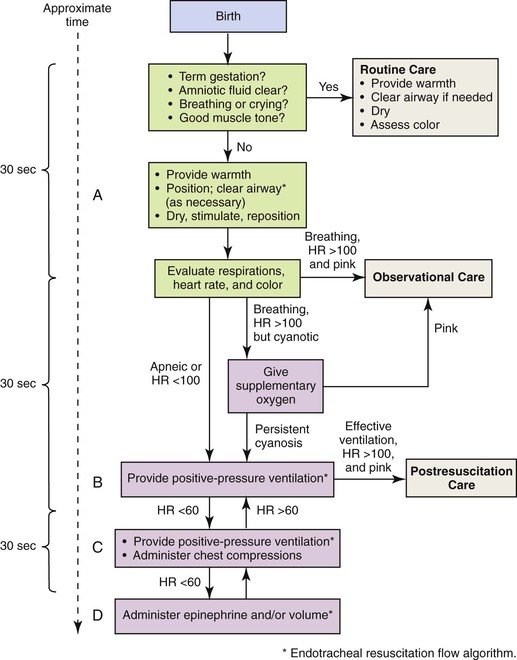
• Compare and contrast the physical characteristics of preterm, late preterm, term, and postterm neonates. • Discuss respiratory distress syndrome and the approach to treatment. • Compare methods of oxygen therapy for the high risk infant. • Describe nursing interventions for nutritional care of the preterm infant. • Discuss the pathophysiologic mechanism of retinopathy of prematurity and bronchopulmonary dysplasia (chronic lung disease), and identify the predisposing risk factors. • Describe the treatment of the infant with meconium aspiration. • Describe risk factors associated with the birth and transition of an infant of a mother with diabetes. • Summarize the assessment and care of the newborn with soft-tissue, skeletal, and nervous system injuries caused by birth trauma. • Describe methods used to identify clinical signs of infection in the newborn. • Identify the effects of maternal use of alcohol, heroin, methadone, marijuana, methamphetamine, cocaine, and smoking tobacco on the fetus and newborn. • Describe the assessment of a newborn exposed to recreational drugs in utero. • Compare characteristics of neonatal Rh and ABO incompatibility. • Plan developmentally appropriate care for the high risk infant. • Develop a plan to address the unique needs of parents of high risk infants. • Describe emotional, behavioral, cognitive, and physical responses commonly experienced during the grieving process associated with perinatal loss. • Identify specific nursing interventions to meet the special needs of parents and their families related to perinatal loss and grief. late preterm (near term) infant An infant born between Additional information related to the content in Chapter 24 can be found on the companion website at http://evolve.elsevier.com/Lowdermilk/maternity/ • Critical Thinking Exercise: Patent Ductus Arteriosus • Critical Thinking Exercise: Fetal Alcohol Syndrome • Nursing Care Plan: The Drug-Exposed Newborn • Nursing Care Plan: Fetal Death: 24 Weeks of Gestation • Nursing Care Plan: The High Risk Preterm Newborn • Nursing Care Plan: The Infant of a Mother with Diabetes Mellitus • Spanish Guidelines: Intensive Care Nursery: Parent Teaching on First Visit High risk infants are most often classified according to birth weight, gestational age, and predominant pathophysiologic problems (Box 24-1). Intrauterine growth rates may differ among infants; factors such as heredity, placental insufficiency, and maternal disease influence intrauterine growth and birth weight. The classification system in the box encompasses birth weight and gestational age. The potential problems and care needs of the preterm infant weighing 2000 g differ from those of the term or postterm infant of equal weight. The presence of physiologic disorders and anomalies affects the infant’s response to treatment. These conditions include necrotizing enterocolitis, growth failure, bronchopulmonary dysplasia, intraventricular-periventricular hemorrhage, and retinopathy of prematurity. (See Table 24-2 on pp. 762-763). With the advent of managed care, attempts to cut health care costs were made. Infants who appeared to be “near” term began to be treated much the same as term infants, thus avoiding the excess costs of neonatal intensive care for infants who appeared to be healthy. Late-preterm infants (infants born between Late-preterm infants comprise approximately 70% of the total preterm infant population and the mortality rate for this group is significantly higher than that of term infants (7.9 per 1000 live births versus 2.4 per 1000 live births, respectively) (Tomashek, Shapiro-Mendoza, Davidoff, & Petrini, 2007). Because birthweights of late-preterm infants often range from 2000 to 2500 g and they appear relatively mature in comparison to the smaller less mature infant they may be cared for in the newborn nursery; within this setting, risk factors for late-preterm infants may be overlooked. The Association of Women’s Health, Obstetric, and Neonatal Nurses (AWHONN) published a Late-Preterm Assessment Guide (Santa-Donato, Medoff-Cooper, Bakewell-Sachs, Frazer Askin, & Rosenberg, 2007) for the education of perinatal nurses regarding the late-preterm infant’s risk factors and appropriate care and follow up (Table 24-1) (see also Chapter 18). TABLE 24-1 Late-Preterm Infant Assessment and Interventions *This list is not exhaustive of nursing interventions; additional interventions include those discussed under the care of the high risk infant in this chapter. Source: Santa-Donato, A., Medoff-Cooper, B., Bakewell-Sachs, S., Frazer Askin, D., & Rosenberg, S. (2007). Late preterm infant assessment guide. Washington, DC: Association of Women’s Health, Obstetric and Neonatal Nurses. For the high risk infant, an accurate assessment of gestational age (see Chapter 17) is critical in helping the nurse identify the potential problems the newborn can experience. The response of preterm, late preterm, and postterm infants to extrauterine life is different from that of term infants. By understanding the physiologic basis of these differences the nurse can assess these infants; determine the response of the preterm, late-preterm, or postterm infant; and anticipate potential problems. • Decreased number of functional alveoli • Decreased tracheal cartilage • Greater obstruction of respiratory passages • Insufficient calcification of the bony thorax • Circulating hormones (prostaglandins) that may affect cardiovascular function • Immature and fragile pulmonary vasculature • Distance between functional alveoli and capillary bed, especially in ELBW infants • Large surface area in relation to body weight • Minimal insulating subcutaneous fat • Limited stores of brown fat (an internal source for the generation of heat present in normal term infants) • Decreased or absent reflex control of skin capillaries (vasoconstriction) • Inadequate muscle mass activity • Poor muscle tone, resulting in more body surface area being exposed to the cooling effects of the environment • An immature temperature regulation center in the brain • Increased insensible water loss • Decreased ability to increase oxygen consumption The goal of thermoregulation is a neutral thermal environment (NTE), which is the environmental temperature at which oxygen consumption and metabolic rate are minimal but adequate to maintain the body temperature (Blackburn, 2007). The NTE range for preterm infants weighing less than 1000 g is very narrow, and the prediction of NTE for each infant is impossible. Extremely immature infants may require environmental temperatures equal to skin and core temperature or possibly higher to achieve thermoneutrality (Blackburn). With knowledge of the four mechanisms of heat transfer (i.e., convection, conduction, radiation, evaporation) the nurse can create an environment for the preterm infant that promotes temperature stability (see Chapter 16). Given that overheating produces an increase in oxygen and calorie consumption, the infant is also jeopardized if he or she becomes hyperthermic (apnea and flushed color may indicate hyperthermia). The preterm infant is not able to sweat and thus dissipate heat. • Birth trauma causing damage to immature intracranial structures • Bleeding from fragile capillaries • Impaired coagulation process, including prolonged prothrombin time • Recurrent hypoxic and hyperoxic episodes • Predisposition to hypoglycemia • Fluctuating systemic BP with concomitant variation in cerebral blood flow and pressure The preterm infant is predisposed to hematologic problems because of the following conditions: • Increased capillary fragility • Increased tendency to bleed (prolonged prothrombin time and partial thromboplastin time) • Decreased production of red blood cells (RBCs) resulting from physiologic rapid decrease in erythropoiesis after birth • Large amount of fetal hemoglobin (up to 80% of the total volume) • Loss of blood attributable to frequent blood sampling for laboratory tests • Decreased RBC survival related to the relatively larger size of the RBC and its increased permeability to sodium and potassium Neonates are highly susceptible to infection as a result of diminished nonspecific (inflammatory) and specific (humoral) immunity, such as impaired phagocytosis, delayed chemotactic response, minimal or absent immunoglobulin A (IGA) and immunoglobulin M (IgM), and decreased complement levels. Because of the infant’s poor response to pathogenic agents, in most instances, no local inflammatory reaction is seen at the portal of entry to signal an infection, and the resulting symptoms tend to be vague and nonspecific. Consequently, diagnosis and treatment may be delayed. Preterm and term infants exhibit various nonspecific signs and symptoms of infection (Box 24-2). Early identification and treatment of sepsis is essential. • Incubator or radiant warmer to control body temperature (NTE) • Oxygen administration, depending on infant’s pulmonary and circulatory status • Electronic monitoring of respiratory and cardiac function • Assistive devices for positioning the infant • Clustering of care and minimization of stimulation and handling Various metabolic support measures that may be instituted consist of the following: • Parenteral fluids to support nutrition, hydration, and fluid and electrolyte balance • IV access for fluids, parenteral nutrition, and to facilitate medication administration • Blood work to monitor arterial blood gases (ABGs), blood glucose level, and electrolytes and other diagnostic studies (C-reactive protein, white blood cell [WBC] count with differential, hemoglobin, and hematocrit) as indicated The preterm infant is susceptible to heat loss and its complications (see Fig. 16-2 on p. 444). In addition, low-birth-weight (LBW) infants may be unable to increase their metabolic rate because of impaired gas exchange, caloric intake restrictions in relation to high expenditure, or poor thermoregulation. Transepidermal water loss is greater than in the term infant because of skin immaturity in ELBW and VLBW infants (i.e., those weighing less than 1000 g and 1500 g, respectively) and can contribute to temperature instability. The preterm infant should be transferred from the birth room in a prewarmed incubator; ELBW infants may be placed in a polyethylene bag to decrease heat and water loss (Fig. 24-1). Skin-to-skin contact (kangaroo care) between the stable preterm infant and parent is a viable option for interaction because of the maintenance of appropriate body temperature by the infant (see p. 767 for further discussion of kangaroo care). Preterm and other high risk infants are cared for in the NTE created by use of an external heat source. A probe applied to the infant is attached to an external heat source supplied by a radiant warmer or a servo-controlled incubator. Optimal thermoneutrality cannot be predicted for every preterm infant’s needs. The American Academy of Pediatrics and the American Heart Association Neonatal Resuscitation Program recommends that the first axillary temperature not be below 36.5° C (Kattwinkel, 2006). Standard guidelines for maintaining NTE in the LBW infant are published (Blake & Murray, 2006). Further research is needed to define an NTE for the ELBW infant. The goals of oxygen therapy are to provide adequate oxygen to the tissues, prevent lactic acid accumulation resulting from hypoxia, and at the same time avoid the potentially negative effects of hyperoxia and free radicals. Numerous methods have been devised to improve oxygenation (Fig. 24-2). All of these methods require that the gas be warmed and humidified before entering the respiratory tract. If the infant does not require mechanical ventilation, oxygen can be supplied by plastic hood placed over the infant’s head, by nasal cannula, or by nasal continuous positive airway pressure (CPAP) to supply variable concentrations of humidified oxygen. Because oxygen therapy is not without inherent hazards, each infant must be closely monitored to prevent hyperoxemia and hypoxemia. In 2010 the American Heart Association published neonatal resuscitation guidelines (Kattwinkel, Perlman, Aziz, et al., 2010). A rapid assessment of infants can identify those who do not require resuscitation: those born at term gestation, with no evidence of meconium or infection in the amniotic fluid, those who are breathing or crying, and those with good muscle tone. If any of these characteristics is absent, the infant should receive the following actions in sequence: (1) initial steps in stabilization: provide warmth by placing the baby under a radiant warmer, position the head in a position to open the airway, clear the airway with a bulb syringe or suction catheter, dry the baby, stimulate breathing, and reposition the baby; (2) ventilation; (3) chest compressions; and (4) administration of epinephrine or volume expansion or both. The decision to move from one category of action to the next is based on the assessment of respirations, heart rate, and color. Rapid decision making is imperative; 30 seconds are allotted for each step. The condition of the infant is reevaluated and the decision made whether to progress to the next step (Fig. 24-3). Resuscitation of asphyxiated newborns with 21% oxygen rather than 100% oxygen shows promise. Proponents for room air resuscitation suggest that fewer complications are associated with oxidative stress and hyperoxemia when room air is administered. The 2010 American Heart Association resuscitation standards for neonatal resuscitation stress that resuscitation may begin with no supplemental oxygen (i.e., 21% or room air) but that if the infant’s condition does not improve within 90 seconds, supplemental oxygen should be available for use. The stated goal is to minimize oxygen free radicals by preventing hyperoxia using supplemental oxygen at levels less than 100% (Kattwinkel, et al., 2010). A review of several studies indicates that neonatal mortality is reduced by 30% to 40% when room air instead of 100% oxygen is used for neonatal resuscitation (Saugstad, 2007). Fluctuations in oxygen saturation are also deemed harmful. Experts recommend that oxygen saturations for ELBW infants be maintained between 85% and 93% but definitely not exceeding 95% (Saugstad). Rates of retinopathy of prematurity and bronchopulmonary dysplasia are reduced in infants whose arterial oxygen saturation (SaO2) is kept between 93% and 95%. Surfactant can be administered as an adjunct to oxygen and ventilation therapy (see Medication Guide: Surfactant Replacement). Generally, infants born before 32 weeks of gestation do not have adequate amounts of pulmonary surfactant to survive extrauterine life. In many centers the use of prophylactic surfactant is reserved for infants younger than 29 weeks who will likely have respiratory distress syndrome (RDS) (see Table 24-2) (Hagedorn, Gardner, Dickey, & Abman, 2006). The American Academy of Pediatrics (AAP) Committee on Fetus and Newborn (Engle & AAP Committee on Fetus and Newborn, 2008) recommends the use of surfactant in infants with RDS as soon as possible after birth, especially ELBW infants and those not exposed to maternal antenatal steroids. The administration of antenatal steroids to the mother and surfactant replacement has decreased the incidence of RDS and concomitant morbidities. Frequent skin assessments are essential when the infant is receiving supplemental oxygen with any of the methods described herein but particularly in infants with poor perfusion and in those requiring equipment that comes in continuous contact with the infant’s skin (e.g., nasal CPAP, nasal cannula, pulse oximetry probes). A greater-than-normal incidence of skin breakdown is noted in infants and children who require the use of medical devices (e.g., nasal prongs, pulse oximetry probes) (Noonan, Quigley, & Curley, 2006).
The Newborn at Risk
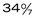 and
and 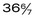 weeks of gestation, regardless of birth weight
weeks of gestation, regardless of birth weight
Web Resources
![]()
Preterm Infant

Late-Preterm Infant
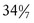 and
and 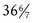 weeks of gestation) may be able to make an effective transition to extrauterine life; however, such infants, by nature of their limited gestation, remain at risk for problems related to thermoregulation, hypoglycemia, hyperbilirubinemia, sepsis, and respiratory function (Bakewell-Sachs, 2007). Experts now recommend that infants born between 34 and
weeks of gestation) may be able to make an effective transition to extrauterine life; however, such infants, by nature of their limited gestation, remain at risk for problems related to thermoregulation, hypoglycemia, hyperbilirubinemia, sepsis, and respiratory function (Bakewell-Sachs, 2007). Experts now recommend that infants born between 34 and 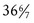 weeks gestation be called late-preterm infants rather than near-term infants (Engle, 2006; Engle, Tomashek, & Wallman, 2007).
weeks gestation be called late-preterm infants rather than near-term infants (Engle, 2006; Engle, Tomashek, & Wallman, 2007).
RISK FACTORS
ASSESSMENT
INTERVENTIONS*
Respiratory distress (RD)
Assess for cardinal signs of RD (nasal flaring, grunting, tachypnea, central cyanosis, retractions), for presence of apnea especially during feedings, and for hypothermia, hypoglycemia.
Perform gestational age assessment; observe for signs of RD; monitor oxygenation by pulse oximetry; provide supplemental oxygen judiciously.
Thermal instability
Monitor axillary temperature every 30 min immediately postpartum until stable; thereafter every 1-4 hr, depending on gestational age and ability to maintain thermal stability.
Provide skin-to-skin care in immediate postpartum period for stable infant; implement measures to prevent excess heat loss (adjust environmental temperature, avoid drafts); bathe only after thermal stability has been maintained for 1 hr.
Hypoglycemia
Monitor for signs and symptoms of hypoglycemia; assess feeding ability (latch on, nipple feeding); assess thermal stability, signs and symptoms of RD; monitor bedside glucose in infants with additional risk factors (mother with diabetes, prolonged labor, RD, poor feeding).
Initiate early feedings of human milk or formula; avoid dextrose water or water feedings; provide intravenous dextrose as necessary for hypoglycemia.
Jaundice
Observe for jaundice in first 24 hr; evaluate maternal-fetal history for additional risk factors that may cause increased hemolysis and circulating levels of unconjugated bilirubin (Rh, ABO, spherocytosis, bruising); assess feeding method, voiding, stooling patterns.
Monitor transcutaneous bilirubin, and note risk zone on hour-specific nomogram (Fig. 17-8).
Feeding problems
Assess suck-swallow and breathing; assess for RD, hypoglycemia, thermal stability; assess latch-on, maternal comfort with feeding method; weight loss no more than 10% of birth weight.
Initiate early feedings—human milk or formula; ensure maternal knowledge of feeding method and signs of inadequate feeding (sleepiness, lethargy, color changes during feeding, apnea during feeding, decreased or absent urinary output).
Care Management

Respiratory function
Body temperature
Central nervous system function
Hematologic status
Infection prevention
Interventions
Physical care
Maintain body temperature
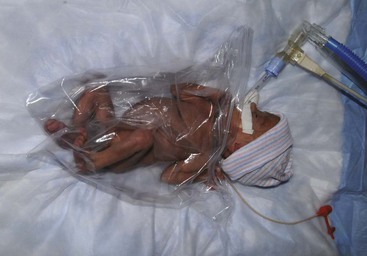
Oxygen therapy
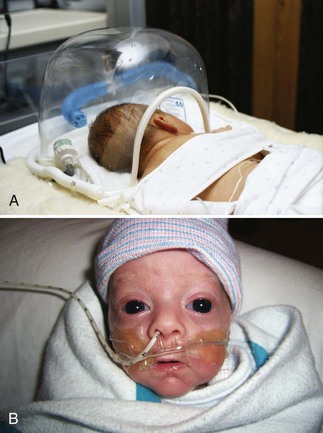
Neonatal resuscitation
Surfactant replacement therapy
Weaning from respiratory assistance
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


The Newborn at Risk
Get Clinical Tree app for offline access

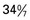 and
and 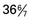 weeks of gestation, regardless of birth weight
weeks of gestation, regardless of birth weight