Chapter 1 The nursing role
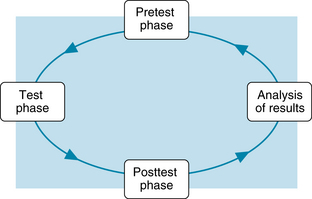
The process of laboratory or diagnostic testing can be conceptualized as a cycle that has four phases of operation: (1) the pretest phase, (2) the test phase, (3) the posttest phase, and (4) analysis of the results (Figure 1). Appropriate nursing roles and responsibilities for the test or procedure are pertinent in each phase.
Procedural role and nursing responsibilities
Pretest phase
Scheduling of a diagnostic test
When multiple tests in various departments are prescribed, it is sometimes necessary to prioritize the test schedule because the method of conducting one test can interfere with the results of another. For example, x-ray studies that use contrast medium are performed before x-ray studies that use barium contrast material. Residual barium remains in the intestine for several days, and its opacity would obscure the view of the other tissues, such as the biliary tract and abdominal vasculature. Likewise, blood tests that use a radioimmunoassay method of analysis must be performed before or 7 days after a nuclear scan because the radioisotopes of the scan would interfere with the radioimmunoassay method of analysis of the blood and alter the test results. When these interfering factors involve tests performed by a single department, such as the laboratory, the priorities are routinely sorted out by the laboratory personnel.
Requisition forms
The laboratory requisition form is the major way to request the needed tests for the patient. The physician or health care provider who orders the test is also responsible to complete the requisition form. This simplified method avoids potential transcription errors that can occur when someone else completes the written request (McPherson & Pincus, 2007). Usually, the requisition is sent to the laboratory electronically, but it may be written manually.
The needed tests are selected by the physician or health care provider. Specific information may be included, such as pertinent medical history, suspected diagnosis, date of last menstrual period, or pregnancy status. This additional information is used to help with diagnosis and to establish the correct reference values based on age, gender, or other biologic variables.
Test phase
Identification procedures
It is essential to perform a correct identification before collecting the specimen or starting a diagnostic procedure, such as bone marrow or other biopsy, culture, or any other specimen. The Joint Commission (2010) requires that the person who collects the specimen must obtain the patient’s identification from two different sources. One source is the name on the patient’s wristband and it is the same name as that on the requisition. The second source is that the patient states his or her full name. The patient’s room number or physical location cannot be used for identification purposes.
Infection control: standard precautions
Standard precautions must be used when obtaining or handling a blood or body fluid specimen. Gloves must be worn during the collection procedure. If splashing or contact with a mucous membrane is anticipated, the nurse wears a mask, protective eyewear, and a gown or protective clothing in addition to the gloves.
Point-of-care testing
Point-of-care (POC) testing refers to methods of testing and analysis that bring the laboratory services nearer to the patient. This type of testing also is known as bedside testing, near-patient testing, or a rapid test. Satellite laboratories may be established near operating rooms, intensive care units, and emergency departments. Desktop analyzers may be used in these sites as well and in a clinic, an ambulatory care setting, a physician’s office, and long-term care facility. There is rapid turn-around time between obtaining the specimen and receiving the test results. Medical diagnosis and treatment is based on real-time values and will be more accurate, avoiding a long delay before the central laboratory results could be available. There has been a dramatic expansion of these rapid tests during the past decade and this trend is likely to continue in the future (Lewandrowski, 2009a; Nichols, 2007). A partial list of the point-of-care tests is presented in Box 1.
Box 1 Partial List of Tests Available With Point-of-Care Testing
From Lewandrowski, K. (2009). Point of care testing: An overview and a look to the future (circa 2009, United States). Clinics in Laboratory Medicine, 29(3), 427.
Another category of point-of-care testing refers to the testing that is performed with small handheld instruments that analyze the patient’s blood in a few minutes. The specimen is obtained and the blood analyzed wherever the patient is located, including the home or the workplace. The patient is usually the person who performs the test as part of self-care responsibilities. Blood testing monitors are used for timely measurement of glucose levels in diabetics and more recently for monitoring the prothrombin time-international normalized ratio (INR) in those who are maintained on anticoagulant medication.
Expanded nursing role
The laboratory is responsible for point-of-care testing, policies, procedures, and maintenance of the equipment. Nurses, as the providers of direct patient care, may use the automated analyzers. Alternatively, multitasked nursing assistants may perform the point-of-care testing. As part of laboratory accreditation requirements, the person who uses this equipment must have formal training for each test, certification that the training has been completed satisfactorily, and an annual reevaluation of performance (Ehrmeyer & Laessig, 2009). Without the training there is a high incidence of inconsistent performance and inaccurate test results.