Chapter 41 Senescence, or aging, is characterized by several physiologic changes. Incidence of disease and chronic conditions increases in older adults, although physiologic decline occurs independently of disease and is responsible for development of symptoms at an earlier stage of disease in older adults than in their younger counterparts.1 Changes in physiologic function are important to consider when caring for older adult patients. Various physiologic changes that occur with aging warrant special physical examination techniques.2 Clinicians must distinguish between changes in health caused by physiologic or pathologic processes. Comprehensive physiologic, psychologic, and environmental nursing assessment of the older adult and significant others/family members is vital in critical care and subsequent coordination of care.3 The purpose of this chapter is to familiarize the critical care nurse with literature and research on care of the critically ill older adult. The senescent population is increasing worldwide. In 2009 there were approximately 39.6 million American residents over the age of 65. Average life expectancy in 2009 was 84 years.4 In 2030 the number of Americans older than 65 years will be close to 70 million.2 Demographic shifts and overall population increases have resulted in changes in provision and reimbursement of health care delivery and services. Medicare and Medicaid have become major payers of hospital services, creating challenges for health care providers and organizations.5 In 2008 the Medicare program had 45 million enrollees and expenditures of $468 billion, which was a $36 billion increase over 2007.4 The Patient Protection and Affordable Care Act (PPACA) is estimated to increase coverage to an additional 32 million uninsured individuals by 2014.5 Health care services rendered to persons age 65 and over in 2007 included 289.7 million ambulatory visits, 13.9 million hospital in-patient care discharges, 1.3 million nursing home residents, 1 million home health patients, and 868,100 hospice discharges.4 Patients older than 65 years currently account for 42% to 52% of admissions to critical care units and almost 60% of all critical care unit days.2 Recent estimates of critical care costs account for 4% of national health expenditures, or approximately $81.7 billion.6 Health conditions experienced by older persons include hypertension (64% to 80%), heart disease (31% to 46%), cancer (17% to 23%), and diabetes (27%). Causes of mortality in persons over age 65 include heart disease (28%), cancer (22%), stroke (7%), chronic lower respiratory diseases (6%), and Alzheimer’s disease (4%).4 All mortality rates declined between 1997 and 2007 except Alzheimer’s disease, which increased more than 50%.7 Many of these conditions result in admission to critical care units; therefore critical care nurses need to understand key components in caring for older adult patients. Advancing age has many effects on the cardiovascular system (Table 41-1). Age-related anatomic and cellular changes in the myocardium and peripheral vasculature have significant impact on the critically ill older adult.2 Age is a major risk factor for cardiovascular disease in older adults, leading to more cardiovascular events in this population when admitted to the critical care unit.8 Cardiac and arterial system changes include atherosclerosis, hypertension, myocardial infarction, and stroke. Pathologic alterations of aging include hypertrophy, altered left ventricular (LV) function, increased arterial stiffness, and impaired endothelial function.8 TABLE 41-1 AGE-RELATED CARDIOVASCULAR CHANGES LV, Left ventricular; PUFA, polyunsaturated fatty acids; VSMC, vascular smooth muscle cells. Adapted from Strait JB, Lakatta EG. Aging-associated cardiovascular changes and their relationship to heart failure. Heart Failures Clinic. 2012;8(1):143. Changes in the electrocardiogram (ECG) include decreased R-wave and S-wave amplitude, increased P-R interval, and increased Q-T duration reflective of prolonged rate of relaxation (Table 41-2).9 The QRS axis shifts leftward with age, perhaps due to increased LV wall thickness or hypertrophy.9 Intrinsic heart rate decreases with age in the deficiency of parasympathetic influences; however, increases in atrial and ventricular arrhythmias increase in prevalence.9 Cardiac dysrhythmias include atrial fibrillation, paroxysmal supraventricular tachycardia, with the most common dysrhythmia of premature ventricular contraction (PVC).9 Reports show that 3% to 4% of patients older than 60 years of age experience atrial fibrillation9; incidence may be as high 10% in octogenarians.8 A study of healthy geriatric patients with 24-hour ambulatory ECG recordings showed that 13% to 50% experienced paroxysmal supraventricular tachycardia in frequency with exercise.9 Older individuals also have a high prevalence of left ventricular hypertrophy (LVH), which predisposes them to ventricular arrhythmias and sudden death. Atrial fibrillation is thought to be related to increased arterial stiffness, reduced LV compliance and rate control, and anticoagulation is recommended for most patients.10 Age-associated cardiac conduction changes (even if asymptomatic) are predictive of future cardiac morbidity and mortality older adults.9 TABLE 41-2 AGE-RELATED CHANGES IN RESTING ELECTROCARDIOGRAPHIC VARIABLES Modified from Strait JB, Lakatta EG. Aging-associated cardiovascular changes. Heart Failure Clin. 2012;8:143. Collagen is the principal noncontractile protein occupying the cardiac interstitium. Since myocardial collagen content increases with age, increased myocardial collagen content renders the myocardium less compliant and may be responsible for increased loading of blood vessels.11 Slipping of myocytes can adversely affect diastolic filling, and structural changes that occur in the walls of blood vessels can lead to increased systolic blood pressure (SBP).11 Collagen type I is more plentiful in the aging heart than the young heart, which contains more collagen type III. Collagen type I is less distensible than type III collagen, and both have different physical and mechanical properties.11 Consequently, the left ventricle must develop a higher filling pressure for a given increase in ventricular volume. Decreased LV compliance may be evident in the older adult by the presence of an S4 heart sound.12 Chronic heart failure (CHF) affects approximately 5.7 million people in the United States of America and may be a contributing cause of death in 282,000 individuals per year, at a predicted cost of $39 billion annually.13 Approximately 50% of heart failure (HF) cases are found within the 6% of U.S. population older than age 75.9 Incidence of myocardial infarction (MI) in the Framingham Heart Study demonstrated that the rate of MI doubles for men and increases more than five-fold in women from the age group of 55 to 64 years to the age group of 85 to 94 years.13 Similar results were seen in the Atherosclerosis Risk in Communities (ARIC) surveillance study.13 Critical care nurses should recognize that older adult patients are not only more likely than young patients to experience an MI, but are more likely to die from an MI. MI is one of the most common causes of CHF leading to HF via an intricate process known as left ventricular (LV) remodeling.13 Age-associated changes in arterial structure and function cause large vessels to become less distensible, leading to prolonged systolic contraction, lengthened diastolic relaxation, increased myocardial oxygen demand, and diminished organ perfusion.9 Additionally, atherosclerotic coronary arteries may limit blood flow to the myocardium, creating a higher risk of developing myocardial ischemia or infarction. MI in older adults is often associated with ST-segment depression rather than ST elevation. Sensation of chest pain may be altered and may be less intense and of shorter duration.2 Other atypical symptomatology may include dyspnea, confusion, and failure to thrive, which results in unrecognized signs and symptoms of cardiac problems and delays in diagnosis and treatment.2 The aging heart undergoes a modest degree of hypertrophy and thickening of the LV wall without significant changes in LV cavity size.9 Increase in LV wall thickness is primarily due to growth in muscle cell size, which can lead to delayed early diastolic filling and increased cardiac pressures.9 The early diastolic filling period and isovolumic phase of myocardial relaxation are prolonged in the older adult human myocardium. These changes may suggest diastolic dysfunction, but do not translate into decreases in end-diastolic volume or stroke volume.9 Myocardial contraction is dependent on intracellular levels of free calcium and the sensitivity of the contractile proteins for calcium.9 The senescent myocardium may have a lower threshold for atrial and ventricular arrhythmias due to changes in gene expression of proteins that regulate calcium.9 Prolonged duration of contraction (systole) is caused in part by a slowed or delayed rate of myocardial relaxation. This may be an adaptive mechanism to preserve contractile function compromised by age-related increases in afterload.9 Aging is associated with a decline in exercise performance due to many physiologic variables. Cardiac performance is dependent on the heart’s ability to increase and maintain cardiac output (CO), allowing oxygen delivery to the peripheral tissues. During exercise CO is increased by several mechanisms, the most important of which are increased heart rate, increased inotropic state of the myocardium, and decreased aortic impedance.9 The Baltimore Longitudinal Study of Aging reported that older adults’ diminished ability to exercise was not related to a difference in CO response.9 Maximal heart rate achieved during exercise is attenuated in older adults; however, decreased heart rate response is accompanied by increased LV end-diastolic volume. As adults age, there is a progressive decline in VO2max, which begins in the second decade of life and falls by roughly 10% per decade. In summary, an older individual’s ability to exercise may be limited by reduction in cardiac reserve, increased vascular afterload, arterial-ventricular load mismatching, pulmonary function, reduced intrinsic myocardial contractility, impaired autonomic regulation, and physical deconditioning.9 The aging effects on the peripheral vascular system are revealed in a gradual but linear rise in SBP up until age 80 years when values tend to plateau.2 Diastolic blood pressure (BP) is less affected by age and generally remains the same or decreases.2 Important determinants of SBP include vascular compliance and blood volume within the system. Endothelial function and compliance of the vasculature is determined by cell type and tissue composition. In older adults, the intimal layer of the large and distal arteries thickens due to an increase in smooth muscle cells and connective tissue.8 This gradual decrease in arterial compliance, or “stiffening of the arteries,” is sometimes referred to as arteriosclerosis. Arteriosclerotic changes are also accompanied by changes caused by atherosclerosis. Atherosclerosis is the accumulation of lipoproteins and fibrinous products such as platelets, macrophages, and leukocytes within a vessel.8 Arteriosclerotic and atherosclerotic processes cause arteries to become progressively less distensible and alter the vascular pressure-volume relationship. These changes are clinically significant because small changes in intravascular volume are accompanied by disproportionate increases in SBP, leading to increases in afterload and development of concentric (pressure-induced) ventricular hypertrophy in the older adult.8 Improved cardiovascular care has led to delay of cardiovascular disease; however, monitoring of cardiovascular risk factors such as cholesterol, BP, and obesity is now needed in the older adult population.14 Lipoprotein levels increase with advancing age, adding to risk factors for development and progression of atherosclerosis. Serum lipoproteins are particles that contain various amounts of cholesterol, triglycerides, phospholipids, and apoproteins. The classification of lipoproteins is based on their size and relative concentration of cholesterol, triglycerides, and apoproteins. The five principal serum lipoproteins are chylomicrons, low-density lipoproteins (LDLs), very-low-density lipoproteins (VLDLs), intermediate-density lipoproteins (IDLs), and high-density lipoproteins (HDLs). Lipoprotein (Lp) (a) is composed of an LDL-like particle that in elevated levels has been implicated as a strong risk factor for coronary heart disease (CHD) and stroke.15 Timely identification of high-risk individuals with high Lp(a) may allow for therapy such as statins to reduce LDL cholesterol to guideline-recommended levels.15 Another condition that may warrant attention in persons older than 65 years is metabolic syndrome. Metabolic syndrome is characterized by a group of risk factors, including abdominal obesity, high triglycerides, low HDLs, small LDLs, elevated BP, proinflammatory, prothrombotic states, and high plasma insulin (insulin resistance).14 Metabolic syndrome has been reported to increase cardiovascular and mortality risk in middle-aged populations due to glucose metabolism and insulin resistance that is linked to cholesterol metabolism.14 Reduction of risk associated with diabetes and metabolic syndrome may be modulated by manipulation of cholesterol absorption, leading to fewer events and improved survival in older adult cardiovascular patients.14 The population is aging, with hypertension affecting most individuals older than 65 years of age.10 Pathophysiology of hypertension in older adults results from changes in arterial structure and function that decrease distensibility of large vessels, reduce forward circulation flow, increase pulse wave velocity, cause late SBP augmentation, and increase myocardial oxygen demand, all of which limit organ perfusion.10 Older adults with poor BP control have an increased risk of cerebrovascular disease (CVD), coronary artery disease (CAD), disorders of LV structure and function, aortic and peripheral arterial disease, chronic kidney disease (CKD), ophthalmologic disorders, and quality of life (QOL) issues.10 Secondary issues related to hypertension include renal artery stenosis, obstructive sleep apnea, primary aldosteronism, and thyroid disorders. Older adult patients with higher baseline SBP or longer duration of hypertension often experience autonomic dysfunction, microvascular damage, and CKD related to reduced renal tubular mass, fewer transport pathways for potassium excretion, and hyperkalemia.10 Glomerulosclerosis and interstitial fibrosis lead to progressive renal dysfunction and reduction in GFR, increased intracellular sodium, reduced sodium–calcium exchange, and volume expansion.10 Older adults generally have contracted intravascular volumes and impaired baroreflexes, which may be exacerbated by diuretics, sodium, and water depletion, causing orthostatic hypotension. Volume overload is commonly due to excessive salt intake, inadequate kidney function, or insufficient diuretic therapy. An age-related decline in plasma renin activity, tubular function, and glomerular filtration rate affects overall sodium and water homeostasis.2 Risk stratification tools like the Framingham Risk Score can be used to predict MI, stroke, or CVD.10 Evaluation of older adult patients with known or suspected hypertension should include identification of reversible and/or treatable causes, evaluation of organ damage, assessment for other risk factors, and identification of treatment barriers. Recommended laboratory testing for hypertensive patients includes urinalysis, serum chemistries, lipid profile, blood glucose testing (including hemoglobin A1c), and ECG/echocardiography.10 Treatment of cardiovascular risk factors in older adults includes aggressive treatment of dyslipidemia with lipid-lowering medication, control of blood glucose, and lifestyle modification. There should be consideration that QOL issues such as cognitive function, physical activity, and sexual function are reduced by aging and disease.10 Drug treatment is recommended for older adult hypertensive patients with attention to alterations in medication distribution and disposal, changes in homeostatic CV control, and QOL factors. Lifestyle modification may be the only treatment necessary for milder forms of hypertension in older adults. Smoking cessation, weight reduction, increased physical activity, and sodium restriction result in greater benefit in older individuals than in young adults.10 Antihypertensive treatment in older adults should be started at the lowest dose and gradually increased to the maximum dose needed to achieve an SBP 140 mm Hg. A second medication from another class should be added if the initial therapeutic response does not achieve an SBP 140 mm Hg. A third medication from another class can be added after maximum doses of two classes of medications are reached. Recommended initial medications include thiazide diuretics (hydrochlorothiazide), calcium antagonists (diltiazem, nicardipine), angiotensin-converting enzyme inhibitors (captopril, lisinopril), angiotensin-receptor blockers (losartan), and beta-blockers (metoprolol, carvedilol).10 Refer to cardiovascular chapter for more information on cardiac medications. Older adult patients who have CAD, hypertension, stable angina, or previous MI should be prescribed a beta-blocker such as metroprolol for initial therapy. A long-acting calcium antagonist (CA) such as verapamil may be added to initial therapy if the BP remains elevated or if angina persists. An angiotensin-converting enzyme inhibitor (ACEI) such as benzapril is indicated if LV ejection fraction is reduced and/or if HF is present. Verapamil and diltiazem are not recommended if significant LV systolic dysfunction or conduction system disease is present.10 A BP of 130/80 mm Hg should be targeted in patients with HF and CAD.10 Older adult patients with hypertension and systolic HF should receive a diuretic, beta-blocker, ACEI, and aldosterone antagonist. Limited studies support use of ARBs and ACEIs in the presence of chronic renal dysfunction. Older, black hypertensive patients with HF may benefit from a regimen including isosorbide dinitrate and hydralazine. Older adult patients with hypertension and asymptomatic LV systolic dysfunction should be treated with ACEIs and beta-blockers. Because HF may improve in hypertensive older adult patients with renal artery stenosis after renal revascularization, this should be considered when HF patients are refractory to conventional management of hypertension. Older adults with diabetes mellitus, hypertension, and nephropathy should be treated initially with ACEIs or ARBs. In older adults with prediabetes/metabolic syndrome, attempts should be made to reduce BP using lifestyle modification. If medications are needed, thiazide diuretics increase risk for incident diabetes mellitus and hyperglycemia.10 Medication regimens for hypertensive older adult patients with CKD include angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers (ARBs). ACEIs should be considered for patients with nondiabetic nephropathy and ACEIs or ARBs are indicated if proteinuria is present. Older adult patients with hypertension and diabetes mellitus have a higher mortality risk than similarly aged nondiabetic controls.10 Hypertension and HF are both associated with a more pronounced decline in renal function in older age. With the recognition of early renal dysfunction, more patients should benefit from aggressive therapy.10 Baroreceptors are mechanoreceptors that respond to stretch and other changes in the blood vessel wall. They are located at the bifurcation of the common carotid artery and the aortic arch.16 Impulses arising in the baroreceptor region project to the vasomotor center (nucleus of tractus solitarius) in the medulla. Abrupt changes in BP caused by increases in peripheral resistance, CO, or blood volume are sensed by baroreceptors, resulting in an increase in impulse frequency to the vasomotor center within the medulla. This increase inhibits vasoconstrictor impulses arising from the vasoconstrictor region within the medulla, resulting in a decrease in heart rate and peripheral vasodilation returning BP to within normal limits.16 The baroreflex can be tested by measuring heart rate response (i.e., increase or decrease in heart rate) after administration of a pressor or a depressor agent and by changing the patient’s position from lying to standing. Baroreflex-mediated tachycardia response to depressor agents is also attenuated in older adults. There are several reports of attenuation in heart rate response of older adults after changes in position.16 When an individual changes position from supine to standing, distribution of blood volume changes, resulting in a reduction in CO and BP. This is known as orthostatic hypotension.16 Simultaneously baroreceptors increase heart rate and maintain BP by increasing CO. The baroreceptor-reflex response also mediates changes in peripheral resistance and force of myocardial contraction, which offsets the drop in BP. Prevalence of orthostatic hypotension is greater in geriatric patients; therefore, judicious use of antihypertensive medications is recommended.16 Treatment with medication should be considered when nonpharmacologic interventions are unsuccessful. Medication therapy for hypertension should target a SBP of 140 mm Hg to 145 mm Hg and diastolic of greater than 95 mm Hg in patients older than 80 years of age.10 Heart rate control can be managed with beta-blockers and calcium channel blockers. Amiodarone can be used for conversion and maintenance of sinus rhythm with atrial fibrillation.10 Aging increases the risk of major hemorrhage in patients with atrial fibrillation, with or without warfarin therapy.10 Anticoagulation therapy can be complicated by polypharmacy, simultaneous use of antiplatelet medications, uncontrolled hypertension, and poorly controlled anticoagulation therapy.17 Ventricular dysrhythmias are best managed with cardiac-resynchronization therapy and pharmacologic therapy in patients with advanced HF.18 Cardiac resynchronization therapy (CRT) is a type of pacemaker or pacemaker/defibrillator that resynchronizes the contractions of the heart’s ventricles by sending electrical impulses to the heart muscle, assisting blood flow and vascular remodeling. CRT has become an established therapy for advanced heart failure LV reverse remodeling, with significant reduction in LV volumes and improvement in LVEF.18 Combination pharmacologic therapy in older adult patients provides opportunity for enhanced efficacy, avoidance of adverse effects, enhanced convenience, and compliance.17 Older adult patients will often discontinue or take medications inappropriately, resulting in failure to reach recommended targets and outcomes.17 The average older adult patient takes six prescription medications; therefore, daily polypharmacy, nonadherence, and potential medication interactions are important concerns.17 Nonadherence to medication regimens may be due to competing health problems, socioeconomic status, treatment complexity, side effects, and cost of medications.19 Clinical and fiscal consequences of medication nonadherence can lead to increased hospital readmissions and adverse medical events.19 Assessing factors that affect medication adherence such as literacy, eyesight, understanding of directions, education level, severity of disease, and comorbid conditions may assist in understanding barriers to compliance.17 Patient management is most effective when intraprofessional health care teams collaborate to achieve and maintain goals. Novel opportunities for therapeutic management and compliance include smart phones, telemedicine, and computerized technologies. Other collaborative efforts include effective communication with local pharmacies, medication reconciliation at discharge, follow-up discharge phone calls, and timely provider handoff. Many changes in the pulmonary system that occur with aging are reflected in tests of pulmonary function and include changes in compliance of the chest wall, in static elastic recoil of the lung, and in strength of respiratory muscles.20 Progressive changes due to age should not alter the older adult’s ability to breathe effortlessly; however, factors such as repeated exposure to environmental pollutants and frequent pulmonary infections may accelerate age-related changes. Changes in tests of pulmonary function are listed in Tables 41-3 and 41-4. TABLE 41-3 AGE-RELATED CHANGES IN COMMONLY PERFORMED PULMONARY FUNCTION TESTS TABLE 41-4 PROGRESSIVE CHANGES IN ARTERIAL OXYGEN TENSION AND CARBON DIOXIDE TENSION Paco2, Carbon dioxide tension; Pao2, arterial oxygen tension. Data from Sorbini CA, et al. Arterial oxygen tension in relation to age in healthy subjects. Respiration. 1968;25:3. The aging thorax has a greater anterior–posterior diameter than in younger adults and there is some degree of dorsal kyphosis due to osteoporosis.20 Rib mobility declines because of contractures of intercostal muscles and calcification of costal cartilage. Progressive decreases in chest wall compliance and changes in the shape of the thorax change chest wall mechanics and lead to deterioration in respiratory function (Fig. 41-1).20 Strength of the diaphragm and intercostal muscles decreases with age. The diaphragm is the most important inspiratory muscle because its movement accounts for 75% of change in intrathoracic volume during quiet respiration. Other factors, such as an increase in abdominal girth and change in posture, also decrease thoracic excursion. Respiratory muscle function is affected by skeletal muscle and peripheral muscle strength.20 During aging, skeletal muscle progressively atrophies and its energy metabolism decreases, which may partially explain the declining strength of the respiratory muscles.20 Handgrip strength testing can be a simple and useful measurement in assessment of muscle function.20 Respiratory muscle performance is impaired concomitantly by the age-related geometric modifications of the rib cage, decreased chest wall compliance, and increase in functional residual capacity (FRC) resulting from decreased elastic recoil of the lung. Changes in chest wall compliance lead to a greater contribution to breathing from the diaphragm and abdominal muscles and a lesser contribution from thoracic muscles.20 These anatomic changes are reflected by an increase in residual volume and decrease in vital capacity (see Table 41-3). Age-related decline in chest wall compliance of the respiratory system is 20% less in a 60-year-old subject compared with a 20-year-old.20 Table 41-5 summarizes age-related changes in the chest. TABLE 41-5 AGE-RELATED CHANGES IN THE CHEST Adapted from Bonomo L, et al. Aging and the respiratory system. Radiologic Clin North Am. 2008;46(4). Maximum inspiratory and expiratory pressures may decrease by as much as 50% because of decline in respiratory muscle strength, resulting in a decrease in thoracic wall excursion.2 The age-related reductions in maximal inspiratory pressure and maximal expiratory pressure are likely a consequence of impaired respiratory mechanics and sarcopenia.21 Sarcopenia describes reduced muscle mass and function, which may be related to decreased muscle protein synthesis, increased muscle proteolysis, motor neuron loss, and increased muscle fat content.21 Respiratory muscle strength is related to nutritional status, often deficient in older adults.20 Additionally, prevalence of vertebral fractures increases with age, particularly in women.20 The accessory inspiratory muscles (sternocleidomastoid, scalene, and trapezius) facilitate inspiration during exercise. Reports have described that ventilatory muscle strength improved after older adult men and women received ventilatory muscle training.2 There is an age-associated decrease in effectiveness of the cough reflex and decrease in ciliary responsiveness and motion that predisposes older adult patients to aspiration and hospital-acquired infections.2 These changes emphasize the importance of dysphagia screening, deep breathing, and coughing for the older patient in the critical care unit. Age-associated changes in pulmonary function do not alter the older adult’s ability to breathe effortlessly, although a decrease in respiratory muscle strength may be a limiting factor during exercise. The application of conventional quality control standards to objective assessment of pulmonary function in older subjects may prove difficult because of mood alterations, fatigability, lack of cooperation, or cognitive impairment.21 A diminished recoil of the lung occurs with aging, causing increased lung volume, distention of the alveolar spaces, and a decrease in surface area of airspace occurs starting in the third decade of life.20 Reduced lung elasticity results from changes in the ratio of elastic to support tissue that occur with advancing age.22 Ventilation and diffusion depend on numerous factors, including lung surface area. Displacement of inhaled air volume away from the alveoli limits the surface area available for gas exchange. This may in part explain the progressive and linear decrease in pulmonary diffusion capacity, which depends on surface area and capillary blood volume.22 There are reports that capillary blood volume and surface area also decrease with advancing age. Changes in pulmonary circulation result in a ventilation/perfusion (V/Q) mismatch.21 V/Q mismatch leads to a decline in arterial oxygen tension of approximately 0.3 mm Hg per year from the age of 30 years.2 The typical Pao2 for healthy persons older than 65 years is approximately 89 mm Hg, compared with 100 mm Hg for younger adults aged 18 to 24 years.21 Age-related changes in ventilation and arterial tension for carbon dioxide (Paco2) occur across the adult life span. Total minute ventilation (VE) must increase with advancing age to maintain arterial tension for carbon dioxide (Paco2). Paco2 is largely dependent on the VE, which is the sum of alveolar ventilation (VA) and dead space ventilation. V/Q inequality may exist with decreased diffusion capacity of the lung for carbon monoxide and the transfer capacity of oxygen across the alveolar–capillary interface.21 The impact of exposure to air and inhaled pollutants creates a challenge in differentiating the true impact of normal physiologic aging from that of environmental exposure.20 Control of ventilatory responses to hypoxia and hypercapnia falls by 50% and 40%, respectively, in the older adult.2 This may be due to declining chemoreceptor function.5 Decreases in Pao2 could be the result of an increase in closing volume in dependent lung zones during resting tidal breathing in older subjects.22a,22b Consequently, dependent lung zones may be ventilated intermittently, leading to regional differences. Alterations in blood volume and vascular resistance within the pulmonary circulation may also contribute to V/Q mismatching. Important considerations for critical care nurses with regard to older adult patients includes recognition of decreased respiratory reserve and more rapid decompensation than in younger patients.2 Other factors, such as exposure to environmental pollutants and chronic pulmonary disease, have an impact on the ability to compensate for respiratory conditions or wean from mechanical ventilation.2 Pulmonary function studies may be the best way to assess respiratory impairment in older adults, because muscle strength, ventilator control, and gaseous exchange may impair respiratory mechanics.21 Dynamic lung volumes and flow rates depend on resistance of airways and chest wall compliance and are limited by collapse of small airways during forced expiration. Age-related changes in respiratory mechanics lead to airflow restrictions, which are reflected in decreased 1-second forced expiratory volume (FEV1).21 Maximal expiratory flow rate and maximal midexpiratory flow rate are also decreased.21 Age-related decrease in dynamic lung volume is probably caused by decreased chest wall compliance, small airways closure during forced expiration, and decreased strength of expiratory muscles. Breathing exercises generate lung volumes of forced vital capacity (FVC), which is an untimed lung volume and FEV1, which is a timed lung volume. These spirometry tests can assist in assessing restrictive respiratory patterns.21 Reduced timed lung volume is exhibited in chronic obstructive pulmonary disease (COPD), asthma, bronchiectasis, and cystic fibrosis.21 Other conditions that display reductions in the timed and untimed lung volumes include kyphosis, scoliosis, myasthenia gravis, diaphragmatic paralysis, pleural effusions or fibrosis, and pulmonary hypertension.21 Older individuals are less able to protect against environmental injury and infection of the respiratory system. Decreases in T-cell function, decline in mucociliary clearance, and a decrease swallowing ability with loss of cough reflex can increase frequency and severity of pneumonia in older adults. Poor dentition, nutrition, and oral hygiene also play a role in the incidence of oropharyngeal colonization with gram-negative bacteria and aspiration pneumonia.2 Noncritical conditions of the respiratory system such as bronchitis, emphysema, COPD, and lung cancer should also be assessed in older adults, as pathology and normal aging processes are often difficult to differentiate.21
The Older Adult Patient
Overview
Health Care Statistics
Health Conditions and the Older Adult
Cardiovascular System
Age-Related Changes of the Cardiovascular System
PHYSIOLOGIC ALTERATION
MECHANISM
PATHOLOGIC CHANGE
Cardiovascular Structural Remodeling
↑ Vascular intimal thickness
Early stages of atherosclerosis
↑ Vascular stiffness
↑ LV wall thickness
↑ Left atrial size
↑ Left atrial pressure/volume
↑ Prevalence of lone atrial fibrillation and other atrial arrhythmias
Cardiovascular Functional Changes
Altered regulation of vascular tone
↓ NO production/effects
Reduced threshold for cell Ca2+ overload
Changes in gene expression of proteins that regulate Ca2+ handling; ↑ PUFA ration in cardiac membranes
↑ Cardiovascular reserve
Lower threshold for increased severity of heart failure
Reduced physical activity
Learned lifestyle
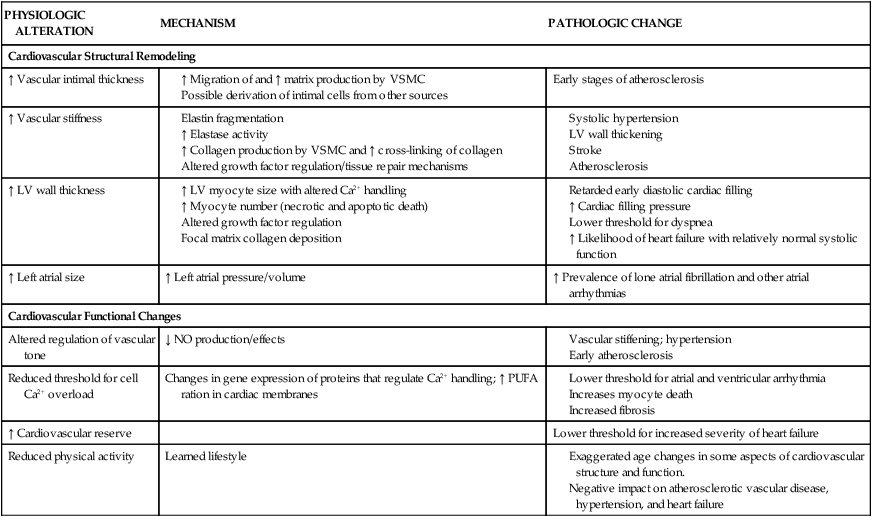
Age-Related Changes in Electrocardiogram
ECG VARIABLE
CHANGE WITH AGE
R–R internal
No change
P-wave duration
Minor increase
PR interval
Increase
QRS duration
No change
QRS axis
Leftward shift
QRS voltage
Decrease
QT interval
Minor increase
T-wave voltage
Decrease
Age-Related Changes in Myocardial Structure and Function
Myocardial Infarction and Heart Failure
Age-Related Changes in Left Ventricular Function During Exercise
Peripheral Vascular System
Hypertension
Management Recommendations.
Pharmacologic Management of Uncomplicated Hypertension.
Pharmacologic Management of Complicated Hypertension.
Age-Related Changes in Baroreceptor Function
Cardiac Medication Considerations in Older Adults
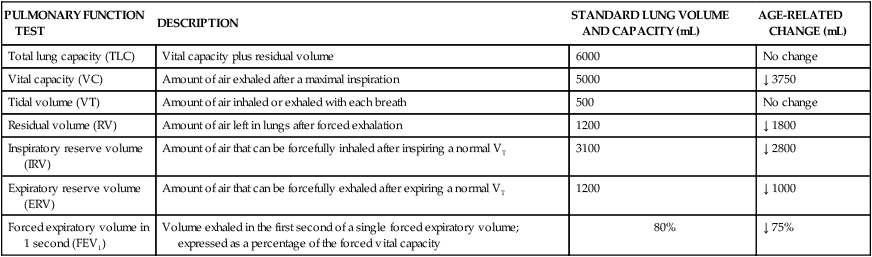
Pulmonary System
PULMONARY FUNCTION TEST
DESCRIPTION
STANDARD LUNG VOLUME AND CAPACITY (mL)
AGE-RELATED CHANGE (mL)
Total lung capacity (TLC)
Vital capacity plus residual volume
6000
No change
Vital capacity (VC)
Amount of air exhaled after a maximal inspiration
5000
↓ 3750
Tidal volume (VT)
Amount of air inhaled or exhaled with each breath
500
No change
Residual volume (RV)
Amount of air left in lungs after forced exhalation
1200
↓ 1800
Inspiratory reserve volume (IRV)
Amount of air that can be forcefully inhaled after inspiring a normal VT
3100
↓ 2800
Expiratory reserve volume (ERV)
Amount of air that can be forcefully exhaled after expiring a normal VT
1200
↓ 1000
Forced expiratory volume in 1 second (FEV1)
Volume exhaled in the first second of a single forced expiratory volume; expressed as a percentage of the forced vital capacity
80%
↓ 75%
AGE GROUP (YR)
Pao2 (mm Hg)
Paco2 (mm Hg)
≤30
94
39
31-40
87
38
41-50
84
40
51-60
81
39
>60
74
40
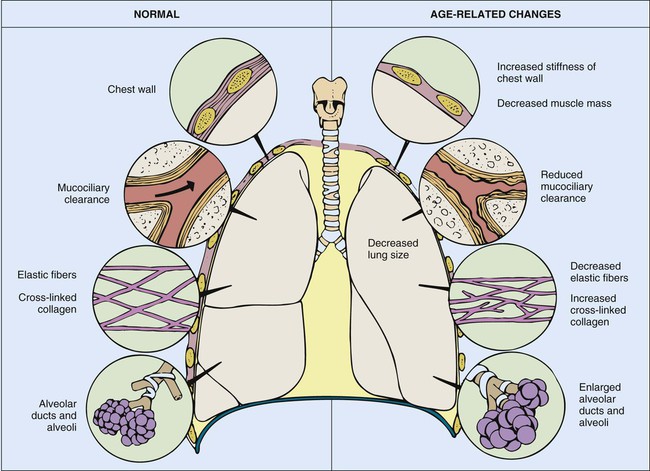
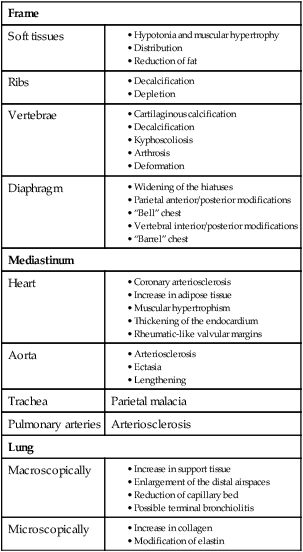
Thoracic Wall and Respiratory Muscles
Frame
Soft tissues
Ribs
Vertebrae
Diaphragm
Mediastinum
Heart
Aorta
Trachea
Parietal malacia
Pulmonary arteries
Arteriosclerosis
Lung
Macroscopically
Microscopically
Pulmonary Gas Exchange
Lung Volumes and Capacities
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Nurse Key
Fastest Nurse Insight Engine
Get Clinical Tree app for offline access