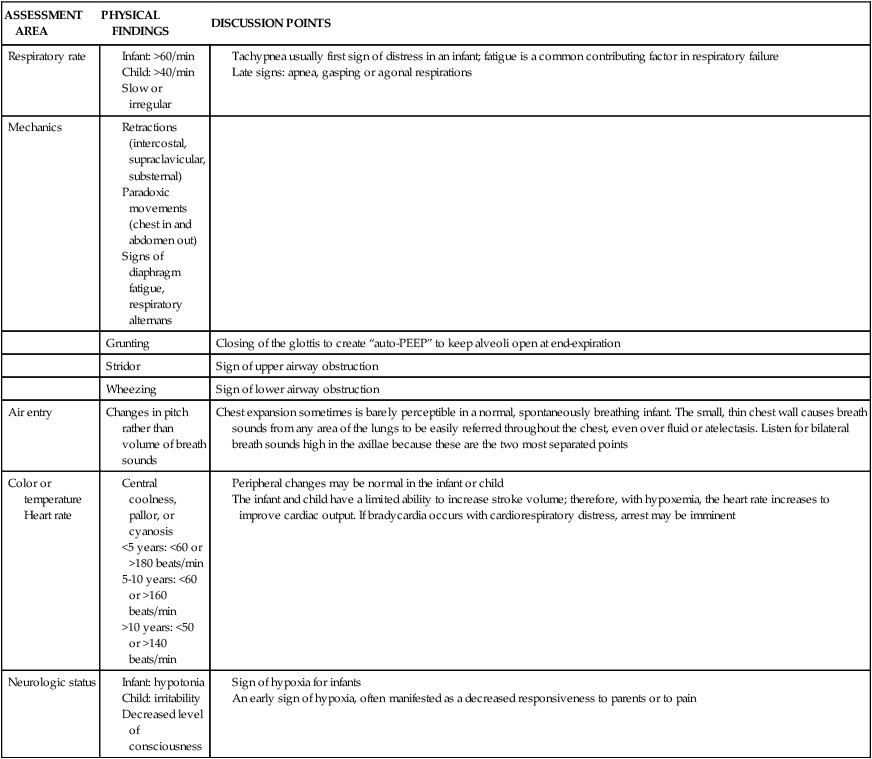
ASSESSMENT AREA
PHYSICAL FINDINGS
DISCUSSION POINTS
Respiratory rate
Mechanics
Grunting
Closing of the glottis to create “auto-PEEP” to keep alveoli open at end-expiration
Stridor
Sign of upper airway obstruction
Wheezing
Sign of lower airway obstruction
Air entry
Changes in pitch rather than volume of breath sounds
Chest expansion sometimes is barely perceptible in a normal, spontaneously breathing infant. The small, thin chest wall causes breath sounds from any area of the lungs to be easily referred throughout the chest, even over fluid or atelectasis. Listen for bilateral breath sounds high in the axillae because these are the two most separated points
Color or temperature
Heart rate
Neurologic status
Airway Positioning
Supplemental Oxygen Devices
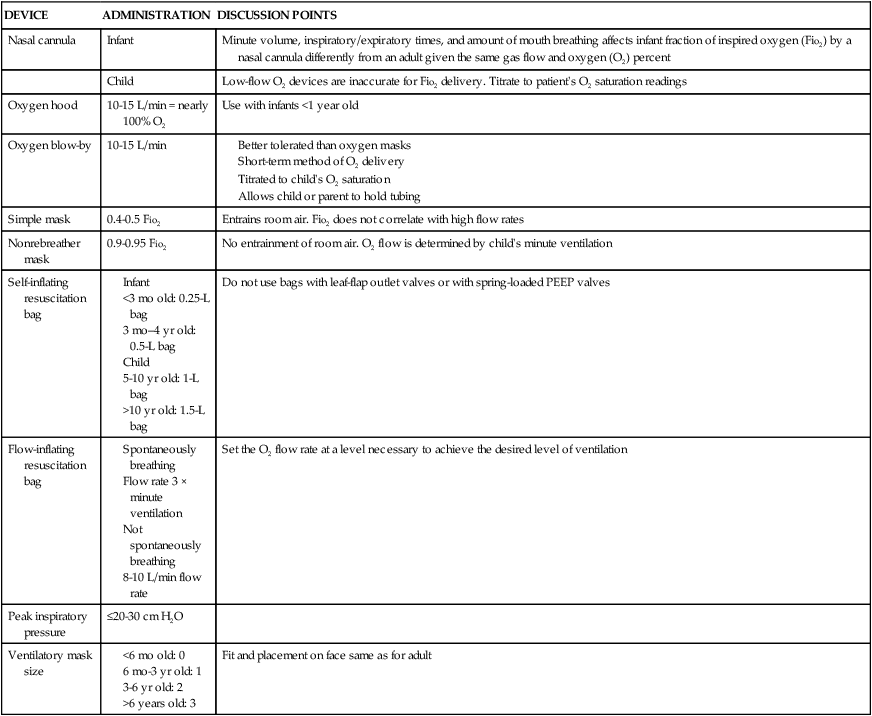
DEVICE
ADMINISTRATION
DISCUSSION POINTS
Nasal cannula
Infant
Minute volume, inspiratory/expiratory times, and amount of mouth breathing affects infant fraction of inspired oxygen (Fio2) by a nasal cannula differently from an adult given the same gas flow and oxygen (O2) percent
Child
Low-flow O2 devices are inaccurate for Fio2 delivery. Titrate to patient’s O2 saturation readings
Oxygen hood
10-15 L/min = nearly 100% O2
Use with infants <1 year old
Oxygen blow-by
10-15 L/min
Simple mask
0.4-0.5 Fio2
Entrains room air. Fio2 does not correlate with high flow rates
Nonrebreather mask
0.9-0.95 Fio2
No entrainment of room air. O2 flow is determined by child’s minute ventilation
Self-inflating resuscitation bag
Do not use bags with leaf-flap outlet valves or with spring-loaded PEEP valves
Flow-inflating resuscitation bag
Set the O2 flow rate at a level necessary to achieve the desired level of ventilation
Peak inspiratory pressure
≤20-30 cm H2O
Ventilatory mask size
Fit and placement on face same as for adult
Endotracheal Intubation
Procedure
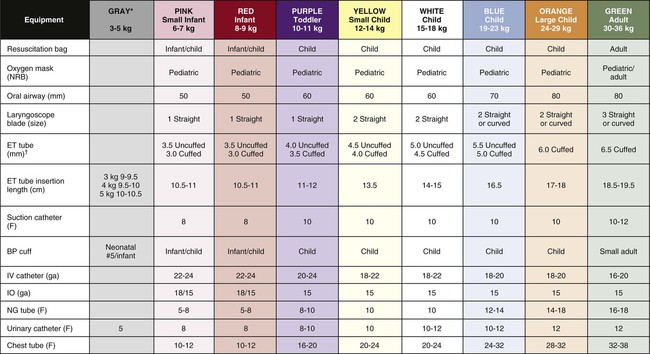
BP, Blood pressure; ET, endotracheal; F, French; IO, intraosseous; IV, intravenous; NG, nasogastric; NRB, nonrebreathing.
*For Gray column, use Pink or Red equipment sizes if no size is listed.
†Per 2010 AHA Guidelines, in the hospital, cuffed or uncuffed tubes may be used. (Adapted from Broselow Pediatric Emergency Tap. Distributed by Armstrong Medical Industries. Lincolnshire, IL. Copyright 2007 Vital Signs, Inc. All rights reserved).
Securing Endotracheal and Nasotracheal Tubes
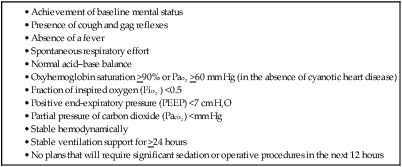
Mechanical Ventilation
Tracheostomies
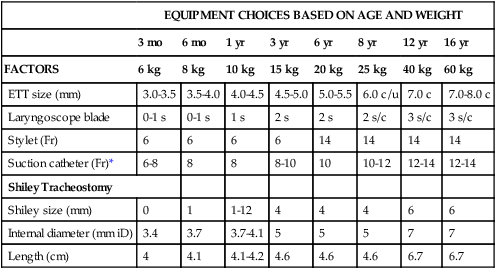
EQUIPMENT CHOICES BASED ON AGE AND WEIGHT
3 mo
6 mo
1 yr
3 yr
6 yr
8 yr
12 yr
16 yr
FACTORS
6 kg
8 kg
10 kg
15 kg
20 kg
25 kg
40 kg
60 kg
ETT size (mm)
3.0-3.5
3.5-4.0
4.0-4.5
4.5-5.0
5.0-5.5
6.0 c/u
7.0 c
7.0-8.0 c
Laryngoscope blade
0-1 s
0-1 s
1 s
2 s
2 s
2 s/c
3 s/c
3 s/c
Stylet (Fr)
6
6
6
6
14
14
14
14
Suction catheter (Fr)*
6-8
8
8
8-10
10
10-12
12-14
12-14
Shiley Tracheostomy
Shiley size (mm)
0
1
1-12
4
4
4
6
6
Internal diameter (mm iD)
3.4
3.7
3.7-4.1
5
5
5
7
7
Length (cm)
4
4.1
4.1-4.2
4.6
4.6
4.6
6.7
6.7
Bronchiolitis
Pathophysiology
Clinical Assessment
Treatment
Status Asthmaticus
Pathophysiology
Clinical Assessment
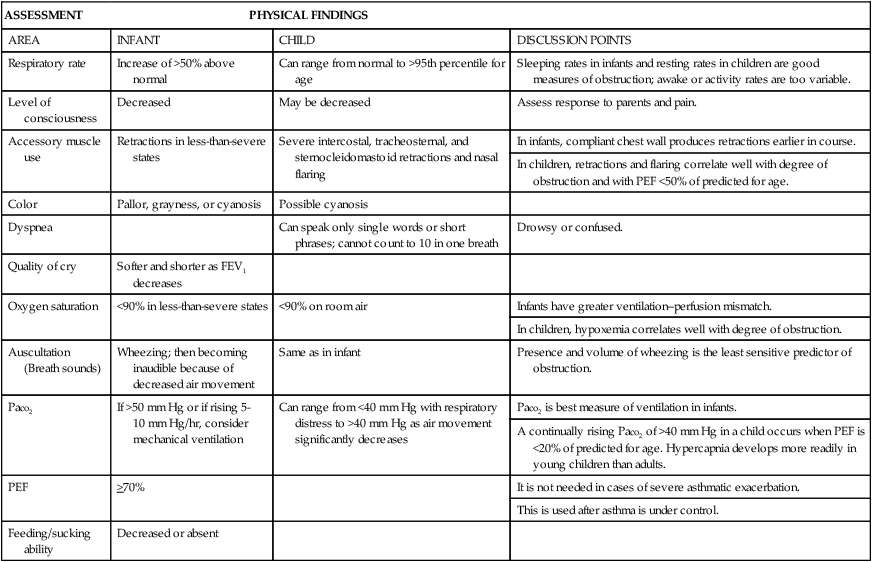
ASSESSMENT
PHYSICAL FINDINGS
AREA
INFANT
CHILD
DISCUSSION POINTS
Respiratory rate
Increase of >50% above normal
Can range from normal to >95th percentile for age
Sleeping rates in infants and resting rates in children are good measures of obstruction; awake or activity rates are too variable.
Level of consciousness
Decreased
May be decreased
Assess response to parents and pain.
Accessory muscle use
Retractions in less-than-severe states
Severe intercostal, tracheosternal, and sternocleidomastoid retractions and nasal flaring
In infants, compliant chest wall produces retractions earlier in course.
In children, retractions and flaring correlate well with degree of obstruction and with PEF <50% of predicted for age.
Color
Pallor, grayness, or cyanosis
Possible cyanosis
Dyspnea
Can speak only single words or short phrases; cannot count to 10 in one breath
Drowsy or confused.
Quality of cry
Softer and shorter as FEV1 decreases
Oxygen saturation
<90% in less-than-severe states
<90% on room air
Infants have greater ventilation–perfusion mismatch.
In children, hypoxemia correlates well with degree of obstruction.
Auscultation (Breath sounds)
Wheezing; then becoming inaudible because of decreased air movement
Same as in infant
Presence and volume of wheezing is the least sensitive predictor of obstruction.
Paco2
If >50 mm Hg or if rising 5-10 mm Hg/hr, consider mechanical ventilation
Can range from <40 mm Hg with respiratory distress to >40 mm Hg as air movement significantly decreases
Paco2 is best measure of ventilation in infants.
A continually rising Paco2 of >40 mm Hg in a child occurs when PEF is <20% of predicted for age. Hypercapnia develops more readily in young children than adults.
PEF
>70%
It is not needed in cases of severe asthmatic exacerbation.
This is used after asthma is under control.
Feeding/sucking ability
Decreased or absent
Treatment
Apparent Life-Threatening Event
Pathophysiology
Monitoring
Treatment
Discharge Education
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


The Pediatric Patient
Get Clinical Tree app for offline access